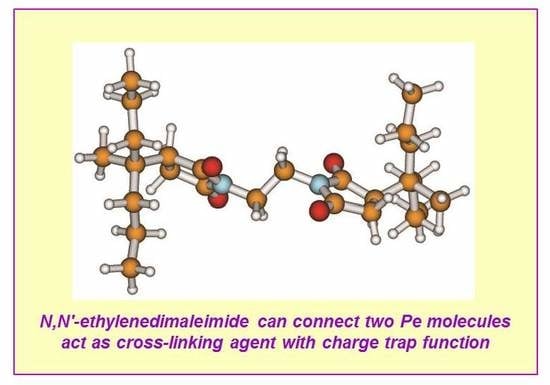
Theoretical Study on the Grafting Reaction of Maleimide to Polyethylene in the UV Radiation Cross-Linking Process
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
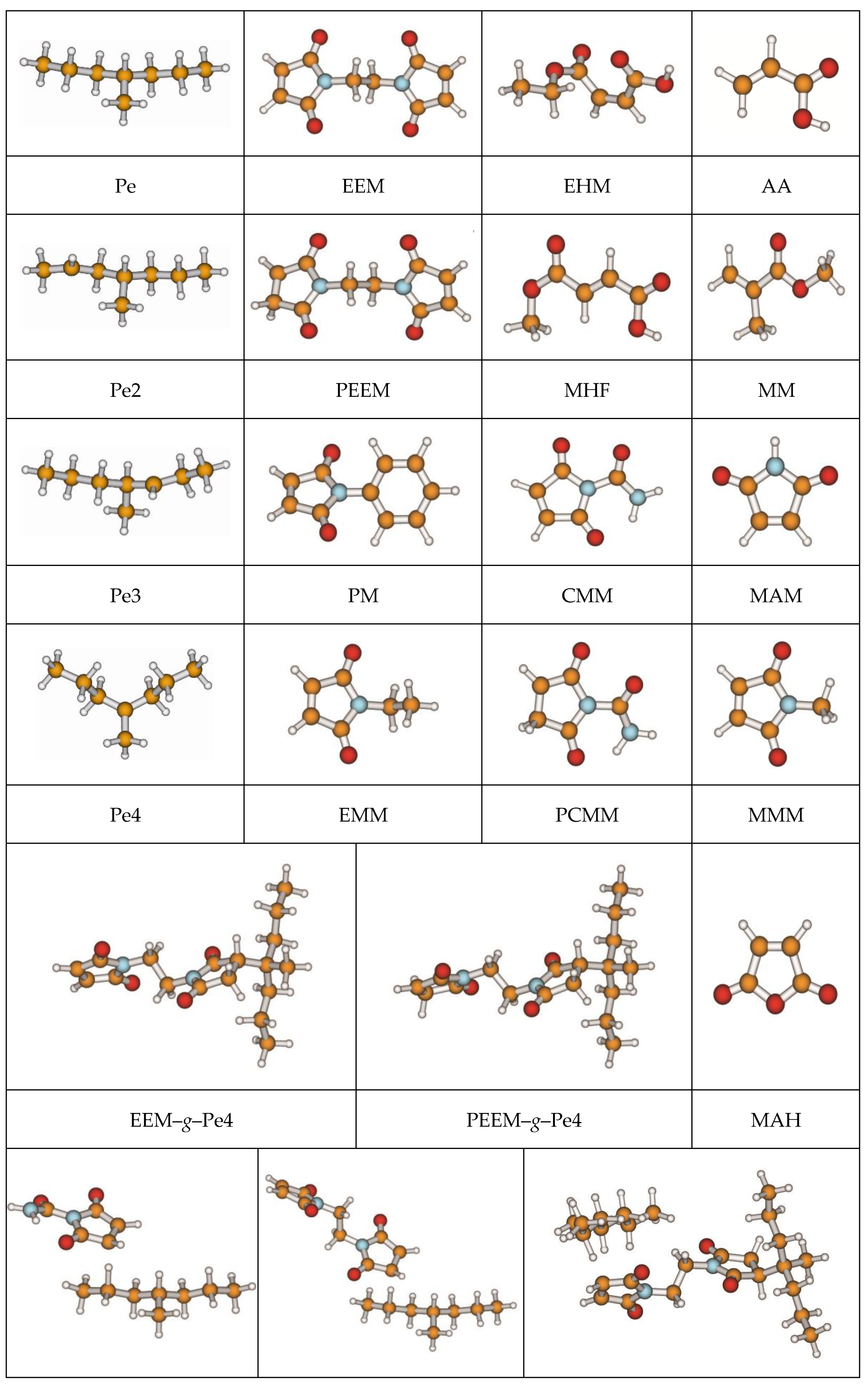
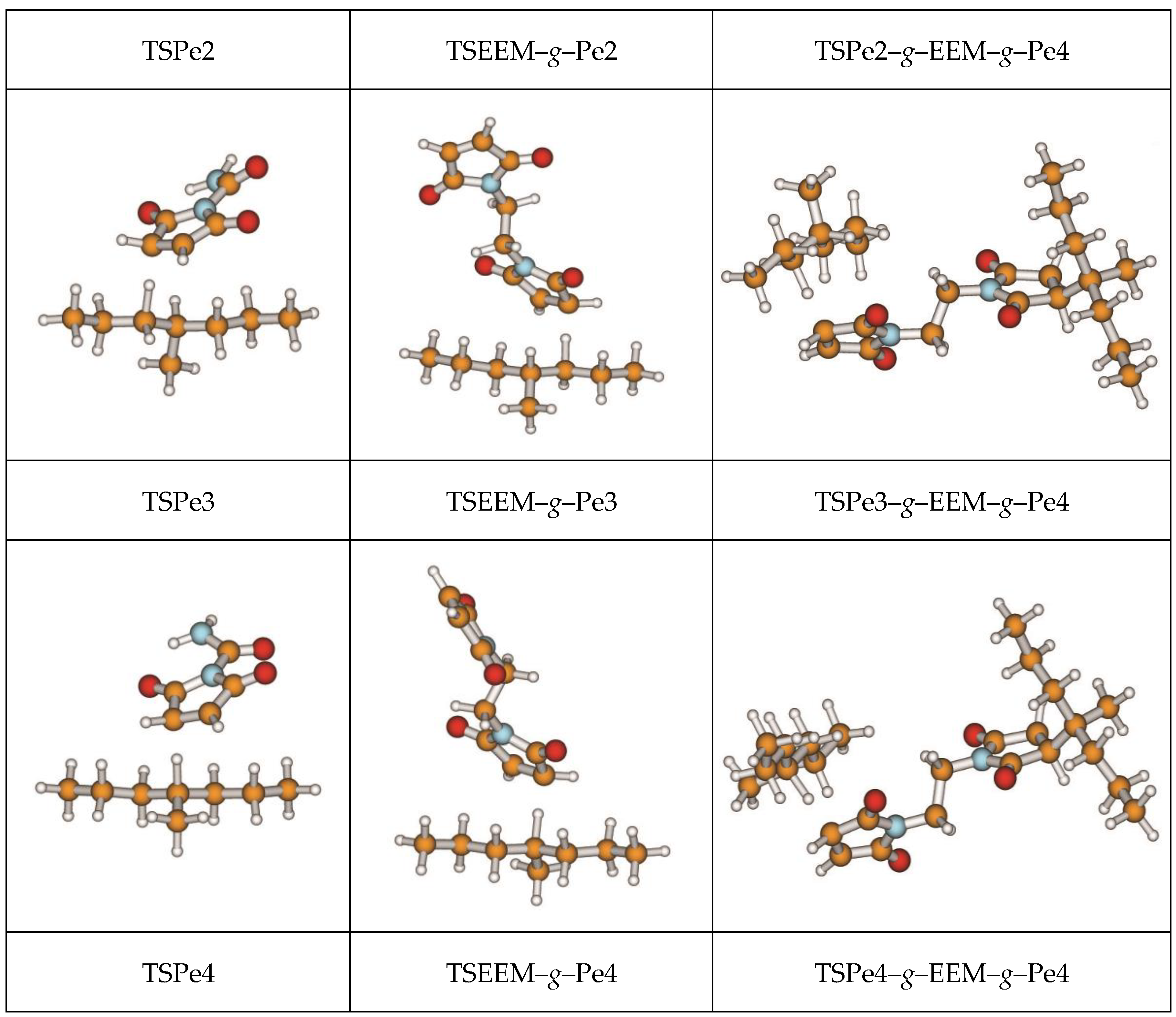
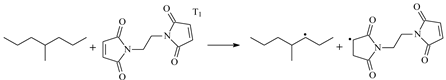
3.1. Stationary Point Geometries
3.2. Frontier MOs and NBO Charge Population
3.3. Energetics
4. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Montanari, G.C.; Laurent, C.; Teyssedre, G.; Laurent, C.; Teyssedre, G.; Campus, A.; Nilsson, U.H. From LDPE to XLPE: Investigating the change of electrical properties. Part I. space charge, conduction and lifetime. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 438–446. [Google Scholar] [CrossRef]
- Mazzanti, G.; Montanari, G.C.; Dissado, L.A. Electrical aging and life models: the role of space charge. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 876–890. [Google Scholar] [CrossRef]
- Montanari, G.C.; Mazzanti, G.; Palmieri, F.; Motori, A.; Perego, G.; Serra, S. Space-charge trapping and conduction in LDPE, HDPE and XLPE. J. Phys. D 2001, 34, 2902–2911. [Google Scholar] [CrossRef]
- Lau, K.Y.; Vaughan, A.S.; Chen, G.; Hosier, I.L.; Holt, A.F.; Ching, K.Y. On the space charge and DC breakdown behavior of polyethylene/silica nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 340–351. [Google Scholar] [CrossRef]
- Pitsa, D.; Danikas, M.G.; Vardakis, G.E.; Tanaka, T. Influence of homocharges and nanoparticles in electrical tree propagation under DC voltage application. Electr. Eng. 2012, 94, 81–88. [Google Scholar] [CrossRef]
- Danikas, M.G.; Tanaka, T. Nanocomposites-a review of electrical treeing and breakdown. IEEE Electr. Insul. Mag. 2009, 25, 19–25. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.K.; Han, J.H.; Suh, K.S. Space charge and electrical conduction in maleic anhydride-grafted polyethylene. IEEE Trans. Dielectr. Electr. Insul. 1995, 2, 1132–1139. [Google Scholar]
- Zha, J.W.; Wu, Y.H.; Wang, S.J.; Wu, D.H.; Yan, H.D.; Dang, Z.M. Improvement of space charge suppression of polypropylene for potential application in HVDC cables. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2337–2343. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.; Dang, B.; He, J.L. Mechanism of highly improved electrical properties in polypropylene by chemical modification of grafting maleic anhydride. J. Phys. D 2016, 49, 415301. [Google Scholar] [CrossRef]
- Pan, B.; Viswanathan, K.; Hoyle, C.E.; Moore, R.B. Photoinitiated grafting of maleic anhydride onto polypropylene. J. Polym. Sci. Part A 2004, 42, 1953–1962. [Google Scholar] [CrossRef]
- Qu, B.; Bao, W.; Wu, Q.; Shi, W. Recent developments on photoinitiated crosslinking of polyethylene and its applications for manufacturing insulated wire and cable. In Proceedings of the 9th IEEE international conference on the properties and applications of Dielectric Materials, Harbin, China, 19–23 July 2009; pp. 33–36. [Google Scholar]
- Deng, J.P.; Wang, L.F.; Liu, L.Y.; Yang, W.T. Progress in polymer science developments and new applications of UV-induced surface graft polymerizations. Prog. Polym. Sci. 2009, 34, 156–193. [Google Scholar] [CrossRef]
- Qu, B.J.; Rånby, B. Photocross-linking of low-density polyethylene. I. kinetics and reaction parameters. J. Appl. Polym. Sci. 1993, 48, 701–709. [Google Scholar] [CrossRef]
- Wu, Q.H.; Qu, B.J. Photoinitiating characteristics of benzophenone derivatives as new initiators in the photocrosslinking of polyethylene. Polym. Eng. Sci. 2001, 41, 1220–1226. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Y.; Zhao, H.; Wang, X.; Han, B.Z.; Li, Z.S. Theoretical study on the reaction of maleic anhydride in the UV radiation cross-linking process of polyethylene. Polymer 2017, 133, 232–239. [Google Scholar] [CrossRef]
- Hayakawa, K.; Kawase, K.; Yamakita, H. Further studies on the ultraviolet graft copolymerization of maleimide by vapor-phase method. J. Polym. Sci. Part A 1974, 12, 2603–2612. [Google Scholar] [CrossRef]
- Aly, R.O.; Mostafa, T.B.; Mokhtar, S.M. Modification of polyethylene by radiation-induced graft copolymerization of N-phenylmaleimide and p-hydroxy N-phenylmaleimide. Polym. Test. 2002, 21, 857–865. [Google Scholar] [CrossRef]
- Na, H.S.; Kim, T.H. Grafting of maleimide containing 2-hydroxy-benzophenone onto polyethylene: Reaction conditions and photo-stabilization effects. Macromol. Res. 2014, 22, 958–962. [Google Scholar] [CrossRef]
- Mokhtar, S.M.; Sabaa, M.W. Gamma radiation-induced graft copolymerization of N-p-hydroxyphenylmaleimide onto polypropylene Films. J. Polym. Res. 2000, 7, 215–219. [Google Scholar] [CrossRef]
- Silva, R.; Muniz, E.C.; Rubira, A.F. Maleimide immobilized on a PE surface: Preparation, characterization and application as a free-radical photoinitiator. Langmuir 2009, 25, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.N.; Duncan, W.T.; Bell, R.L. Chemical Applications of Density-Functional Theory; American Chemical Society: Washington, DC, USA, 1996; p. 85. ISBN 0-8412-3403-5. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti conelation energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Zhang, H.; Shang, Y.; Li, M.X.; Zhao, H.; Wang, X.; Han, B.Z. Mechanism in the cross-linking process of polyethylene. RSC Adv. 2015, 5, 90343–90353. [Google Scholar] [CrossRef]
- Zangwill, A.; Soven, P. Density-functional approach to local-field effects in finite systems: Photoabsorption in the rare gases. Phys. Rev. A 1980, 21, 1561–1572. [Google Scholar] [CrossRef]
- Levine, Z.H.; Soven, P. Time-dependent local-density theory of dielectric effects in small molecules. Phys. Rev. A 1984, 29, 625–635. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Hammond, G.S. A correlation of reaction rates. J. Am. Chem. Soc. 1955, 77, 334–338. [Google Scholar] [CrossRef]
- Han, B.Z.; Jiao, M.G.; Li, C.Y.; Zhang, C.C.; Wu, Z.J.; Wang, Y.; Zhang, H. QM/MM simulations on the role of SiO2 in polymeric insulation materials. RSC Adv. 2016, 6, 555–562. [Google Scholar] [CrossRef]
- Markert, H.; Wiedenmann, R. Cross-linking of polyethylene in the presence of additives part I: Thermal decomposition of the cross-linking initiators. Siemens Forschungs-und Entwicklungsbericht 1973, 2, 85–91. [Google Scholar]
- Zhao, H.; Chen, J.Q.; Zhang, H.; Shang, Y.; Wang, X.; Han, B.Z.; Li, Z.S. Theoretical study on the reaction of triallyl isocyanurate in the UV radiation cross-linking of polyethylene. RSC Adv. 2017, 7, 37095–37104. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Y.; Zhao, H.; Han, B.Z.; Li, Z.S. Mechanisms on electrical breakdown strength increment of polyethylene by acetophenone and its analogues addition: A theoretical study. J. Mol. Model 2013, 19, 4477–4485. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, N.; Suzuki, T.; Ito, M. Why does intersystem crossing occur in isolated molecules of benzaldehyde, acetophenone, and benzophenone? J. Phys. Chem. 1988, 92, 1086–1093. [Google Scholar] [CrossRef]
| ab. | Molecular Formula | Sn | Excitation Energy | ab. | Molecular Formula | Sn | Excitation Energy |
|---|---|---|---|---|---|---|---|
| BP | 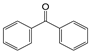 | 1 | 3.5991 | MAH | 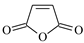 | 1 | 3.6485 |
| 2 | 4.5270 | 2 | 4.3668 | ||||
| 3 | 4.6060 | 3 | 5.3341 | ||||
| CMM |  | 1 | 3.2558 | EH |  | 1 | 4.5740 |
| 2 | 3.7502 | 2 | 4.7433 | ||||
| 3 | 3.9501 | 3 | 5.1899 | ||||
| MAM |  | 1 | 3.4273 | MHF |  | 1 | 3.8208 |
| 2 | 4.2021 | 2 | 4.2214 | ||||
| 3 | 4.5092 | 3 | 5.0101 | ||||
| EEM | 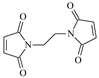 | 1 | 3.4650 | TRCC |  | 1 | 4.6060 |
| 2 | 3.4650 | 2 | 5.7016 | ||||
| 3 | 3.9339 | 3 | 6.0774 | ||||
| MMM |  | 1 | 3.4672 | ACA |  | 1 | 5.1686 |
| 2 | 4.0443 | 2 | 5.9846 | ||||
| 3 | 4.1825 | 3 | 6.1150 | ||||
| EMM | 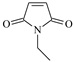 | 1 | 3.4604 | AA | 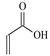 | 1 | 4.6843 |
| 2 | 4.0070 | 2 | 6.3785 | ||||
| 3 | 4.1725 | 3 | 6.6245 | ||||
| PM | 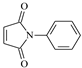 | 1 | 3.1134 | MM | 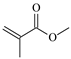 | 1 | 4.8253 |
| 2 | 3.4628 | 2 | 5.8684 | ||||
| 3 | 3.5347 | 3 | 6.4778 |
| Reaction Equation | Reactant | b/f | Product | Frequency |
|---|---|---|---|---|
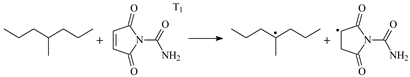 | 1.100 | 1.280/1.479 | 1.098 | 964 i |
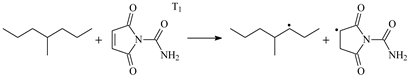 | 1.098 | 1.302/1.438 | 1.098 | 1199 i |
 | 1.097 | 1.303/1.428 | 1.098 | 1185 i |
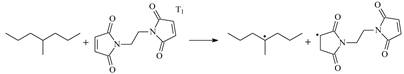 | 1.100 | 1.285/1.469 | 1.097 | 1080 i |
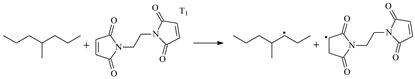 | 1.098 | 1.305/1.435 | 1.097 | 1288 i |
 | 1.097 | 1.305/1.424 | 1.097 | 1280 i |
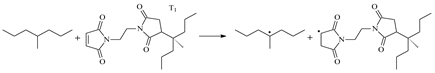 | 1.100 | 1.289/1.465 | 1.097 | 1118 i |
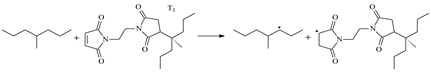 | 1.098 | 1.308/1.433 | 1.097 | 1307 i |
 | 1.097 | 1.300/1.429 | 1.097 | 1257 i |
 | Natural Charge Population | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | N4 | C5 | O6 | O7 | H8 | H9 | |||
| R= | H | S0 | −0.226 | −0.226 | 0.652 | −0.674 | 0.652 | −0.534 | −0.534 | 0.232 | 0.232 |
| S1 | −0.207 | −0.207 | 0.620 | −0.660 | 0.620 | −0.534 | −0.534 | 0.238 | 0.238 | ||
| T1 | −0.298 | −0.297 | 0.538 | −0.614 | 0.537 | −0.382 | −0.382 | 0.237 | 0.237 | ||
| R= |  | S0 | −0.225 | −0.207 | 0.672 | −0.594 | 0.673 | −0.473 | −0.562 | 0.236 | 0.236 |
| S1 | −0.227 | −0.166 | 0.617 | −0.571 | 0.661 | −0.432 | −0.538 | 0.241 | 0.242 | ||
| T1 | −0.278 | −0.297 | 0.525 | −0.530 | 0.592 | −0.264 | −0.610 | 0.231 | 0.235 | ||
| R= |  | S0 | −0.222 | −0.221 | 0.666 | −0.543 | 0.668 | −0.539 | −0.538 | 0.232 | 0.232 |
| −0.222 | −0.221 | 0.666 | −0.543 | 0.668 | −0.539 | −0.538 | 0.232 | 0.232 | |||
| S1 | −0.215 | −0.194 | 0.626 | −0.532 | 0.647 | -0.549 | −0.527 | 0.237 | 0.239 | ||
| −0.222 | −0.221 | 0.665 | −0.543 | 0.668 | -0.537 | −0.539 | 0.232 | 0.232 | |||
| T1 | −0.142 | −0.142 | 0.549 | −0.382 | 0.552 | -0.590 | −0.587 | 0.217 | 0.217 | ||
| −0.220 | −0.220 | 0.666 | −0.539 | 0.669 | -0.536 | −0.533 | 0.233 | 0.233 | |||
| Reaction Equation | B3LYP/6-311+G(d,p) | ||
|---|---|---|---|
| ΔETS+ZPE | Δ | ||
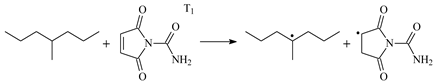 | 0.41 | −0.18 | 3.91 |
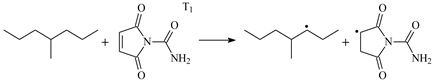 | 0.53 | −0.06 | 4.04 |
 | 0.49 | −0.09 | 4.01 |
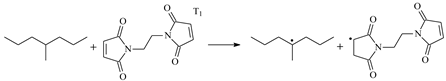 | 0.19 | −0.47 | 3.91 |
 | 0.30 | −0.35 | 4.04 |
 | 0.26 | −0.38 | 4.01 |
 | 0.20 | −0.46 | 3.91 |
 | 0.32 | −0.34 | 4.04 |
 | 0.25 | −0.37 | 4.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Shang, Y.; Zhao, H.; Li, C.; Wang, X.; Han, B.; Li, Z. Theoretical Study on the Grafting Reaction of Maleimide to Polyethylene in the UV Radiation Cross-Linking Process. Polymers 2018, 10, 1044. https://doi.org/10.3390/polym10091044
Zhang H, Shang Y, Zhao H, Li C, Wang X, Han B, Li Z. Theoretical Study on the Grafting Reaction of Maleimide to Polyethylene in the UV Radiation Cross-Linking Process. Polymers. 2018; 10(9):1044. https://doi.org/10.3390/polym10091044
Chicago/Turabian StyleZhang, Hui, Yan Shang, Hong Zhao, Chunyang Li, Xuan Wang, Baozhong Han, and Zesheng Li. 2018. "Theoretical Study on the Grafting Reaction of Maleimide to Polyethylene in the UV Radiation Cross-Linking Process" Polymers 10, no. 9: 1044. https://doi.org/10.3390/polym10091044