Dynamic Optics with Transparency and Color Changes under Ambient Conditions
Abstract
:1. Introduction
2. Experimental
2.1. Materials
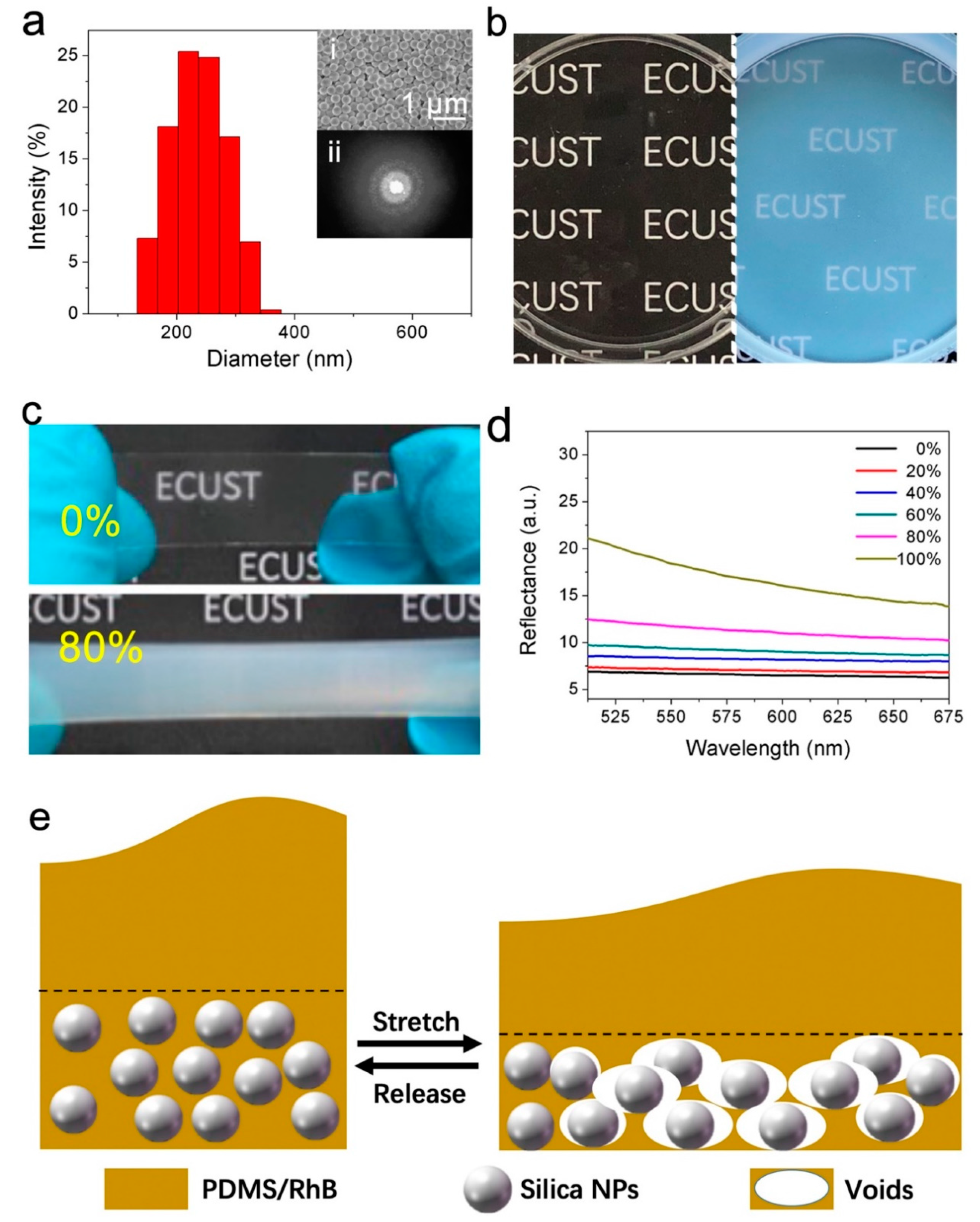
2.2. Synthesis of Silica NPs
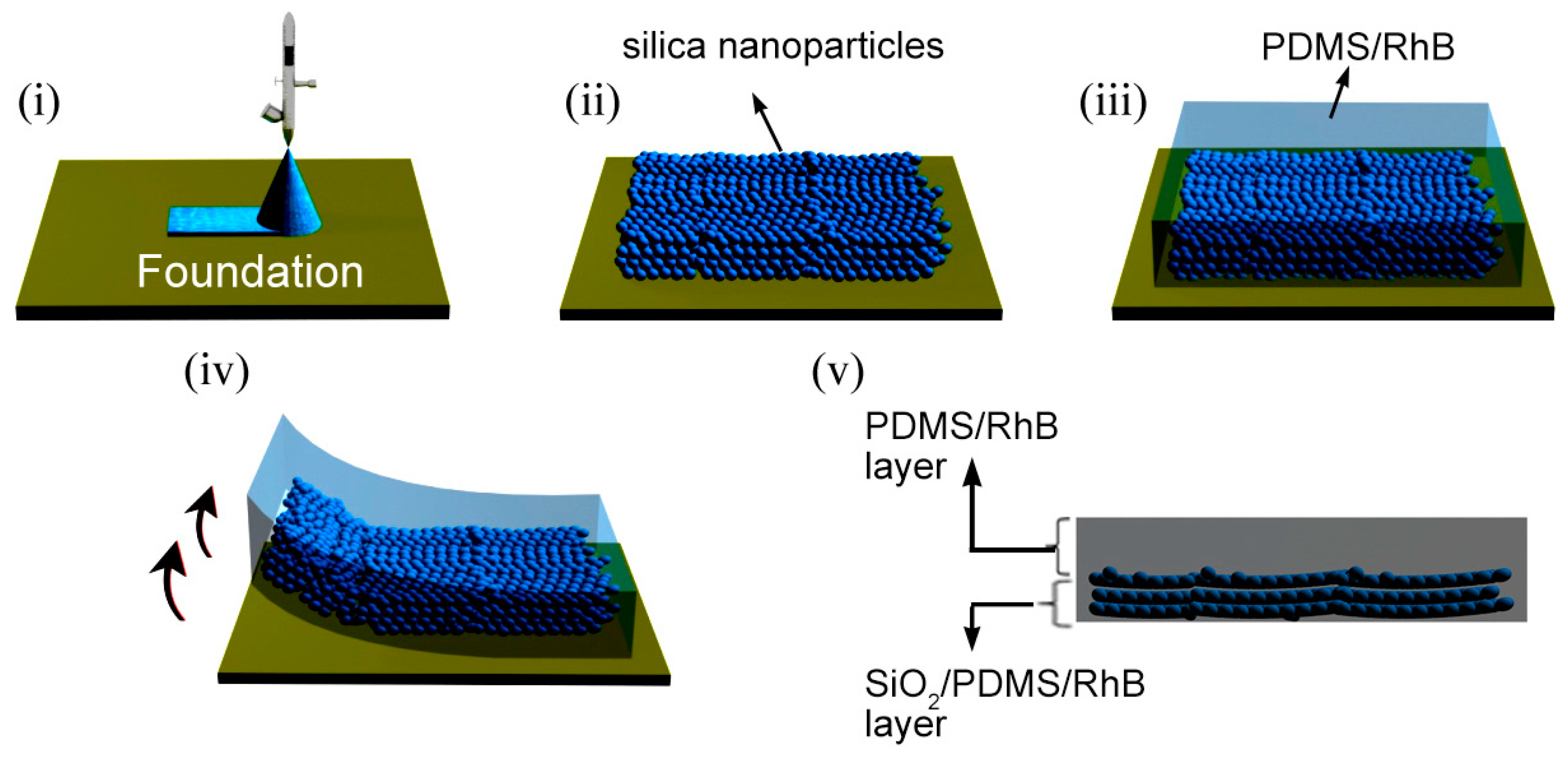
2.3. Fabrication of Hybrid Films
2.4. Characterization
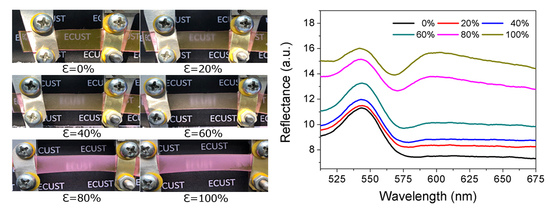
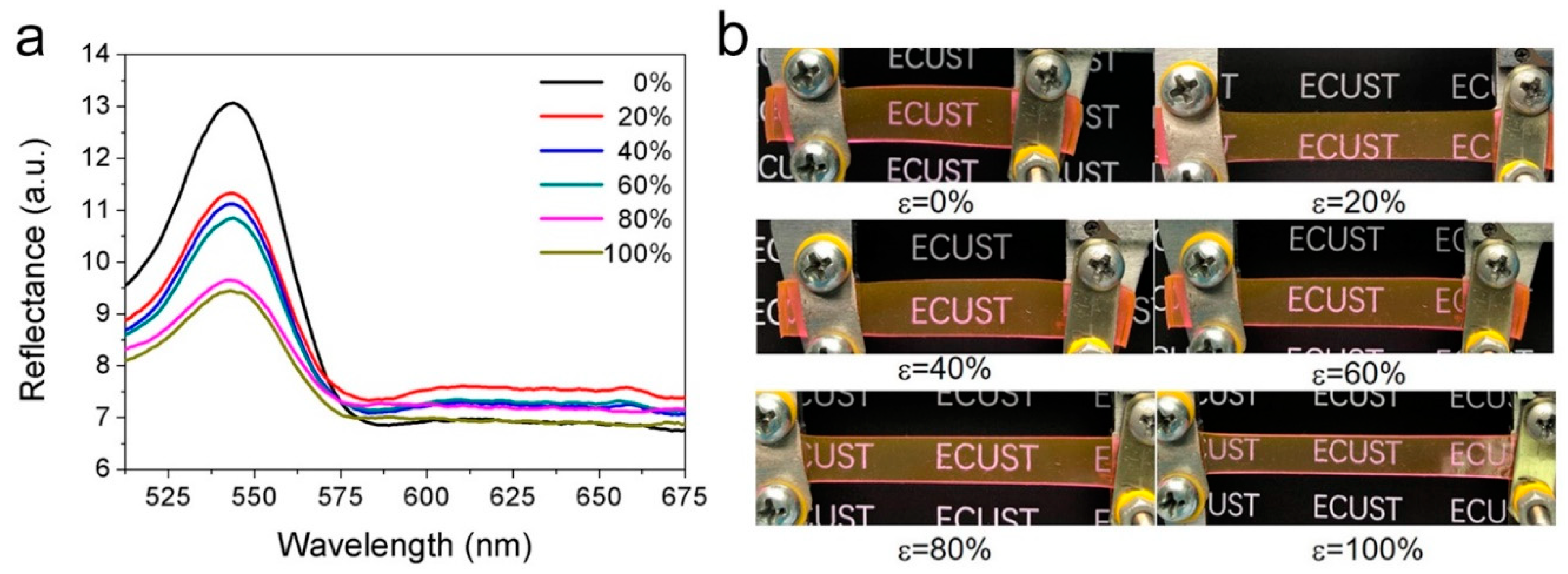
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carpick, R.W.; And, D.Y.S.; Burns, A.R. First Observation of Mechanochromism at the Nanometer Scale. Langmuir 2000, 16, 1270–1278. [Google Scholar] [CrossRef] [Green Version]
- Arsenault, A.C.; Clark, T.J.; Freymann, G.V.; Cademartiri, L.; Sapienza, R.; Bertolotti, J.; Vekris, E.; Wong, S.; Kitaev, V.; Manners, I. From colour fingerprinting to the control of photoluminescence in elastic photonic crystals. Nat. Mater. 2006, 5, 179–184. [Google Scholar] [CrossRef]
- Banisadr, S.; Chen, J. Infrared actuation-induced simultaneous reconfiguration of surface color and morphology for soft robotics. Sci. Rep. 2017, 7, 17521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.G.; Lee, D.Y.; Lim, H.S.; Lee, D.H.; Lee, S.; Cho, K. Switchable transparency and wetting of elastomeric smart windows. Adv. Mater. 2010, 22, 5013–5017. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Lee, E.; Yang, L.; Cho, Y.; Li, M.; Gianola, D.S.; Yang, S. A Robust Smart Window: Reversibly Switching from High Transparency to Angle-Independent Structural Color Display. Adv. Mater. 2015, 27, 2489–2495. [Google Scholar] [CrossRef]
- Zeng, S.; Li, R.; Freire, S.G.; Vmm, G.; Huang, E.Y.; Smith, A.T.; Hu, C.; Wrt, T.; Bian, Z.; Zheng, G. Moisture-Responsive Wrinkling Surfaces with Tunable Dynamics. Adv. Mater. 2017, 29, 1700828. [Google Scholar] [CrossRef]
- Shi, G.; Zhao, Z.; Pai, J.H.; Lee, I.; Zhang, L.; Stevenson, C.; Ishara, K.; Zhang, R.; Zhu, H.; Ma, J. Highly Sensitive, Wearable, Durable Strain Sensors and Stretchable Conductors Using Graphene/Silicon Rubber Composites. Adv. Funct. Mater. 2016, 26, 7614–7625. [Google Scholar] [CrossRef]
- Pang, C.; Lee, G.Y.; Kim, T.; Sang, M.K.; Hong, N.K.; Ahn, S.H.; Suh, K.Y. A flexible and highly sensitive strain-gauge sensor using reversible interlocking of nanofibres. Nat. Mater. 2012, 11, 795–801. [Google Scholar] [CrossRef]
- Wu, H.; Su, Z.; Shi, M.; Miao, L.; Song, Y.; Chen, H.; Han, M.; Zhang, H. Self-Powered Noncontact Electronic Skin for Motion Sensing. Adv. Funct. Mater. 2018, 28, 1704641. [Google Scholar] [CrossRef]
- Wang, Q.; Gossweiler, G.R.; Craig, S.L.; Zhao, X. Cephalopod-inspired design of electro-mechano-chemically responsive elastomers for on-demand fluorescent patterning. Nat. Commun. 2014, 5, 4899. [Google Scholar] [CrossRef] [Green Version]
- Teyssier, J.; Saenko, S.V.; Van, D.D.M.; Milinkovitch, M.C. Photonic crystals cause active colour change in chameleons. Nat. Commun. 2015, 6, 6368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Teng, M.; Wang, Z.; Yang, S.; Jia, X. Mechanically induced multicolor switching based on a single organic molecule. Angew. Chem. 2013, 52, 12268–12272. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, F.; Wang, Z.; Jia, X. Mechanically induced color change based on the chromophores of anthracene and rhodamine 6G. Tetrahedron Lett. 2015, 56, 393–396. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, D.; Huang, W.; Wang, Z.; Freire, S.G.; Yu, X.; Smith, A.T.; Huang, E.Y.; Nguon, H.; Sun, L. Bio-inspired sensitive and reversible mechanochromisms via strain-dependent cracks and folds. Nat. Commun. 2016, 7, 11802. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, C.; Ferrere, S.; Zaban, A.; Sprague, J.; Gregg, B.A. Photoelectrochromic windows and displays. Nature 1996, 383, 608–610. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.F.; Fu, Y.F.; Li, Y.Q.; Huang, P.; Xu, C.H.; Hu, N.; Fu, S.Y. Bio-inspired highly flexible dual-mode electronic cilia. J. Mater. Chem. B 2018, 6, 896–902. [Google Scholar] [CrossRef]
- Cao, D.; Xu, C.; Lu, W.; Qin, C.; Cheng, S. Sunlight-Driven Photo-Thermochromic Smart Windows. So. RRL 2018, 2, 1700219. [Google Scholar] [CrossRef]
- Zhang, B.; Cheng, Y.; Wang, H.; Ye, B.; Shang, L.; Zhao, Y.; Gu, Z. Multifunctional inverse opal particles for drug delivery and monitoring. Nanoscale 2015, 7, 10590–10594. [Google Scholar] [CrossRef]
- Chou, H.T.; Chen, Y.C.; Lee, C.Y.; Chang, H.Y.; Tai, N.H. Switchable transparency of dual-controlled smart glass prepared with hydrogel-containing graphene oxide for energy efficiency. Sol. Energy Mater. Sol. Cells 2017, 166, 45–51. [Google Scholar] [CrossRef]
- Thomas, A.V.; Andow, B.C.; Suresh, S.; Eksik, O.; Yin, J.; Dyson, A.H.; Koratkar, N. Graphene Oxide: Controlled Crumpling of Graphene Oxide Films for Tunable Optical Transmittance. Adv. Mater. 2015, 27, 3256–3265. [Google Scholar] [CrossRef]
- Iwata, M.; Teshima, M.; Seki, T.; Yoshioka, S.; Takeoka, Y. Bio-Inspired Bright Structurally Colored Colloidal Amorphous Array Enhanced by Controlling Thickness and Black Background. Adv. Mater. 2017, 29, 1605050. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Yang, L.; Wu, G.; Yang, S. Angle-independent colours from spray coated quasi-amorphous arrays of nanoparticles: Combination of constructive interference and Rayleigh scattering. J. Mater. Chem. C 2014, 2, 4395–4400. [Google Scholar] [CrossRef]
- Takeoka, Y. Fusion materials for biomimetic structurally colored materials. Polym. J. 2014, 47, 106–113. [Google Scholar] [CrossRef]
- Schäfer, C.G.; Gallei, M.; Zahn, J.T.; Engelhardt, J.; Hellmann, G.P.; Rehahn, M. Reversible Light-, Thermo- and Mechano-Responsive Elastomeric Polymer Opal Films. Chem. Mater. 2013, 25, 2309–2318. [Google Scholar] [CrossRef]
- Schäfer, C.G.; Smolin, D.A.; Hellmann, G.P.; Gallei, M. Fully Reversible Shape Transition of Soft Spheres in Elastomeric Polymer Opal Films. Langmuir 2013, 29, 11275–11283. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Moon, J.H.; Yang, S. Chemical aspects of three-dimensional photonic crystals. Chem. Rev. 2010, 110, 547–574. [Google Scholar] [CrossRef] [PubMed]
- Krishna, N.P.V.; Murthy, T.R.S.; Reddy, K.J.; Sangeeth, K.; Hegde, G.M. Design and Fabrication of Piezoresistive Based Encapsulated Poly-Si Cantilevers for Bio/chemical Sensing. Phys. Procedia 2011, 19, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Malitson, I.H. Interspecimen Comparison of the Refractive Index of Fused Silica. J. Opt. Soc. Am. 1965, 55, 1205–1208. [Google Scholar] [CrossRef]
- Beaumont, P.C.; Johnson, D.G.; Parsons, B.J. Photophysical properties of laser dyes: Picosecond laser flash photolysis studies of rhodamine 6G, rhodamine b and rhodamine 101. J. Chem. Soc. Faraday Trans. 1993, 89, 4185–4191. [Google Scholar] [CrossRef]
- Ramette, R.W.; Sandell, E.B. Rhodamine B Equilibria. J. Am. Chem. Soc. 1956, 78, 4872–4878. [Google Scholar] [CrossRef]
- Seghir, R.; Arscott, S. Extended PDMS stiffness range for flexible systems. Sens. Actuators A Phys. 2015, 230, 33–39. [Google Scholar] [CrossRef]
- Stockman, A.; Sharpe, L.T.; Fach, C. The spectral sensitivity of the human short-wavelength sensitive cones derived from thresholds and color matches. Vis. Res. 1999, 39, 2901–2927. [Google Scholar] [CrossRef] [Green Version]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Zeng, S.; Yao, Y.; Xu, S.; Dong, Q.; Chen, P.; Wang, Z.; Zhang, M.; Zhu, M.; Xu, G.; et al. Dynamic Optics with Transparency and Color Changes under Ambient Conditions. Polymers 2019, 11, 103. https://doi.org/10.3390/polym11010103
Jiang Y, Zeng S, Yao Y, Xu S, Dong Q, Chen P, Wang Z, Zhang M, Zhu M, Xu G, et al. Dynamic Optics with Transparency and Color Changes under Ambient Conditions. Polymers. 2019; 11(1):103. https://doi.org/10.3390/polym11010103
Chicago/Turabian StyleJiang, Yejia, Songshan Zeng, Yu Yao, Shiyu Xu, Qiaonan Dong, Pingxu Chen, Zhaofeng Wang, Monica Zhang, Mengting Zhu, Gefan Xu, and et al. 2019. "Dynamic Optics with Transparency and Color Changes under Ambient Conditions" Polymers 11, no. 1: 103. https://doi.org/10.3390/polym11010103