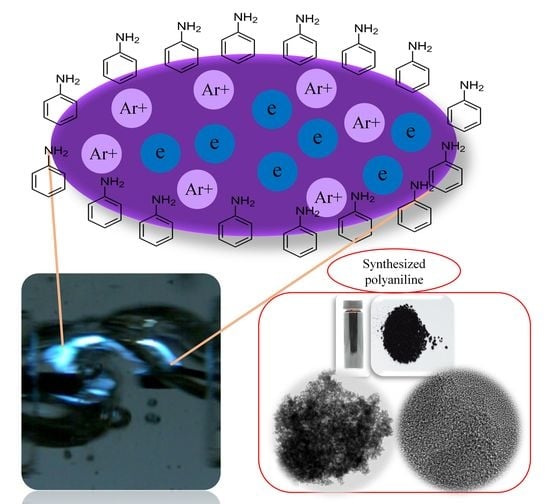
Synthesis of a Polyaniline Nanoparticle Using a Solution Plasma Process with an Ar Gas Bubble Channel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Preparation of Polyaniline
2.3. Voltage–Current (V–I) Measurement
2.4. Optical Emission Spectroscopy
2.5. High Speed Camera
2.6. Scanning Electron Microscopy
2.7. Dynamic Light Scattering
2.8. Transmission Electron Microscopy
2.9. Fourier Transform Infrared Spectroscopy
2.10. Nuclear Magnetic Resonance Spectroscopy
2.11. Gel Permeation Chromatography
2.12. X-ray Photoelectron Spectroscopy
3. Results and Discussion
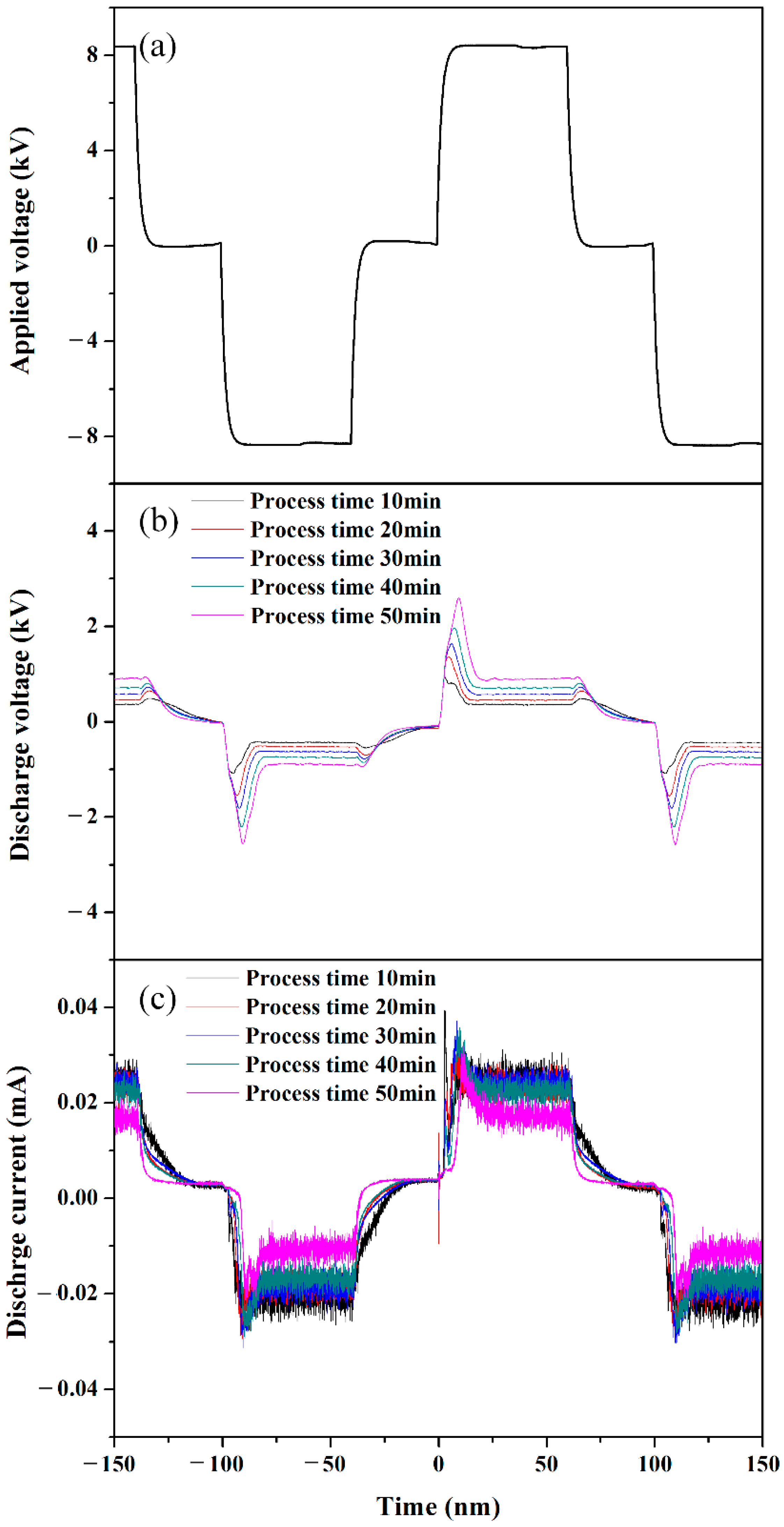
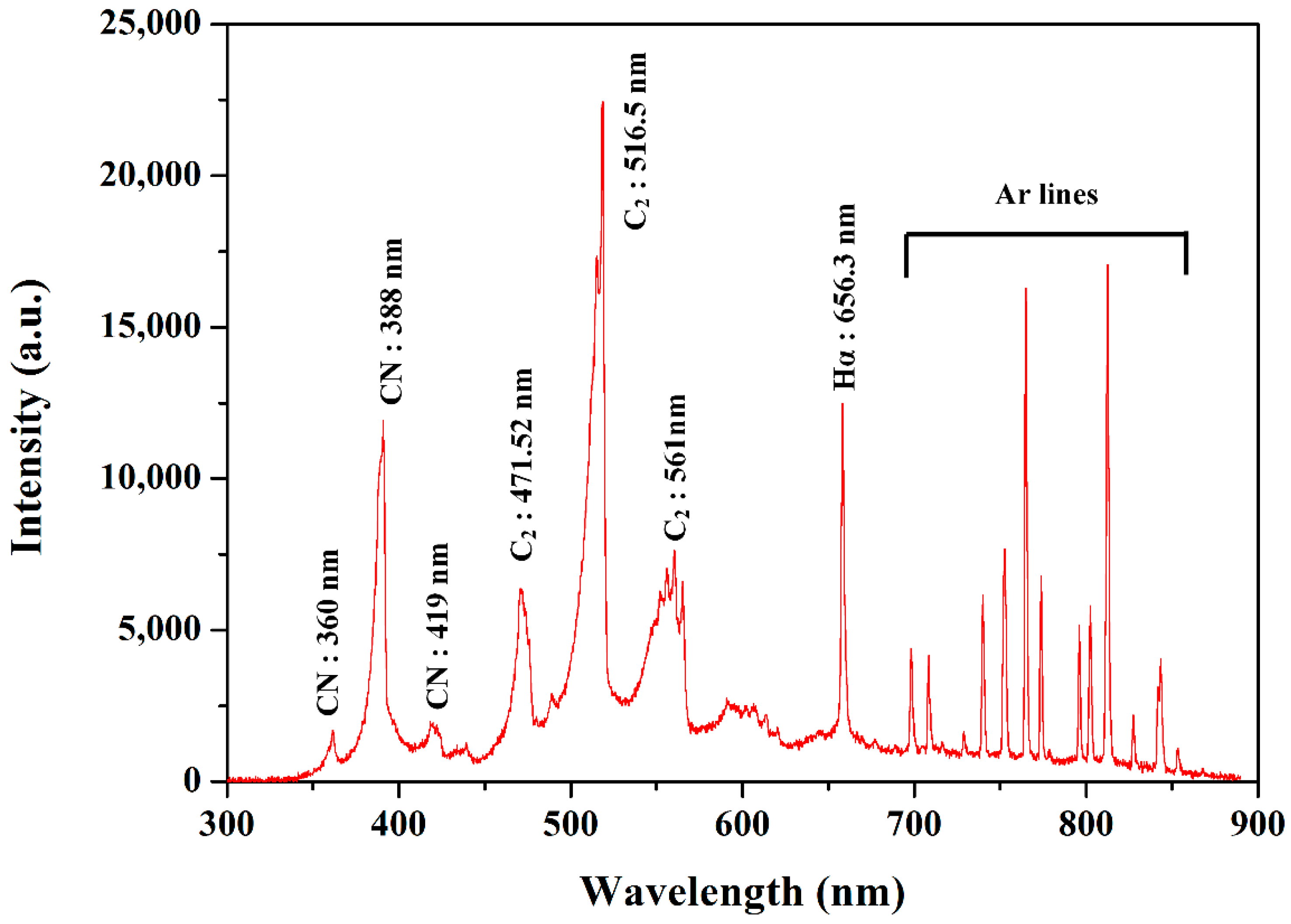
3.1. Discharge Properties with Ar Gas Bubble Channel in Liquid Aniline Monomer
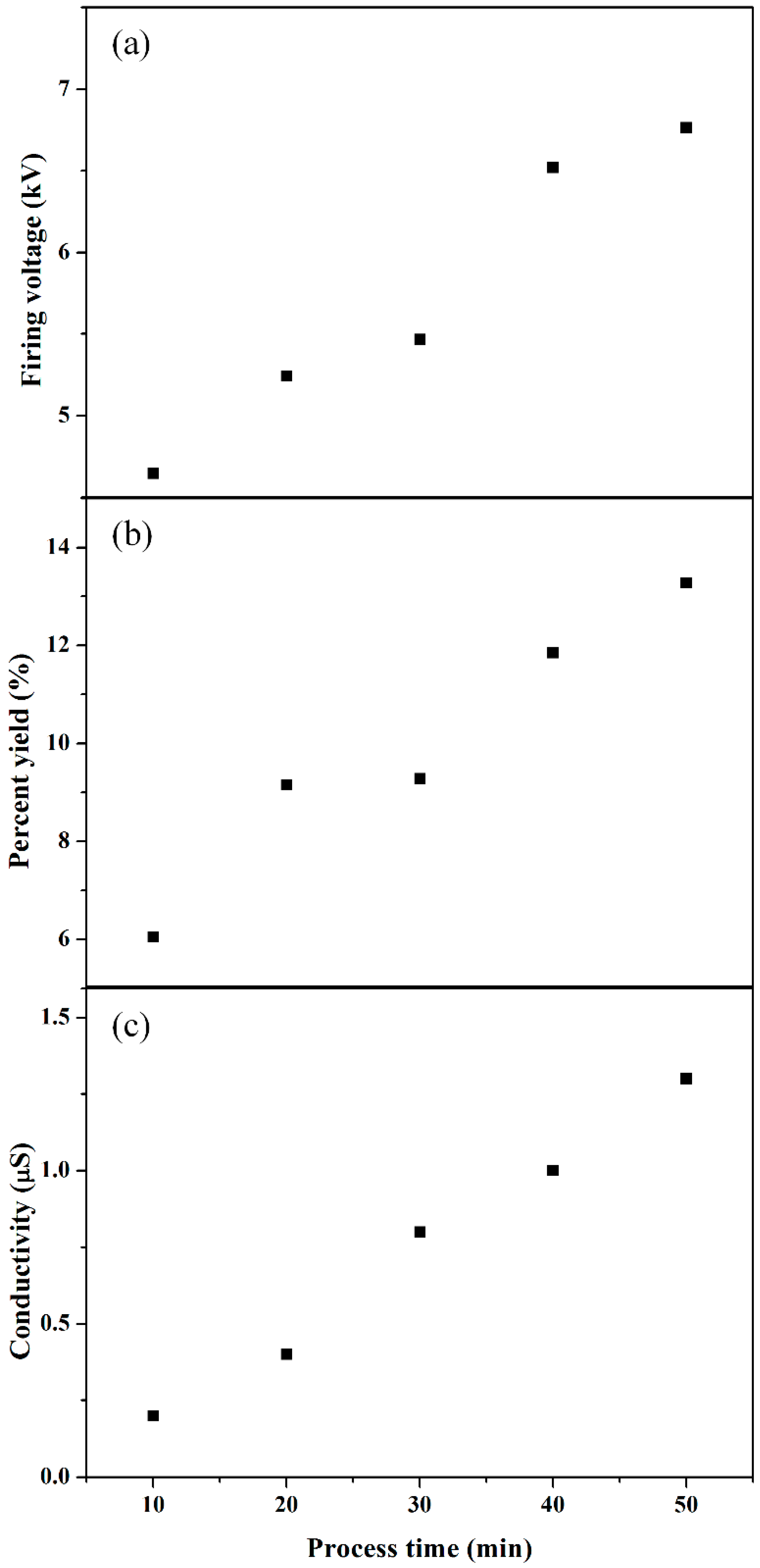
3.2. Properties of Synthesized Polyaniline
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Long, Y.-Z.; Li, M.-M.; Gu, C.; Wan, M.; Duvail, J.-L.; Liu, Z.; Fan, Z. Recent advances in synthesis, physical properties and applications of conducting polymer nanotubes and nanofibers. Prog. Polym. Sci. 2011, 36, 1415–1442. [Google Scholar] [CrossRef]
- Wang, G.; Viverk, R.; Wang, J.-Y. Polyaniline nanoparticles: Synthesis, dispersion and biomedical applications. Mini-Rev. Org. Chem. 2016, 14, 56–64. [Google Scholar] [CrossRef]
- Ciric-Marjanovic, G. Recent advances in polyaniline research: Polyaniline mechanisms, structural aspects, properties and applications. Synth. Met. 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Goktas, H.; Demircioglu, Z.; Sel, K.; Gunes, T.; Kaya, I. The optical properties of plasma polymerized polyaniline thin films. Thin Solid Films 2013, 548, 81–85. [Google Scholar] [CrossRef]
- Du, P.; Lin, L.; Wang, H.; Liu, D.; Wei, W.; Li, J.; Liu, P. Fabrication of polyaniline modified MWNTs core-shell structure for high performance supercapacitors with high rate capability. Mater. Des. 2017, 127, 76–83. [Google Scholar] [CrossRef]
- Bafandeh, N.; Larijani, M.M.; Shafinekhani, A.; Hantehzadeh, M.R.; Sheikh, N. Synthesis of polyaniline films: Case study on post gamma irradiation dose. J. Mater. Sci. Electron. 2016, 27, 10566–10572. [Google Scholar] [CrossRef]
- Zhan, C.; Yu, G.; Lu, Y.; Wang, L.; Wujcik, E.; Wei, S. Conductive polymer nanocomposites: A critical review of modern advanced devices. J. Mater. Chem. C 2017, 5, 1569–1585. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Hu, N.; Yang, Z.; Su, Y.; Xu, S.; Li, M.; Yao, L.; Hong, M.; Zhang, Y. Rational design of sandwiched polyaniline nanotube/layered graphene/polyaniline nanotube papers for high-volumetric supercapacitors. Chem. Eng. J. 2017, 309, 89–97. [Google Scholar] [CrossRef]
- Gomes, E.C.; Oliveira, M.A.S. Chemical polymerization of aniline in hydrochloric acid (HCl) and formic acid (HCOOH) media. Differences between the two synthesized polyanilines. Am. J. Polym. Sci. 2012, 2, 5–13. [Google Scholar] [CrossRef]
- Qin, Q.; Tao, J.; Yang, Y. Preparation and characterization of polyaniline film on stainless steel by elecrochemical polymerization as a counter electrode of DSSC. Synth. Met. 2010, 160, 1167–1172. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Feng, Y.; Hu, W.; Feng, W. Hierachical graphene oxide/polyaniline nanocomposites prepared by interfacial electrochemical polymerization for flexible solid-state supercapacitors. J. Mater. Chem. A 2015, 3, 2135–2143. [Google Scholar] [CrossRef]
- Lin, Q.; Li, Y.; Yang, M. Polyaniline nanofiber humidity sensor prepared by electrospinning. Sens. Actuators B Chem. 2011, 161, 967–972. [Google Scholar] [CrossRef]
- Miao, Y.-E.; Fan, W.; Chen, D.; Liu, T. High-performance superconductors based on hollow polyaniline nanofibers by electrospinning. Appl. Mater. Interfaces 2013, 5, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Merlini, C.; Pegoretti, A.; Araujo, T.M.; Ramoa, S.D.A.S.; Schreiner, W.H.; de Oliveira Barra, G.M. Electrospinning of doped and undoped-polyaniline/poly(vinylidene fluoride) blends. Synth. Met. 2016, 213, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Ma, F.; Qi, S.; Song, B. Synthetic and electromagnetic characterization of polyaniline nanorods using Schiff base through ‘seeding’ polymerization. Synth. Met. 2010, 160, 2077–2084. [Google Scholar] [CrossRef]
- Yang, M.; Cao, L.; Tan, L. Synthesis of sea urchin-like polystyrene/polyaniline microspheres by seed swelling polymerization and their catalytic application. Colloids Surf. A 2014, 441, 678–684. [Google Scholar] [CrossRef]
- Guan, H.; Fan, L.-Z.; Zhang, H.; Qu, X. Polyaniline nanofibers obtained by interfacial polymerization for high-rate supercapacitors. Electrochim. Acta 2010, 56, 964–968. [Google Scholar] [CrossRef]
- Xiang, B.; Zhang, J. Using ultrasound-assisted dispersion and in situ emulsion polymerization to synthesize TiO2/ASA (acrylonitrile-styrene-acrylate) nanocomposites. Compos. Part B 2016, 99, 96–202. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Sonawane, S.H. Ultrasound assisted in situ emulsion polymerization for polymer nanocomposite: A review. Chem. Eng. Process. 2014, 85, 86–107. [Google Scholar] [CrossRef]
- Abdolahi, A.; Hamzah, E.; Ibrahim, Z.; Hashim, S. Synthesis of uniform polyaniline nanofibers through interfacial polymerization. Materials 2012, 5, 1487–1494. [Google Scholar] [CrossRef]
- Yoshida, T.; Yamamoto, N.; Mizutani, T.; Yamamoto, M.; Ogawa, S.; Yagi, S.; Nameki, H.; Yoshida, H. Synthesis of Ag nanoparticles prepared by a solution plasma method and application as a catalyst for photocatalytic reduction of carbon dioxide with water. Catal. Today 2018, 303, 320–326. [Google Scholar] [CrossRef]
- Lee, S.; Saito, N. Enhancement of nitrogen self-doped nanocarbons electrocatalyst via tine-up solution plasma synthesis. RSC Adv. 2018, 8, 35503–35511. [Google Scholar] [CrossRef]
- Pootawang, P.; Saito, N.; Takai, O.; Lee, S.Y. Rapid synthesis of ordered hexagonal mesoporous silica and their incorporation with Ag nanoparticles y solution plasma. Mater. Res. Bull. 2012, 47, 2726–2729. [Google Scholar] [CrossRef]
- Hu, X.; Cho, S.-P.; Takai, O.; Saito, N. Rapid Synthesis and Structural Characterization of Well-Defined Gold Clusters by Solution Plasma Sputtering. Cryst. Growth Des. 2011, 12, 119–123. [Google Scholar] [CrossRef]
- Cho, S.-P.; Bratescu, M.A.; Saito, N.; Takai, O. Microstructural characterization of gold nanoparticles synthesized by solution plasma processing. Nanotechnology 2011, 22, 455701:1–455701:7. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, C.-J. Synthesis of monodisperse gold nanoparticles in ionic liquid by applying room temperature plasma. Mater. Lett. 2010, 65, 353–355. [Google Scholar] [CrossRef]
- Hyun, K.; Ueno, T.; Saito, N. Synthesis of nitrogen-containing carbon by solution plasma in aniline with high-repetition frequency discharge. Jpn. J. Appl. Phys. 2016, 55, 01AE18:1–01AE18:5. [Google Scholar] [CrossRef]
- Tantiplapol, T.; Singsawat, Y.; Narongsil, N.; Damrongsakkul, S.; Saito, N.; Prasertsung, I. Influences of solution plasma conditions on degradation rate and properties of chitosan. Innov. Food Sci. Emerg. Technol. 2015, 32, 116–120. [Google Scholar] [CrossRef]
- Ma, F.; Li, P.; Zhang, B.; Zhao, X.; Fu, Q.; Wang, Z.; Gu, C. Effect of solution plasma process with bubbling gas on physicochemical properties of chitosan. Int. J. Biol. Macromol. 2017, 98, 201–207. [Google Scholar] [CrossRef]
- Kang, J.; Li, O.L.; Saito, N. A simple synthesis method for nano-metal catalyst supported on mesoporous carbon: The solution plasma process. Nanoscale 2013, 5, 6874–6882. [Google Scholar] [CrossRef]
- Elita Hafizah, M.A.; Bimantoro, A.; Andreas; Manaf, A. Synthesized of conductive polyaniline by solution polymerization technique. Procedia Chem. 2016, 19, 162–165. [Google Scholar] [CrossRef]
- Fazekas, P.; Keszler, A.M.; Bódis, E.; Drotár, E.; Klébert, S.; Károly, Z.; Szépvölgyi, J. Optical emission spectra analysis of thermal plasma treatment of poly(vinyl chloride). Open Chem. 2014, 13, 549–556. [Google Scholar] [CrossRef]
- Clay, K.J.; Speakman, S.P.; Amaratunga, G.A.J.; Silva, S.R.P. Characterization of a-C:H:N deposition from CH4/N2 rf plasmas using optical emission spectroscopy. J. Appl. Phys. 1996, 79, 7227–7233. [Google Scholar] [CrossRef]
- Ndiaye, A.A.; Lacoste, A.; Bès, A.; Zaitsev, A.; Poncin-Epaillard, F.; Debarnot, D. A better understanding of the very low-pressure plasma polymerization of aniline by optical emission spectroscopy analysis. Plsma Chem. Plasma Process 2018, 38, 887–902. [Google Scholar] [CrossRef]
- Venditti, I.; Fratoddi, I.; Russo, M.V.; Bearzotti, A. A nanostructured composite based on polyaniline and gold nanoparticles: Synthesis and gas sensing properties. Nanotechnology 2013, 24, 155503:1–155503:7. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Chen, J.; Lu, X.; Chen, L.; Zhang, W.; Wei, Y. SEM study of the morphology of high molecular weight polyaniline. Synth. Met. 2005, 105, 47–51. [Google Scholar] [CrossRef]
- Saeed, M.; Shakoor, A.; Ahmad, E. Structural and electronic properties of polyaniline/yittrium oxide composites. J. Mater. Sci. Mater. Electron 2013, 24, 3536–3540. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Salunkhe, R.R.; Jamadade, V.S.; Dubal, D.P.; Pawar, S.M.; Lokhande, C.D. Hydrophilic polyaniline nanofibrous architecture using elecrosynthesis method for supercapacitor application. Curr. Appl. Phys. 2010, 10, 904–909. [Google Scholar] [CrossRef]
- Mathai, C.J.; Saravanan, S.; Anantharaman, M.R.; Venkitachalam, S.; Jayalekshmi, S. Effect of iodine doping on the bandgap of plasma polymerized aniline thin films. J. Phys. D Appl. Phys. 2002, 35, 2206–2210. [Google Scholar] [CrossRef]
- Jessop, I.A.; Díaz, F.R.; Terraza, C.A.; Tundidor-Camba, A.; Leiva, Á.; Cattin, L.; Bèrnede, J.-C. PANI branches onto donor-acceptor copolymers: Synthesis, characterization and electroluminescent properties of new 2D-materials. Polymers 2018, 10, 553. [Google Scholar] [CrossRef]
- Padamapriya, S.; Harinipriya, S.; Sudha, V.; Kumar, D.; Pal, S.; Chaubey, B. Polyaniline coated copper for hydrogen storage and evolution in alkaline medium. Int. J. Hydrog. Energy 2017, 42, 20453–20462. [Google Scholar] [CrossRef]
- Abdelkader, R.; Amine, H.; Mohammed, B. 1H-NMR spectra of conductive, anticorrosive and soluble polyaniline exchanged by an eco-catalyst layered (Maghnite-H+). World J. Chem. 2013, 2, 86–92. [Google Scholar] [CrossRef]
- Park, C.-S.; Kim, D.H.; Shin, B.J.; Tae, H.-S. Synthesis and Characterization of nanofibrous polyaniline thin film prepared by novel atmospheric pressure plasma polymerization technique. Materials 2016, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, M.; Zhang, D.; Zhao, Q.; Li, Y.; Qui, S.; Yu, J. P(N-Phenylmaleimide-Alt-Styrene) introduced with 4-carboxyl and its effect on the heat deflection temperature of nylon 6. Materials 2018, 11, 2330. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, D.; Pan, Z. The effect of polyaniline (PANI) coating via dielectric-barrier discharge (DBD) plasma on conductivity and air drag of polyethylene terephthalate (PET) yarn. Polymers 2018, 10, 351. [Google Scholar] [CrossRef]
- Xie, Y.; Cai, S.; Hou, Z.; Li, W.; Wang, Y.; Zhang, X.; Yang, W. Surface hydrophobic modification of microcrystalline cellulose by poly(methylhydro)siloxane using response surface methodology. Polymers 2018, 10, 1335. [Google Scholar] [CrossRef]


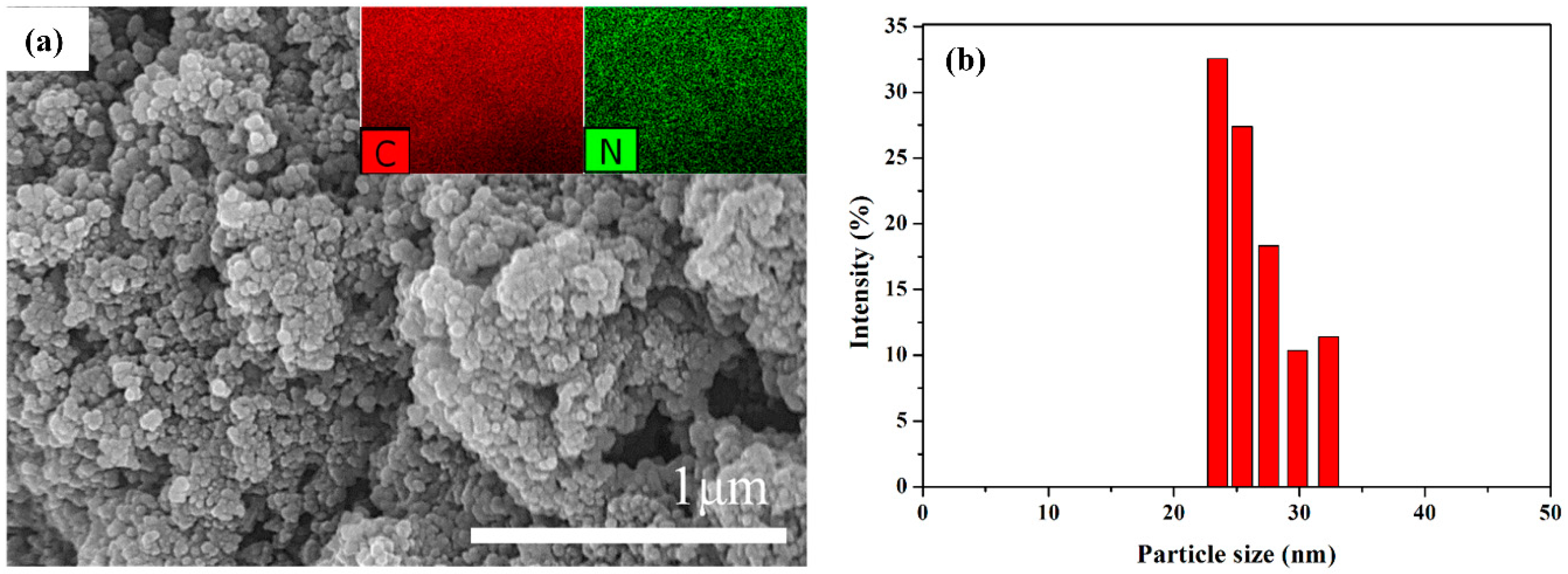
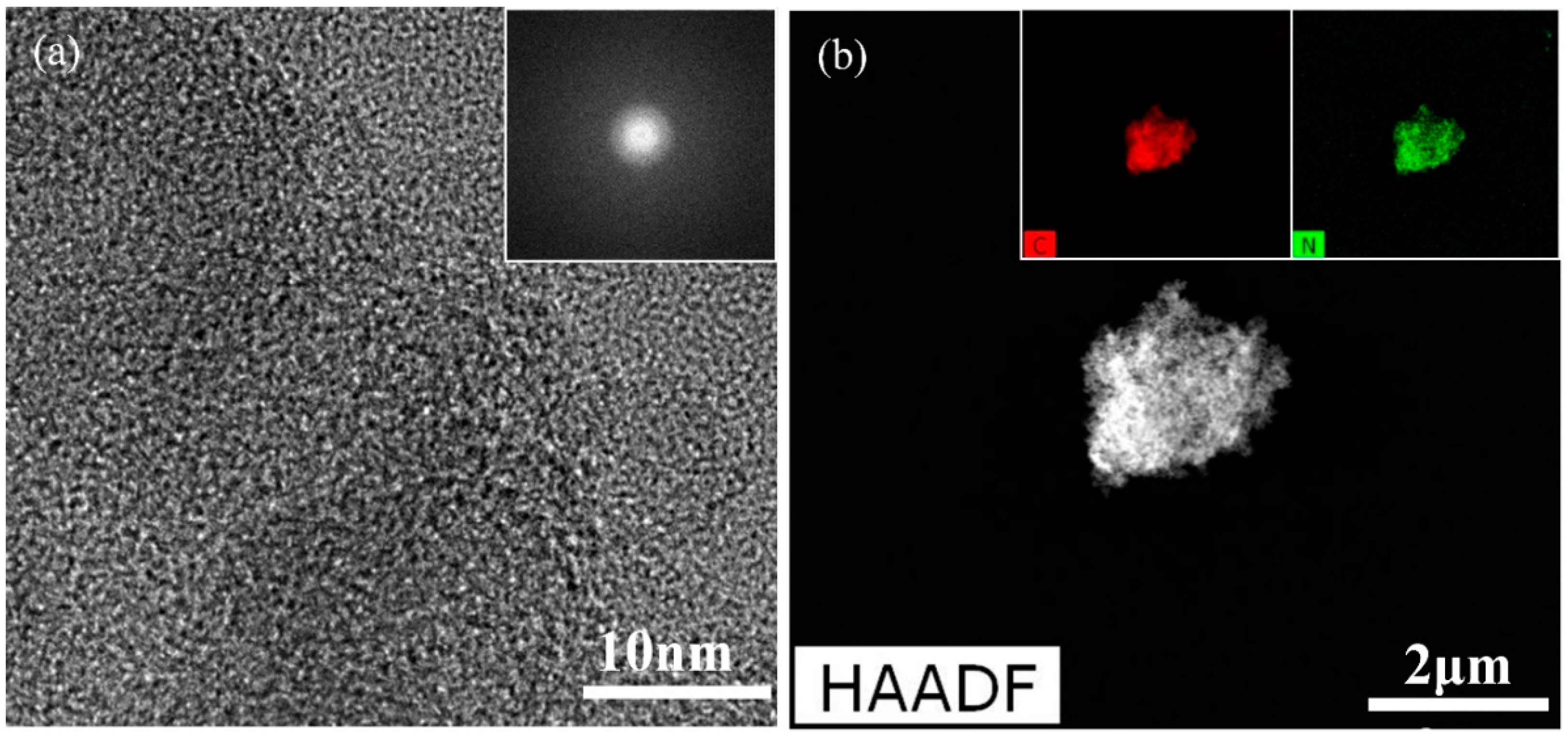
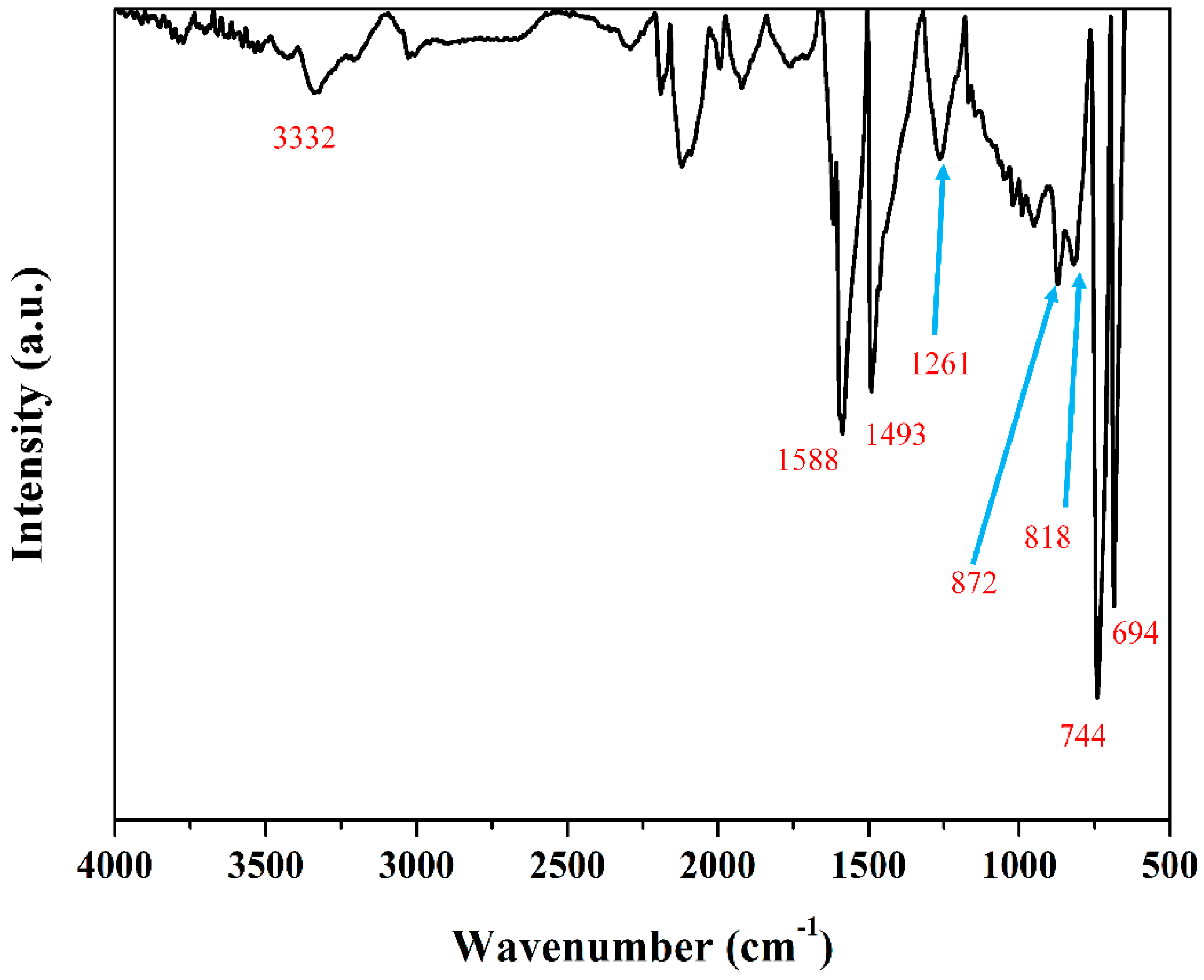
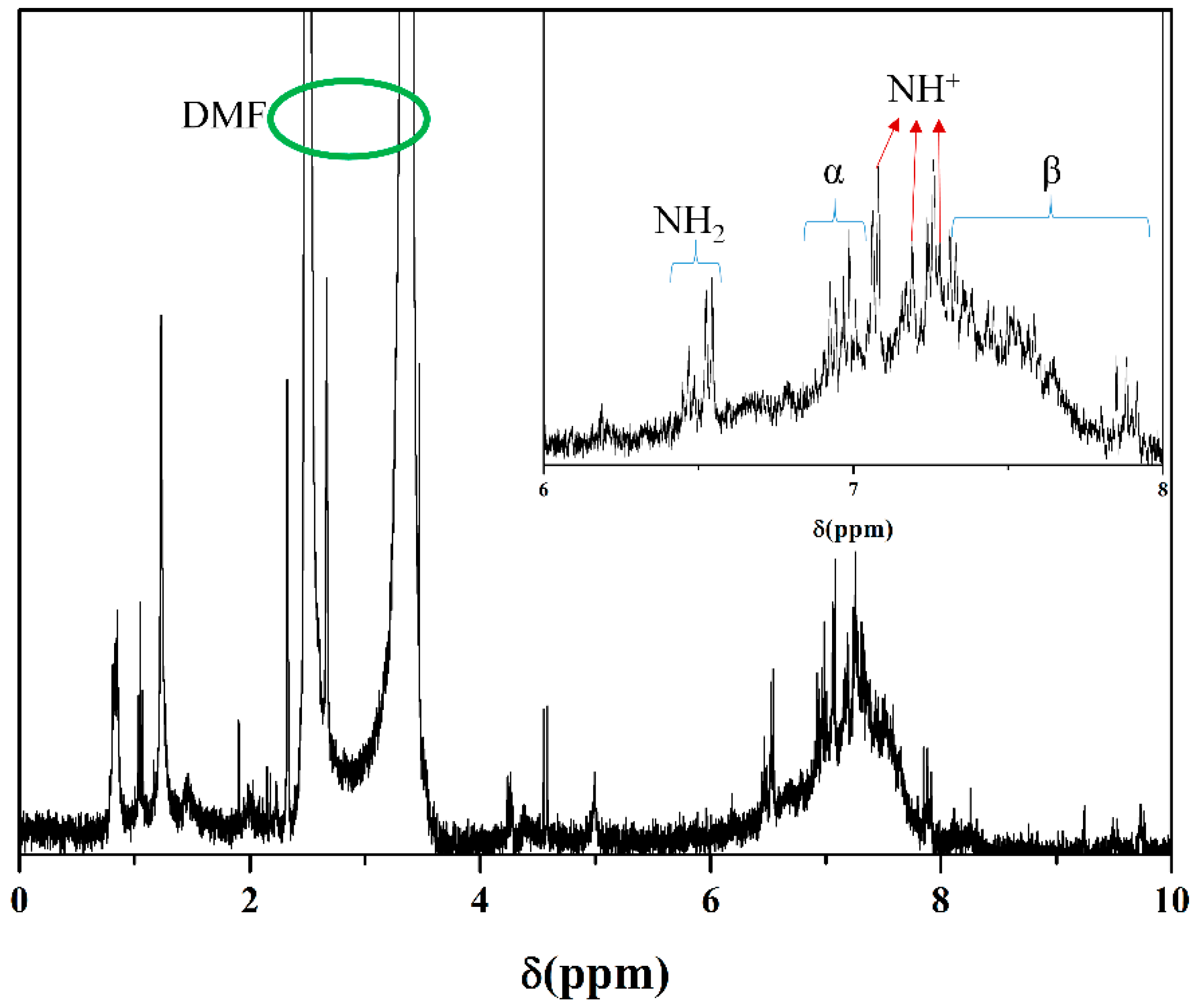
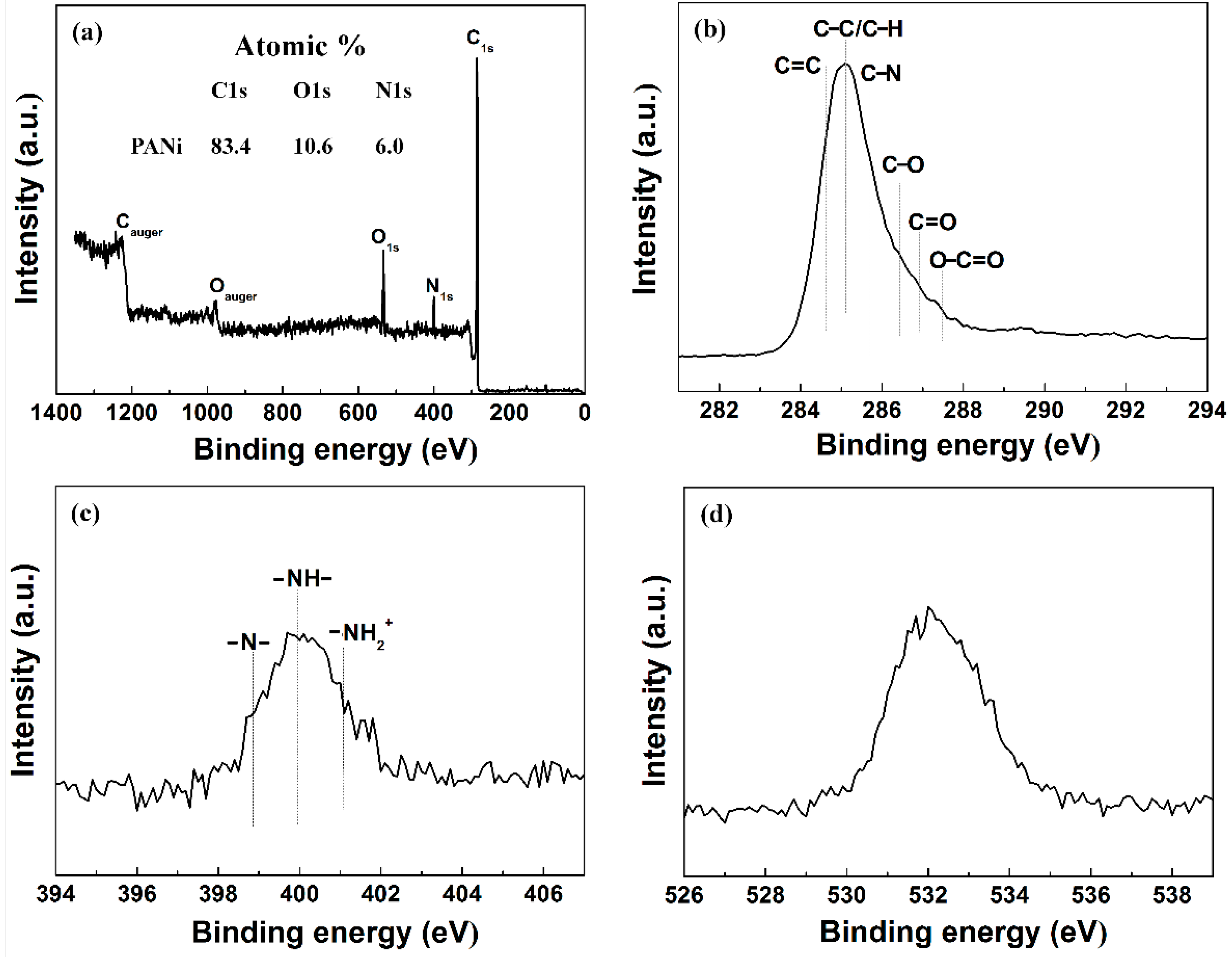
| Samples | Mw (kDa) | Mn (kDa) | Polydispersive Index (PDI) |
|---|---|---|---|
| Aniline monomer | 0.093 | - | - |
| PANI | 9.7059 | 8.1299 | 1.19 |
| Sample | C 1s Peaks Assignment (eV) and Envelopment Composition (%) | |||||
| 284.6 | 285.1 | 285.7 | 286.4 | 286.9 | 287.5 | |
| C=C | C–C/C–H | C–N | C–O | C=O | O–C=O | |
| PANI | 25.4 | 38.7 | 17.2 | 10.7 | 4.3 | 3.7 |
| Sample | N 1s Peaks Assignment (eV) and Envelopment Composition (%) | ||
| 398.7 | 399.7 | 401.1 | |
| –N– | –NH– | –NH2– | |
| PANI | 31.0 | 52.6 | 16.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.-G.; Park, C.-S.; Jung, E.Y.; Shin, B.J.; Tae, H.-S. Synthesis of a Polyaniline Nanoparticle Using a Solution Plasma Process with an Ar Gas Bubble Channel. Polymers 2019, 11, 105. https://doi.org/10.3390/polym11010105
Shin J-G, Park C-S, Jung EY, Shin BJ, Tae H-S. Synthesis of a Polyaniline Nanoparticle Using a Solution Plasma Process with an Ar Gas Bubble Channel. Polymers. 2019; 11(1):105. https://doi.org/10.3390/polym11010105
Chicago/Turabian StyleShin, Jun-Goo, Choon-Sang Park, Eun Young Jung, Bhum Jae Shin, and Heung-Sik Tae. 2019. "Synthesis of a Polyaniline Nanoparticle Using a Solution Plasma Process with an Ar Gas Bubble Channel" Polymers 11, no. 1: 105. https://doi.org/10.3390/polym11010105