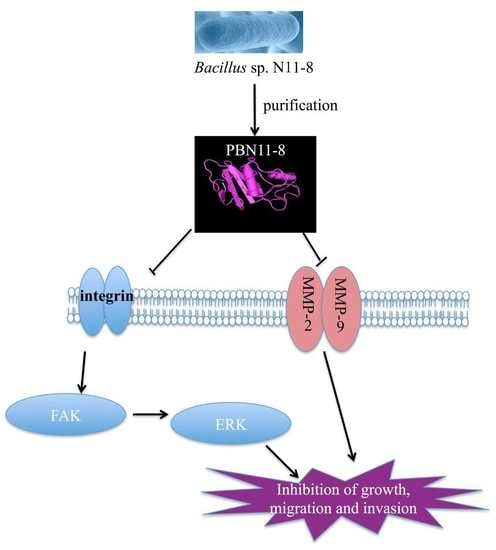
PBN11-8, a Cytotoxic Polypeptide Purified from Marine Bacillus, Suppresses Invasion and Migration of Human Hepatocellular Carcinoma Cells by Targeting Focal Adhesion Kinase Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Materials
2.3. Microorganism and Fermentation
2.4. Purification and Identification of Polypeptide
2.5. Cloning, Sequence Analysis of PBN11-8
2.6. Measurement of Protease Activity
2.7. Cytotoxic Activity Assay
2.8. Crystal Violet Adhesion Assay
2.9. Migration and Invasion Assay on BEL-7402 Cells
2.10. Immunoblotting Assay
2.11. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Assay
- GAPDH forward: 5′-AAGTTCAACGGCACAGTCAAGG-3′,
- GAPDH reverse: 5′-CATACTCAGCACCAGCATCACC-3′;
- Integrin β1forward: 5′-TTCGATGCCATCATGCAAGTTG-3′,
- Integrin β1 reverse: 5′-CCATCTCCAGCAAAGTGAAACC-3′,
- FAK-forward: 5′-ACTCATCGAGAGATCGAGATGG-3′,
- FAK reverse: 5′-GCCCTAGCATTTTCAGTCTTGC-3′.
2.12. Statistical Analysis
3. Results
3.1. Preparation of Crude Extract
3.2. Purification and Identification of Cytotoxic Polypeptide
3.3. Cloning, Sequence Analysis and the Protease Activity of Polypeptide PBN11-8
3.4. PBN11-8 Displayed Cytotoxicity toward Different Cells
3.5. PBN11-8 Affects the Migration and Invasion of BEL-7402 Cells
3.6. PBN11-8 Inhibited the Activation of FAK in BEL-7402 Cells
3.7. PBN11-8 Inhibited the Activation of ERK in BEL-7402 Cells
3.8. Expression of MMP-2 and MMP-9 Was Reduced by PBN11-8 in BEL-7402 Cells
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wu, Y.-H.; Yu, P.-S.; Zhou, Y.-D.; Xu, L.; Wang, C.-S.; Wu, M.; Oren, A.; Xu, X.-W. Muricauda antarctica sp. nov., a marine member of the Flavobacteriaceae isolated from Antarctic seawater. Int. J. Syst. Evol. Microbiol. 2013, 63, 3451–3456. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, B.; Zou, Y.; Zheng, T. Polar microorganisms, a potential source for new natural medicines—A review. Wei Sheng Wu Xue Bao 2008, 48, 695–700. [Google Scholar] [PubMed]
- Xu, Y.; Kersten, R.D.; Nam, S.-J.; Lu, L.; Al-Suwailem, A.M.; Zheng, H.; Fenical, W.; Dorrestein, P.C.; Moore, B.S.; Qian, P.-Y. Bacterial biosynthesis and maturation of the didemnin anti-cancer agents. J. Am. Chem. Soc. 2012, 134, 8625–8632. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yi, Y.; Liu, J.; Lin, X.; Yang, K.; Lv, M.; Zhou, X.; Hao, J.; Liu, J.; Zheng, Y.; et al. Isolation and characterization of marine Brevibacillus sp. S-1 collected from South China Sea and a novel antitumor peptide produced by the strain. PLoS ONE 2014, 9, e111270. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Gundampati, R.K.; Jagannadham, M.V.; Srivastava, S.K. Extracellular l-asparaginase from a protease-deficient Bacillus aryabhattai ITBHU02: purification, biochemical characterization, and evaluation of antineoplastic activity in vitro. Appl. Biochem. Biotechnol. 2013, 171, 1759–1774. [Google Scholar] [CrossRef] [PubMed]
- Bauvois, B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochim. Biophys. Acta 2012, 1825, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-L.; Jeng, L.-B.; Lai, H.-C.; Liao, P.-Y.; Chang, C. Androgen receptor enhances cell adhesion and decreases cell migration via modulating β1-integrin-AKT signaling in hepatocellular carcinoma cells. Cancer Lett. 2014, 351, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.-L.; Ko, B.-S.; Liu, T.-A.; Liang, S.-M.; Liu, C.-C.; Lu, Y.-J.; Tzean, S.-S.; Shen, T.-L.; Liou, J.-Y. Cordycepin suppresses integrin/FAK signaling and epithelial-mesenchymal transition in hepatocellular carcinoma. Anticancer. Agents Med. Chem. 2014, 14, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Eke, I.; Deuse, Y.; Hehlgans, S.; Gurtner, K.; Krause, M.; Baumann, M.; Shevchenko, A.; Sandfort, V.; Cordes, N. β(1)Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J. Clin. Investig. 2012, 122, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Li, J.; Yee, S.-P.; Fellows, G.F.; Goodyer, C.G.; Wang, R. β1 integrin/FAK/ERK signalling pathway is essential for human fetal islet cell differentiation and survival. J. Pathol. 2009, 219, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, V.; Harnois, C.; Demers, M.-J.; Thibodeau, S.; Laquerre, V.; Gauthier, R.; Vezina, A.; Noel, D.; Fujita, N.; Tsuruo, T.; et al. β1 integrin/Fak/Src signaling in intestinal epithelial crypt cell survival: integration of complex regulatory mechanisms. Apoptosis 2008, 13, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yang, K.; Liu, J.; Sun, M.; Zhu, J.; Lv, M.; Kang, D.; Wang, W.; Xing, M.; Li, Z. Screening of microorganisms from Antarctic surface water and cytotoxicity metabolites from Antarctic microorganisms. Food Sci. Nutr. 2016, 4, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.D.; Joyce, J.A. Proteolytic networks in cancer. Trends Cell Biol. 2011, 21, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Christakopoulos, P.; Hatzinikolaou, D.G.; Fountoukidis, G.; Kekos, D.; Claeyssens, M.; Macris, B.J. Purification and mode of action of an alkali-resistant endo-1, 4-β-glucanase from Bacillus pumilus. Arch. Biochem. Biophys. 1999, 364, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhang, Y.; Liu, F. Purification and characterization of a approximately 43 kDa antioxidant protein with antitumor activity from Pholiota nameko. J. Sci. Food Agric. 2016, 96, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Dong, Y.; Chen, G.; Hu, Q. Extraction, purification, characterization and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydr. Polym. 2010, 80, 783–789. [Google Scholar] [CrossRef]
- Lv, S.; Gao, J.; Liu, T.; Zhu, J.; Xu, J.; Song, L.; Liang, J.; Yu, R. Purification and Partial Characterization of a New Antitumor Protein from Tegillarca granosa. Mar. Drugs 2015, 13, 1466–1480. [Google Scholar] [CrossRef] [PubMed]
- Montsko, G.; Tarjanyi, Z.; Mezosi, E.; Kovacs, G.L. A validated method for measurement of serum total, serum free, and salivary cortisol, using high-performance liquid chromatography coupled with high-resolution ESI-TOF mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Ling, P.; Wang, Z.; Niu, R.; Hu, C.; Zhang, T.; Lin, X. A novel polypeptide from shark cartilage with potent anti-angiogenic activity. Cancer Biol. Ther. 2007, 6, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Corbett, T.H. Total structure determination of apratoxin A, a potent novel cytotoxin from the marine cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc. 2001, 123, 5418–5423. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Luo, J.; Jiang, B.; Wang, L.; Wang, S.; Wang, C.; Fu, C.; Li, J.; Shi, D. Marine bromophenol bis (2,3-dibromo-4,5-dihydroxy-phenyl)-methane inhibits the proliferation, migration, and invasion of hepatocellular carcinoma cells via modulating β1-integrin/FAK signaling. Mar. Drugs 2015, 13, 1010–1025. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, A.E.; Bumann, M.; Hege, T.; Russo, S.; Baumann, U. Metzincin’s canonical methionine is responsible for the structural integrity of the zinc-binding site. Biol. Chem. 2009, 390, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-L.; Hung, J.-Y.; Liang, Y.-Y.; Lin, Y.-S.; Tsai, M.-J.; Chou, S.-H.; Lu, C.-Y.; Kuo, P.-L. S100P interacts with integrin α7 and increases cancer cell migration and invasion in lung cancer. Oncotarget 2015, 6, 29585–29598. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Timpson, P.; Horvath, L.G.; Daly, R.J. FAK signaling in human cancer as a target for therapeutics. Pharmacol. Ther. 2015, 146, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Sarode, S.C.; Sarode, G.S.; Choudhary, S.; Patil, S. FAK is overexpressed in keratocystic odontogenic tumor: A preliminary study. J. Oral. Pathol. Med. 2017, 46, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Rudakova, N.L.; Balaban, N.P.; Danilova, Y.V.; Rudenskaya, G.N.; Sharipova, M.R. Characteristics of a novel secreted zinc-dependent endopeptidase of Bacillus intermedius. Biochemistry 2010, 75, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Sabirova, A.R.; Rudakova, N.L.; Balaban, N.P.; Ilyinskaya, O.N.; Demidyuk, I.V.; Kostrov, S.V.; Rudenskaya, G.N.; Sharipova, M.R. A novel secreted metzincin metalloproteinase from Bacillus intermedius. FEBS Lett. 2010, 584, 4419–4425. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-Q.; Liu, X.-F.; Yao, L.; Chen, C.-Q.; Lin, J.-F.; Gu, Z.-D.; Ni, P.-H.; Zheng, X.-M.; Fan, Q.-S. Focal adhesion kinase regulates the phosphorylation protein tyrosine phosphatase-alpha at Tyr789 in breast cancer cells. Mol. Med. Rep. 2015, 11, 4303–4308. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; O’Brien, L.E.; Kwon, S.-H.; Mostov, K.E. STAT1 is required for redifferentiation during Madin-Darby canine kidney tubulogenesis. Mol. Biol. Cell 2010, 21, 3926–3933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hochwald, S.N. The role of FAK in tumor metabolism and therapy. Pharmacol. Ther. 2014, 142, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Montagut, C.; Settleman, J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009, 283, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-L.; Hsieh, M.-J.; Yang, J.-S.; Lin, C.-W.; Lue, K.-H.; Lu, K.-H.; Yang, S.-F. Nobiletin inhibits human osteosarcoma cells metastasis by blocking ERK and JNK-mediated MMPs expression. Oncotarget 2016, 7, 35208–35223. [Google Scholar] [CrossRef] [PubMed]
- Jablonska-Trypuc, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lustosa, S.A.S.; de Viana, L.S.; Affonso, R.J.J.; Silva, S.R.M.; Denadai, M.V.A.; de Toledo, S.R.C.; Oliveira, I.D.; Matos, D. Expression profiling using a cDNA array and immunohistochemistry for the extracellular matrix genes FN-1, ITGA-3, ITGB-5, MMP-2, and MMP-9 in colorectal carcinoma progression and dissemination. Sci. World J. 2014, 2014, 102541. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Zhu, X.; Yang, K.; Zhu, M.; Farooqi, A.A.; Kang, D.; Sun, M.; Xu, Y.; Lin, X.; Feng, Y.; et al. PBN11-8, a Cytotoxic Polypeptide Purified from Marine Bacillus, Suppresses Invasion and Migration of Human Hepatocellular Carcinoma Cells by Targeting Focal Adhesion Kinase Pathways. Polymers 2018, 10, 1043. https://doi.org/10.3390/polym10091043
Zheng L, Zhu X, Yang K, Zhu M, Farooqi AA, Kang D, Sun M, Xu Y, Lin X, Feng Y, et al. PBN11-8, a Cytotoxic Polypeptide Purified from Marine Bacillus, Suppresses Invasion and Migration of Human Hepatocellular Carcinoma Cells by Targeting Focal Adhesion Kinase Pathways. Polymers. 2018; 10(9):1043. https://doi.org/10.3390/polym10091043
Chicago/Turabian StyleZheng, Lanhong, Xiangjie Zhu, Kangli Yang, Meihong Zhu, Ammad Ahmad Farooqi, Daole Kang, Mi Sun, Yixin Xu, Xiukun Lin, Yingang Feng, and et al. 2018. "PBN11-8, a Cytotoxic Polypeptide Purified from Marine Bacillus, Suppresses Invasion and Migration of Human Hepatocellular Carcinoma Cells by Targeting Focal Adhesion Kinase Pathways" Polymers 10, no. 9: 1043. https://doi.org/10.3390/polym10091043
APA StyleZheng, L., Zhu, X., Yang, K., Zhu, M., Farooqi, A. A., Kang, D., Sun, M., Xu, Y., Lin, X., Feng, Y., Liang, F., Zhang, F., & Linhardt, R. J. (2018). PBN11-8, a Cytotoxic Polypeptide Purified from Marine Bacillus, Suppresses Invasion and Migration of Human Hepatocellular Carcinoma Cells by Targeting Focal Adhesion Kinase Pathways. Polymers, 10(9), 1043. https://doi.org/10.3390/polym10091043