Influence of Tert-Butylthiol and Tetrahydrofuran on the Holographic Characteristics of a Polymer Dispersed Liquid Crystal: A Research Line Toward a Specific Sensor for Natural Gas and Liquefied Petroleum Gas
Abstract
:1. Introduction
2. Photopolymer Formulation
2.1. Sample Preparation
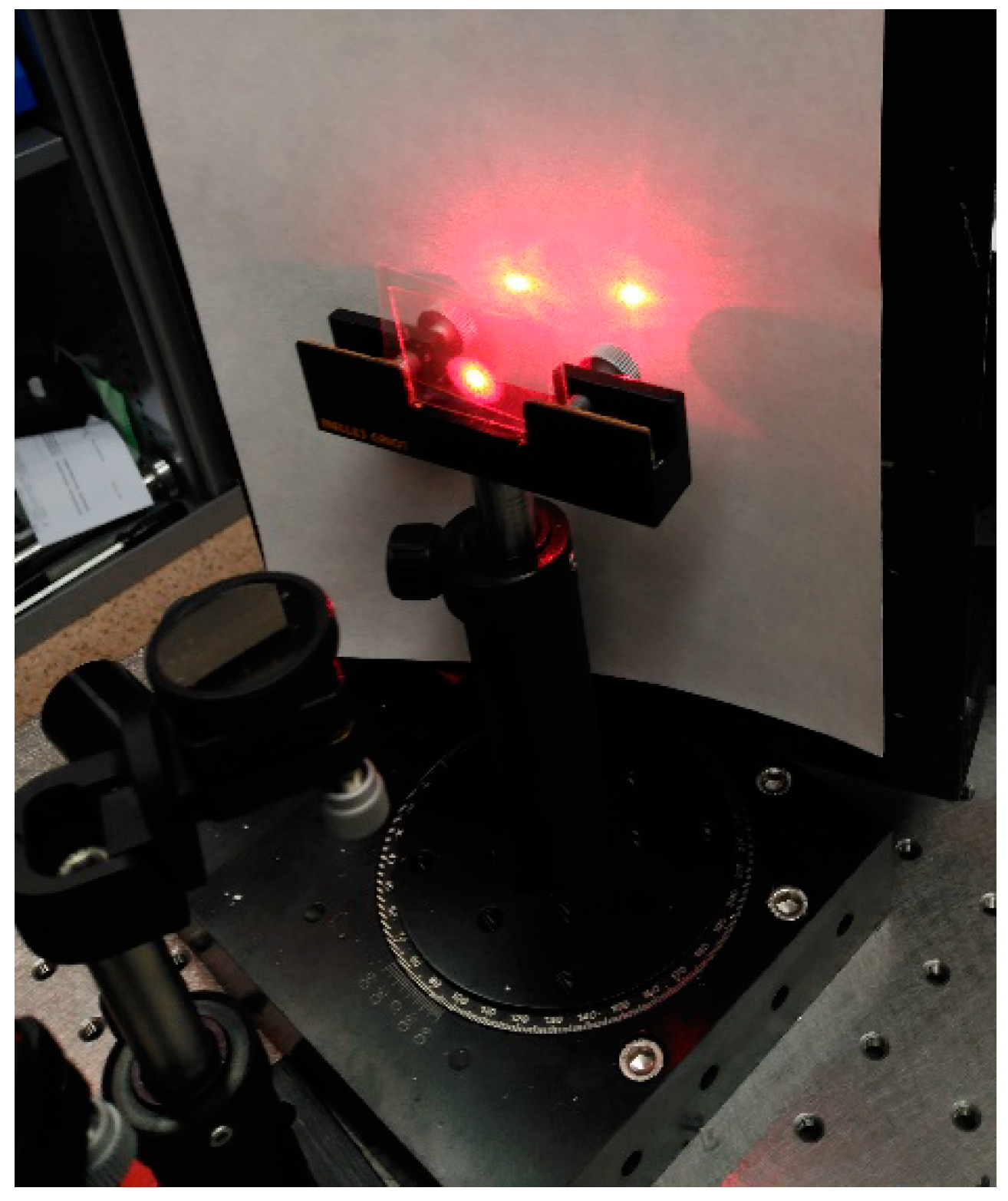
3. Optical Set-Up
4. Results
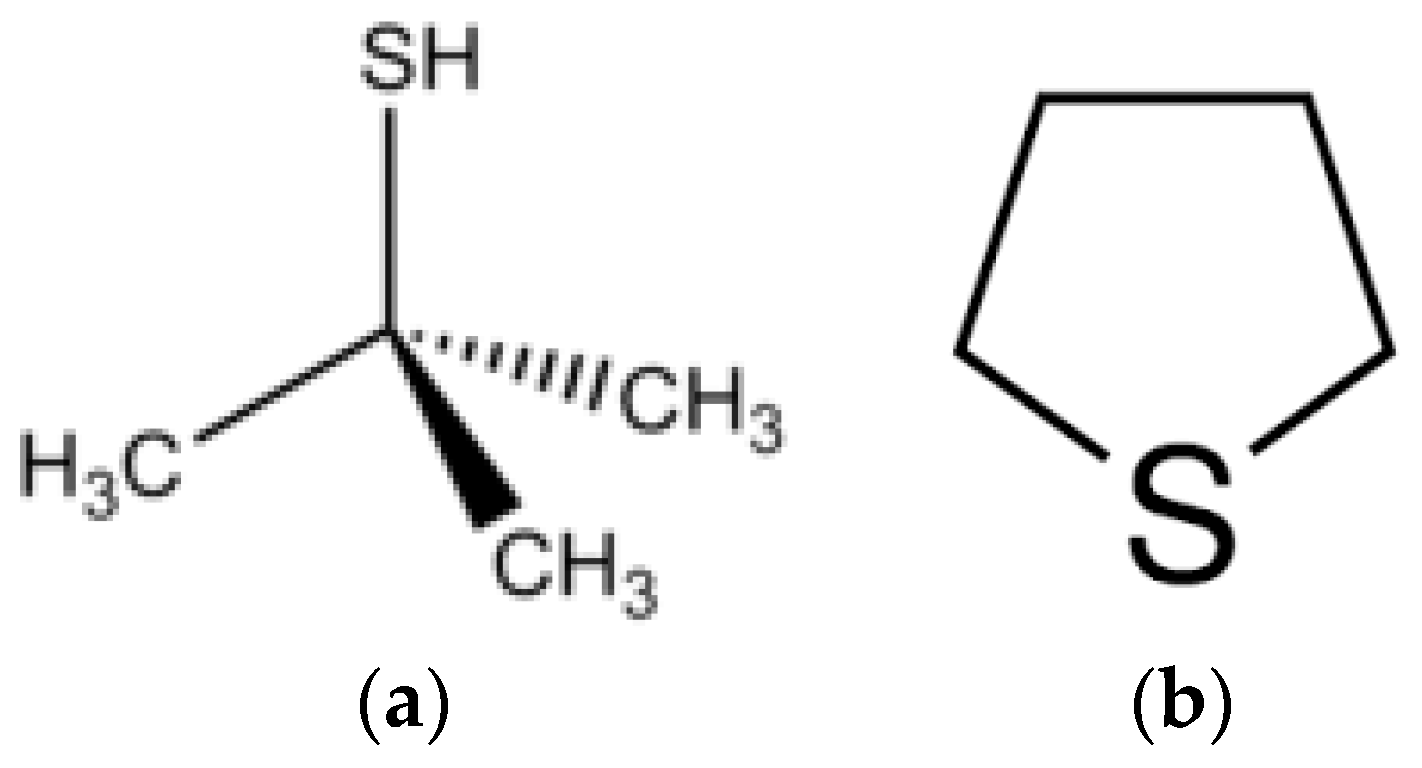
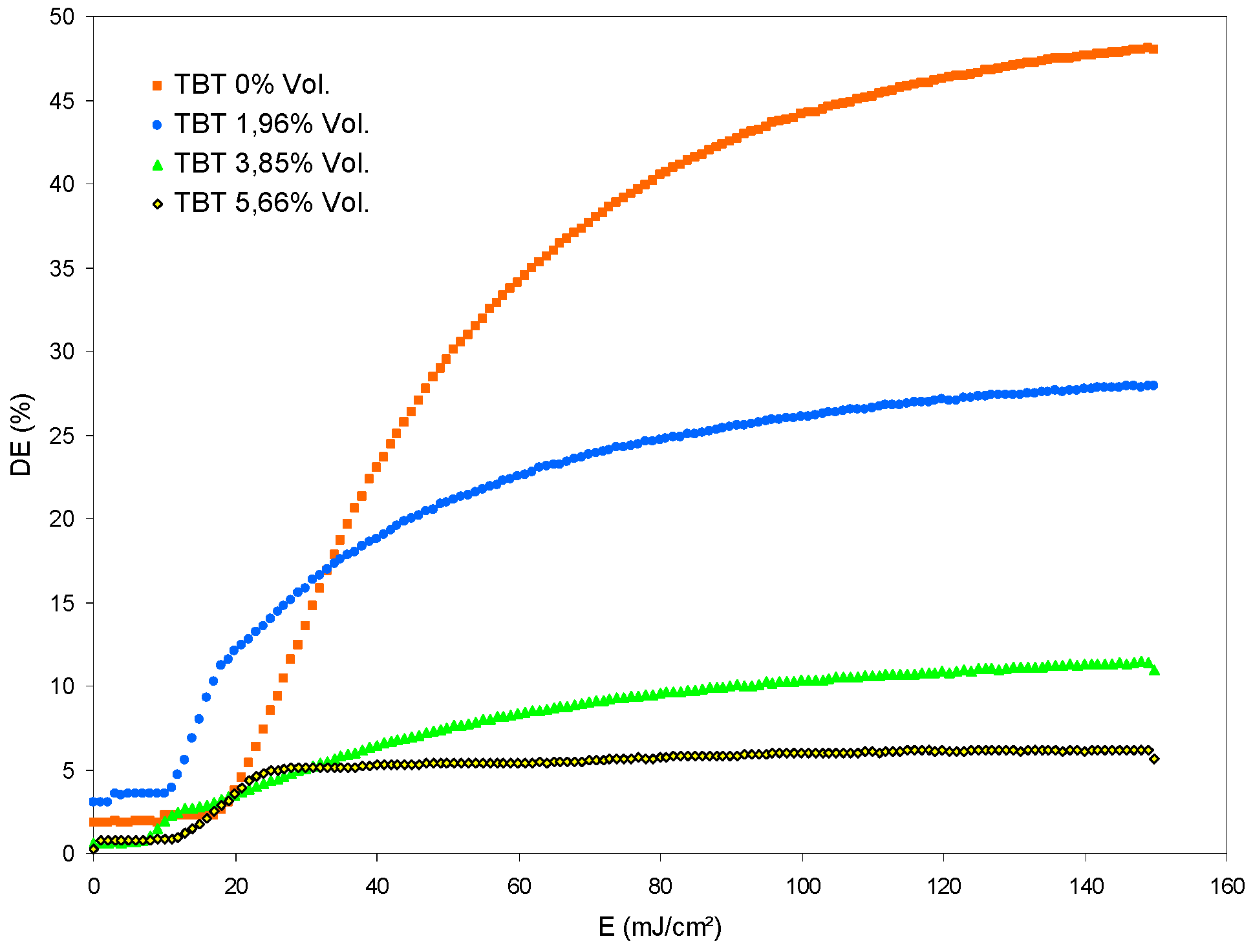
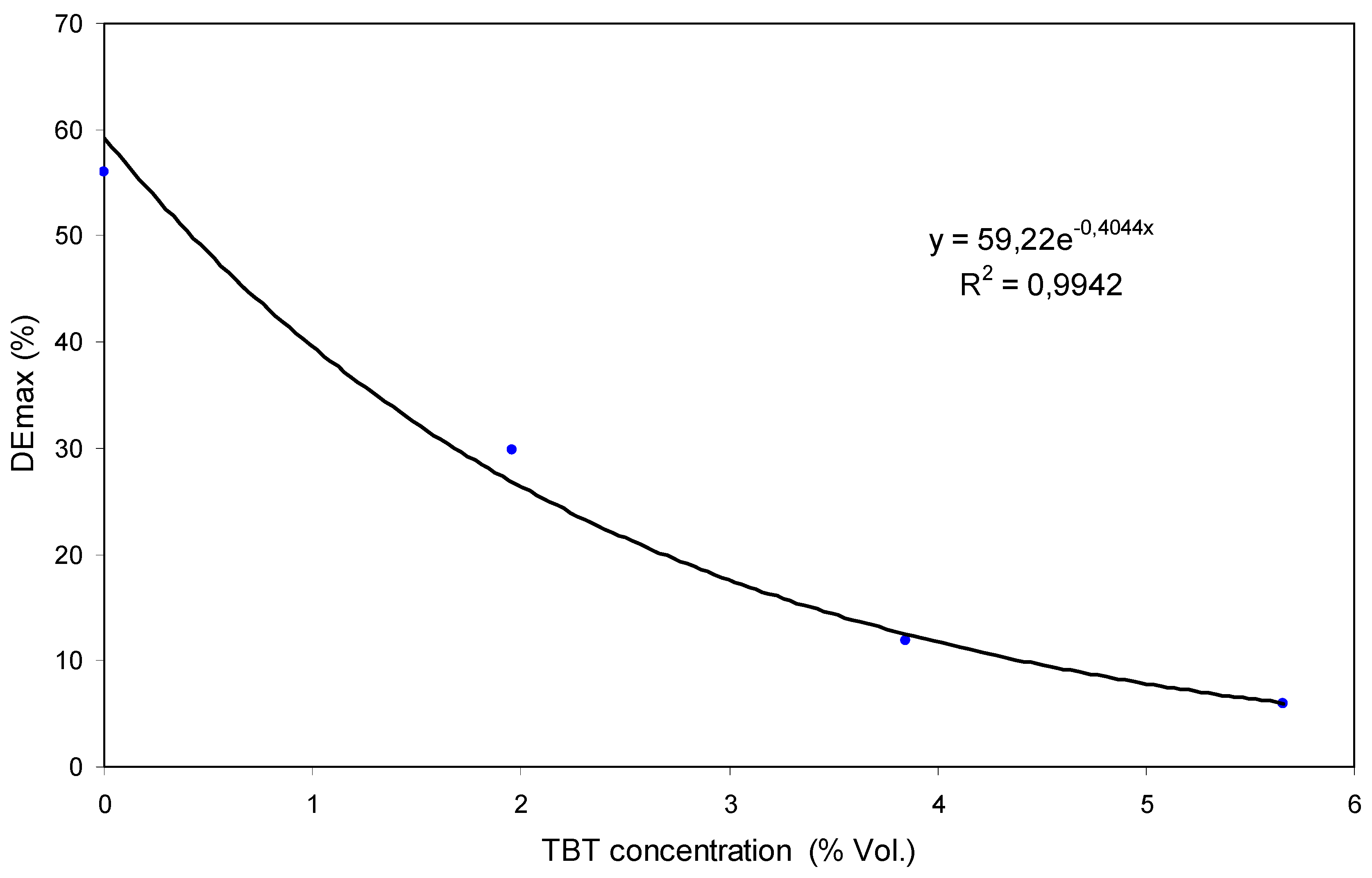
4.1. Experiments with TBT
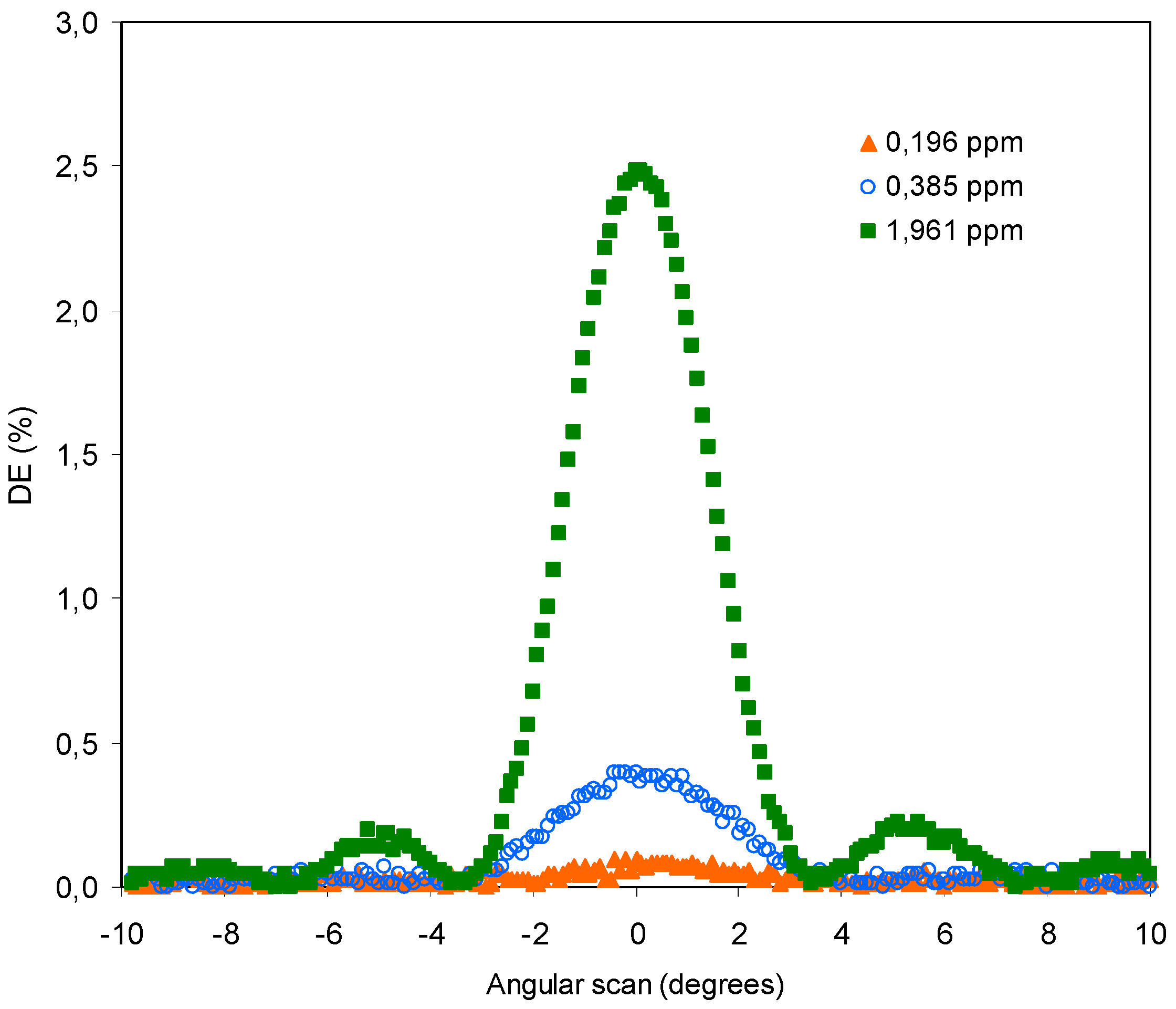
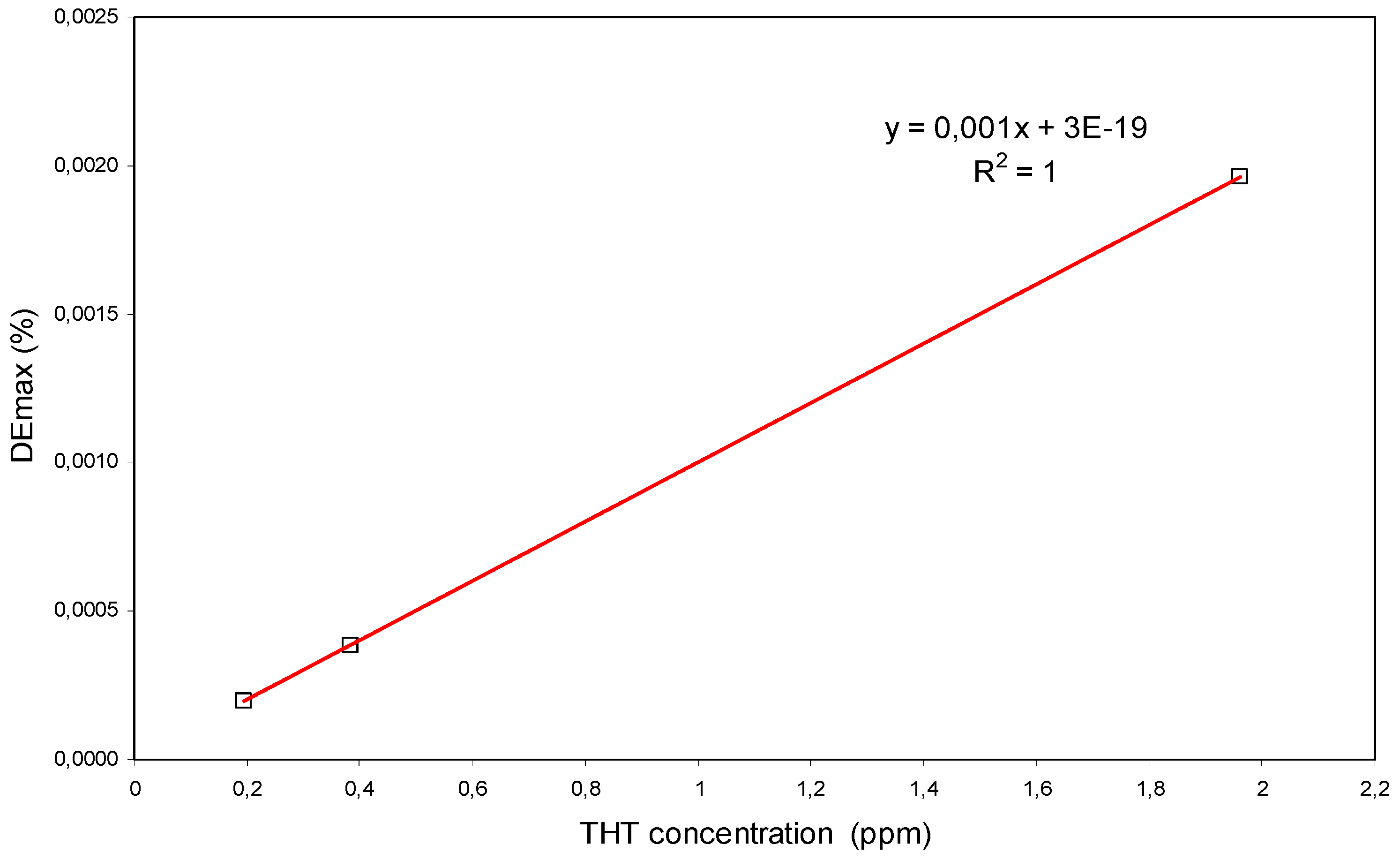
4.2. Experiments with THT
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bogue, R. Recent developments in MEMS sensors: A review of applications, markets and technologies. Sens. Rev. 2013, 33, 300–304. [Google Scholar] [CrossRef]
- Azad, A.M.; Akbar, S.A.; Mhaisalkar, S.G.; Birkefeld, L.D.; Goto, K.S. Solid-State Gas Sensors: A Review. J. Electrochem. Soc. 1992, 139, 3690–3704. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Butt, H.; da Cruz Vasconcellos, D.; Montelongo, Y.; Davidson, C.A.B.; Blyth, J.; Chan, L.; Carmody, J.B.; Vignolini, S.; Steiner, U.; et al. Light-Directed Writing of Chemically Tunable Narrow-Band Holographic Sensors. Adv. Opt. Mater. 2014, 2, 250–254. [Google Scholar] [CrossRef]
- Yetisen, A.K. Holographic Sensors; Springer-Verlag: New York, NY, USA, 2015. [Google Scholar]
- Yetisen, A.K.; Naydenova, I.; da Cruz Vasconcellos, F.; Blyth, J.; Lowe, C.R. Holographic sensors: Three-dimensional analyte-sensitive nanostructures and their applications. Chem. Rev. 2014, 114, 10654–10696. [Google Scholar] [CrossRef]
- Yunusa, Z.; Hamidon, M.N.; Kaiser, A.; Awang, Z. Gas Sensors: A Review. Sens. Transducers J. 2014, 168, 61–75. [Google Scholar]
- Kohl, D. Function and applications of gas sensors. J. Phys. D Appl. Phys. 2001, 34, 19. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef] [Green Version]
- Stetter, J.R.; Li, J. Amperometric Gas Sensors—A Review. Chem. Rev. 2008, 108, 352–366. [Google Scholar] [CrossRef]
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuators B Chem. 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banacha, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; Berg, A.V. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Martínez-Hurtado, J.L.; Davidson, C.A.B.; Lowe, C.R. Monitoring Organic Volatiles and Flammable Gases with a Holographic Sensor; Proceedings SPIE 8024; SPIE: Bellingham, WA, USA, 2011. [Google Scholar]
- Lagerwalla, J.P.F.; Scaliaa, G. A new era for liquid crystal research: Applications of liquid crystals in soft matter nano-, bio- and microtechnology. Curr. Appl. Phys. 2012, 12, 1387–1412. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, L.V.; Brown, D.P.; Wofford, J.M.; Tondiglia, V.P.; Sutherland, R.L.; Lloyd, P.F.; Bunning, T.J. Holographic polymer dispersed liquid crystal reflection gratings formed by visible light initiated thiol-ene photopolymerization. Polymer 2006, 47, 4411–4420. [Google Scholar] [CrossRef]
- Massenot, S.; Kaiser, J.; Chevallier, R.; Renotte, Y. Study of the dynamic formation of transmission gratings recorded in photopolymers and holographic polymer-dispersed liquid crystals. Appl. Opt. 2004, 43, 5489–5497. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, Y.; Tomita, Y. Computer Simulation of Bragg Grating Formation in Holographic Polymer-Dispersed Liquid Crystals Based on the Density Functional Theory; Proceedings SPIE 8429; SPIE: Bellingham, WA, USA, 2012. [Google Scholar]
- Liu, D.; Broer, D.J. Liquid Crystal Polymer Networks: Preparation, Properties, and Applications of Films with Patterned Molecular Alignment. Langmuir 2014, 30, 13499–13509. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.J.; Sun, X.W. Holographic polymer-dispersed liquid crystals: Materials, formation, and applications. Adv. Optoelectron. 2008, 2008, 684349. [Google Scholar] [CrossRef]
- Sarkar, M.; Gill, N.; Whitehead, J.; Crawford, G. Effect of monomer functionality on the morphology and performance of the holographic transmission gratings recorded on polymer dispersed liquid crystals. Macromolecules 2003, 36, 630–638. [Google Scholar] [CrossRef]
- Ortuño, M.; Márquez, A.; Gallego, S.; Pascual, I.; Beléndez, A. Experimental Conditions to Obtain Photopolymerization Induced Phase Separation Process in Liquid Crystal-Photopolymer Composite Materials under Laser Exposure. Int. J. Polym. Sci. 2014, 2014, 386736. [Google Scholar] [CrossRef]
- Ortuño, M.; Riquelme, M.; Gallego, S.; Márquez, A.; Pascual, I.; Beléndez, A. Overmodulation Control in the Optimization of a H-PDLC Device with Ethyl Eosin as Dye. Int. J. Polym. Sci. 2013, 2013, 357963. [Google Scholar] [CrossRef]
- Shih, H.; Lin, C.-C. Visible-Light-Mediated Thiol-Ene Hydrogelation Using Eosin-Y as the Only Photoinitiator. Macromol. Rapid Commun. 2013, 34, 269–273. [Google Scholar] [CrossRef]
- Hata, E.; Tomita, Y. Stoichiometric thiol-to-ene ratio dependences of refractive index modulation and shrinkage of volume gratings recorded in photopolymerizable nanoparticle-polymer composites based on step-growth polymerization. Opt. Mater. Express 2011, 1, 1113–1120. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Gleeson, M.R.; Liu, S.; Guo, J.; Sheridan, J.T. Non-local photo-polymerization kinetics including multiple termination mechanisms and dark reactions: Part III. Primary radical generation and inhibition. J. Opt. Soc. Am. B 2010, 27, 1804–1812. [Google Scholar]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry. Reactions, Mechanisms, and Structure, 5th ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Kogelnik, H. Coupled Wave Theory for Thick Hologram Gratings. Bell Syst. Tech. J. 1969, 48, 2909–2947. [Google Scholar] [CrossRef]
- Meng, S.; Duran, H.; Hu, J.; Kyu, T.; Natarajan, L.V.; Tondiglia, V.P.; Sutherland, R.L.; Bunning, T.J. Influence of photopolymerization reaction kinetics on diffraction efficiency of HPDLC undergoing photopatterning reaction in mixtures of acrylic monomer/nematic liquid crystals. Macromolecules 2007, 40, 3190–3197. [Google Scholar] [CrossRef]
- Navarro-Fuster, V.; Ortuño, M.; Fernández, R.; Gallego, S.; Márquez, A.; Beléndez, A.; Pascual, I. Peristrophic multiplexed holograms recorded in a low toxicity photopolymer. Opt. Mater. Express 2017, 7, 133. [Google Scholar] [CrossRef]
- Neipp, C.; Francés, J.; Martínez, F.J.; Fernández, R.; Alvarez, M.L.; Bleda, S.; Ortuño, M.; Gallego, S. Optimization of Photopolymer Materials for the Fabrication of a Holographic Waveguide. Polymers 2017, 9, 395. [Google Scholar] [CrossRef]
- Howard, J.A.; Korcek, S. Absolute rate constans for hydrocarbon autoxidation. Part XX. Oxidation of some organic sulfides. Can. J. Chem. 1971, 49, 2178–2182. [Google Scholar] [CrossRef]
- Campaigne, E. Chapter: 3.15—Thiophenes and their Benzo Derivatives: (iii) Synthesis and Applications. In Comprehensive Heterocyclic Chemistry; Pergamon Press Ltd.: Oxford, UK, 1984; Volume 4, p. 863. [Google Scholar]
- Chambers, R.C.; Hill, C.L. Comparative study of polyoxometalates and semiconductor metal oxides as catalysts. Photochemical oxidative degradation of thioethers. Inorg. Chem. 1991, 30, 2776–2781. [Google Scholar] [CrossRef]
- Chen, G. Fiber Adsorbents for Tert-Butyl Mercaptan Removal from Pipeline Grade Natural Gas. Master’s Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2013. [Google Scholar]
- Hernández, L.S. Odorization Issues; Department of Transportation Reports; US Department of Transportation: Washington, DC, USA, 2015.
- Tenkrat, D.; Hlincik, T.; Prokes, O. Chapter: 4—Natural Gas Odorization. In Natural Gas; IntechOpen: London, UK, 2010. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Hu, J.; Cai, P.; Lv, Y. A cataluminescence gas sensor based on nanosized V2O5 for tert-butyl mercaptan. Talanta 2010, 82, 733–738. [Google Scholar] [CrossRef]
- Xu, N.Y.; Ying, H.; Zhu, L.; Dai, G. Determination of Tetrahydrothiophene in Ambient Air by Gas Chromatography with a PFPD Detector Coupled to a Preconcentration Technology; Ningbo Environmental Monitoring Centre: Ningbo, China, 2004.







| Component | Polymer A Concentration (wt %) | Polymer B Concentration (wt %) |
|---|---|---|
| DPHPA | 44.27 | 44.10 |
| QYPDLC-036 | 30.43 | 30.31 |
| EE | 0.04 | 0.04 |
| NPG | 0.00 | 0.39 |
| OA | 8.79 | 8.76 |
| NMP | 16.47 | 16.40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora, M.P.; Ramírez, M.G.; Brocal, F.; Ortuño, M.; Beléndez, A.; Pascual, I. Influence of Tert-Butylthiol and Tetrahydrofuran on the Holographic Characteristics of a Polymer Dispersed Liquid Crystal: A Research Line Toward a Specific Sensor for Natural Gas and Liquefied Petroleum Gas. Polymers 2019, 11, 254. https://doi.org/10.3390/polym11020254
Mora MP, Ramírez MG, Brocal F, Ortuño M, Beléndez A, Pascual I. Influence of Tert-Butylthiol and Tetrahydrofuran on the Holographic Characteristics of a Polymer Dispersed Liquid Crystal: A Research Line Toward a Specific Sensor for Natural Gas and Liquefied Petroleum Gas. Polymers. 2019; 11(2):254. https://doi.org/10.3390/polym11020254
Chicago/Turabian StyleMora, María P., Manuel G. Ramírez, Francisco Brocal, Manuel Ortuño, Augusto Beléndez, and Inmaculada Pascual. 2019. "Influence of Tert-Butylthiol and Tetrahydrofuran on the Holographic Characteristics of a Polymer Dispersed Liquid Crystal: A Research Line Toward a Specific Sensor for Natural Gas and Liquefied Petroleum Gas" Polymers 11, no. 2: 254. https://doi.org/10.3390/polym11020254






