Tunable Thermo-Responsive Copolymers from DEGMA and OEGMA Synthesized by RAFT Polymerization and the Effect of the Concentration and Saline Phosphate Buffer on Its Phase Transition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Synthesis of 5.amino-4-methyl-4-(propylthiocarbonothioylthio)-5-oxopentanoic Acid (APP)
2.4. Polymer Synthesis
3. Results and Discussion
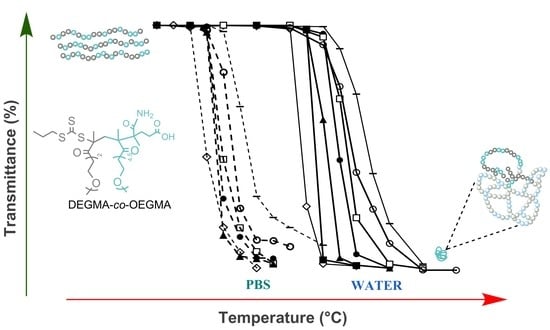
Thermo-Responsive Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Badi, N. Non-linear PEG-based thermoresponsive polymer systems. Prog. Polym. Sci. 2017, 66, 54–79. [Google Scholar] [CrossRef]
- Matanovic, M.R.; Kristl, J.; Grabnar, P.A. Thermoresponsive polymers: Insights into decisive hydrogel characteristics, mechanisms of gelation, and promising biomedical applications. Int. J. Pharm. 2014, 472, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Pietsch, C.; Schubert, U.S.; Hoogenboom, R. Aqueous polymeric sensors based on temperature-induced polymer phase transitions and solvatochromic dyes. Chem. Comm. 2011, 47, 8750–8765. [Google Scholar] [CrossRef] [PubMed]
- Sedláček, O.; Černoch, P.; Kučka, J.; Konefal, R.; Štěpánek, P.; Vetrík, M.; Lodge, T.P.; Hruby, M. Thermoresponsive Polymers for Nuclear Medicine: Which Polymer Is the Best? Langmuir 2016, 32, 6115–6122. [Google Scholar] [CrossRef]
- Taylor, M.J.; Tomlins, P.; Sahota, T.S. Thermoresponsive Gels. Gels 2017, 3, 4. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Deliv. Rev. 2018, 138, 162–192. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Skrabania, K.; Kristen, J.; Laschewsky, A.; Akdemir, Ö.; Hoth, A.; Lutz, J.-F. Design, Synthesis, and Aqueous Aggregation Behavior of Nonionic Single and Multiple Thermoresponsive Polymers. Langmuir 2007, 23, 84–93. [Google Scholar] [CrossRef]
- Becer, C.R.; Hahn, S.; Fijten, M.W.M.; Thijs, H.M.L.; Hoogenboom, R.; Schubert, U.S. Libraries of methacrylic acid and oligo(ethylene glycol) methacrylate copolymers with LCST behavior. J. Polym. Sci. A Polym. Chem. 2008, 46, 7138–7147. [Google Scholar] [CrossRef]
- Vancoillie, G.; Frank, D.; Hoogenboom, R. Thermoresponsive poly(oligo ethylene glycol acrylates). Prog. Polym. Sci. 2014, 39, 1074–1095. [Google Scholar] [CrossRef]
- Han, S.; Hagiwara, M.; Ishizone, T. Synthesis of Thermally Sensitive Water-Soluble Polymethacrylates by Living Anionic Polymerizations of Oligo(ethylene glycol) Methyl Ether Methacrylates. Macromolecules 2003, 36, 8312–8319. [Google Scholar] [CrossRef]
- Ishizone, T.; Seki, A.; Hagiwara, M.; Han, S. Anionic Polymerizations of Oligo (ethylene glycol) Alkyl Ether Methacrylates: Effect of Side Chain Length and ω-Alkyl Group of Side Chain on Cloud Point in Water. Macromolecules 2008, 41, 2963–2967. [Google Scholar] [CrossRef]
- Bergenudd, H.; Coullerez, G.; Jonsson, M.; Malmstrom, E. Solvent effects on ATRP of oligo(ethylene glycol) methacrylate exploring the limits of control. Macromolecules 2009, 49, 3302–3308. [Google Scholar] [CrossRef]
- Ali, M.M.; Sto1ver, H.D.H. Well-Defined Amphiphilic Thermosensitive Copolymers Based on Poly(ethylene glycol monomethacrylate) and Methyl Methacrylate Prepared by Atom Transfer Radical Polymerization. Macromolecules 2004, 37, 5219–5227. [Google Scholar] [CrossRef]
- Oh, J.K.; Min, K.; Matyjaszewski, K. Preparation of Poly(oligo(ethylene glycol) monomethyl ether methacrylate) by Homogeneous Aqueous AGRET ATRP. Macromolecules 2006, 39, 3161–3167. [Google Scholar] [CrossRef]
- Yamamoto, S.; Pietrasik, J.; Matyjaszewski, K. ATRP Synthesis of Thermally Responsive Molecular Brushes from Oligo(ethylene oxide) Methacrylates. Macromolecules 2007, 40, 9348–9353. [Google Scholar] [CrossRef]
- Yamamoto, S.-H.; Pietrasik, J.; Matyjaszewski, K. The effect of structure on the thermoresponsive nature of well-defined poly(oligo(ethylene oxide) methacrylates) synthesized by ATRP. J. Polym. Sci. A Polym. Chem. 2008, 46, 194–202. [Google Scholar] [CrossRef]
- Bebis, K.; Jones, M.W.; Haddleton, D.M.; Gibson, M.I. Thermoresponsive behaviour of poly[(oligo(ethyleneglycol methacrylate)]s and their protein conjugates: Importance of concentration and solvent system. Polym. Chem. 2011, 2, 975–982. [Google Scholar] [CrossRef]
- Moad, G. RAFT polymerization to form stimuli-responsive polymers. Polym. Chem. 2017, 8, 177–219. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process. Aust. J. Chem. 2005, 58, 379–410. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process—A Second Update. Aust. J. Chem. 2009, 62, 1402–1472. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process—A Third Update. Aust. J. Chem. 2012, 65, 985–1076. [Google Scholar] [CrossRef]
- Montolla-Villegas, K.A.; Licea-Claveríe, A.; Zapata-González, I.; Gómez, E.; Ramírez-Jiménez, A. The effect in the RAFT polymerization of two oligo(ethylene glycol) methacrylates when the CTA 4-cyano-4-(propylthiocarbonothioylthio) pentanoic acid is auto-hydrolyzed to its corresponding amide. J. Polym. Res. 2019, 26, 71–81. [Google Scholar] [CrossRef]
- Rizzardo, E.; Chen, M.; Chong, B.; Moad, G.; Skidmore, M.; Thang, S.H. RAFT Polymerization: Adding to the Picture. Macromol. Symp. 2007, 248, 104–116. [Google Scholar] [CrossRef]
- Furchs, A.V.; Thurecht, K.J. Stability of Trithiocarbonate RAFT Agents Containing Both a Cyano and a Carboxylic Acid Functional Group. ACS Macro Lett. 2017, 6, 287–291. [Google Scholar] [CrossRef]
- Porsch, C.; Hansoon, S.; Nordgren, N.; Malmström, E. Thermo-responsive cellulose-based architectures: Tailoring LCST using poly(ethylene glycol) methacrylates. Polym. Chem. 2011, 2, 1114–1123. [Google Scholar] [CrossRef]
- Lutz, J.F.; Hoth, A. reparation of Ideal PEG Analogues with a Tunable Thermosensitivity by Controlled Radical Copolymerization of 2-(2-Methoxyethoxy)ethyl Methacrylate and Oligo(ethylene glycol) Methacrylate. Macromolecules 2006, 39, 893–896. [Google Scholar] [CrossRef]
- Xiao, H.; Pelton, R.; Hamielec, A. Preparation and kinetic characterization of copolymers of acrylamide and poly(ethylene glycol) (meth)acrylate macromonomers. Polymer 1996, 37, 1201–1209. [Google Scholar] [CrossRef]
- Jones, J.A.; Novo, N.; Flanger, K.; Pagnucco, C.D.; Carew, S.; Cheong, C.; Kong, X.Z.; Burke, N.A.D.; Stöver, H.H. Thermoresponsive copolymers of methacrylic acid and poly(ethylene glycol) methyl ether methacrylate. J. Polym. Sci. A Polym. Chem. 2005, 43, 6095–6104. [Google Scholar] [CrossRef]
- Smolne, S.; Weber, S.; Buback, M. Propagation and Termination Kinetics of Poly(Ethylene Glycol) Methyl Ether Methacrylate in Aqueous Solution. Macromol. Chem. Phys. 2016, 217, 2391–2401. [Google Scholar] [CrossRef]
- Dimarzio, E.A.; Gibbs, J.H. Glass temperature of copolymers. J. Polym. Sci. 1959, 40, 121–131. [Google Scholar] [CrossRef]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, T.; Zhong, Q.; Wang, J. Thermoresponsive polymers based on oligo(ethylene glycol) methyl ether methacrylate and modified substrates with thermosensitivity. Macromol. Res. 2017, 25, 206–213. [Google Scholar] [CrossRef]
- Grishkewish, N.; Akhlaghi, S.P.; Yao, Z.; Berry, R.; Tam, K.C. Cellulose nanocrystal-poly(oligo(ethylene glycol) methacrylate) brushes with tunable LCSTs. Carbohydr. Polym. 2016, 144, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, J.P.; Khan, A.; Pasparakis, G.; Saeed, A.O.; Wang, W.; Alexander, C. Ion-Sensitive “Isothermal” Responsive Polymers Prepared in Water. J. Am. Chem. Soc. 2008, 130, 10852–10853. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Mendrek, B.; Żymełka-Miara, I.; Libera, M.; Marcinkowski, A.; Trzebicka, B.; Smet, M.; Dworak, A. Solution behavior of star polymers with oligo(ethylene glycol) methyl ether methacrylate arms. Polymer 2012, 53, 5619–5631. [Google Scholar] [CrossRef]
- Luzon, M.; Boyer, C.; Peinado, C.; Corrales, T.; Whittaker, M.; Tao, L.; Davis, T.P. Water-soluble, thermoresponsive, hyperbranched copolymers based on PEG-methacrylates: Synthesis, characterization, and LCST behavior. J. Polym. Sci. A Polym. Chem. 2010, 48, 2783–2792. [Google Scholar] [CrossRef]















| Sample | OEGMA (%) | Tg (DSC, K) | Tg (GM **, K) |
|---|---|---|---|
| POEGMA | 100 | 213.4 | 213.4 |
| P(OEGMA-co-DEGMA) | 79 * | 218.3 | 218.6 |
| P(OEGMA-co-DEGMA) | 50 * | 224.0 | 225.7 |
| P(OEGMA-co-DEGMA) | 31 * | 228.0 | 230.4 |
| P(OEGMA-co-DEGMA) | 24 * | 230.6 | 232.1 |
| P(OEGMA-co-DEGMA) | 14 * | 236.5 | 234.6 |
| PDEGMA | 0 | 238.0 | 238.0 |
| Sample | Time (min) | OEGMA0: DEGMA0 | OEGMA: DEGMA a | Conv (%) a | Tg (K) b | Mntheob | Mn (g/mol) c | Đc | Tcp (°C) d | Tcp (°C) e | Tcp (°C) f |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PDEGMA | 120 | 0:100 | 0:100 | 69 | 238 | 13281 | 32840 | 1.10 | 25.2 | 21.9 | 21.5 |
| OD 15:85 | 240 | 15:85 | 14:86 | 89 | 236 | 18537 | 39970 | 1.29 | 31.5 | 29.5 | 28.5 |
| OD 25:75 | 240 | 25:75 | 24:76 | 86 | 231 | 20613 | 40360 | 1.23 | 35.9 | 32.3 | 32.0 |
| OD 30:70 | 240 | 30:70 | 31:69 | 95 | 228 | 21360 | 42470 | 1.36 | 41.0 | - | - |
| OD 50:50 | 240 | 50:50 | 50:50 | 81 | 224 | 22996 | 46600 | 1.38 | 45.9 | 41.8 | 42.0 |
| OD 80:20 | 240 | 80:20 | 79:21 | 95 | 216 | 26670 | 58850 | 1.83 | 55.9 | - | - |
| POEGMA | 120 | 100:0 | 100:0 | 38 | 214 | 11795 | 30100 | 1.30 | 66.5 | 61.5 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Jiménez, A.; Montoya-Villegas, K.A.; Licea-Claverie, A.; Gónzalez-Ayón, M.A. Tunable Thermo-Responsive Copolymers from DEGMA and OEGMA Synthesized by RAFT Polymerization and the Effect of the Concentration and Saline Phosphate Buffer on Its Phase Transition. Polymers 2019, 11, 1657. https://doi.org/10.3390/polym11101657
Ramírez-Jiménez A, Montoya-Villegas KA, Licea-Claverie A, Gónzalez-Ayón MA. Tunable Thermo-Responsive Copolymers from DEGMA and OEGMA Synthesized by RAFT Polymerization and the Effect of the Concentration and Saline Phosphate Buffer on Its Phase Transition. Polymers. 2019; 11(10):1657. https://doi.org/10.3390/polym11101657
Chicago/Turabian StyleRamírez-Jiménez, Alejandro, Kathleen Abigail Montoya-Villegas, Angel Licea-Claverie, and Mirian Angelene Gónzalez-Ayón. 2019. "Tunable Thermo-Responsive Copolymers from DEGMA and OEGMA Synthesized by RAFT Polymerization and the Effect of the Concentration and Saline Phosphate Buffer on Its Phase Transition" Polymers 11, no. 10: 1657. https://doi.org/10.3390/polym11101657