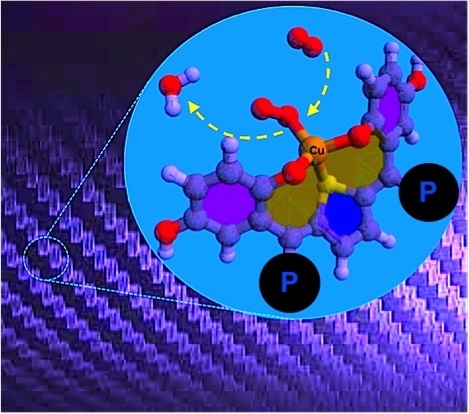
Conducting Copper(I/II)-Metallopolymer for the Electrocatalytic Oxygen Reduction Reaction (ORR) with High Kinetic Current Density
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Synthesis
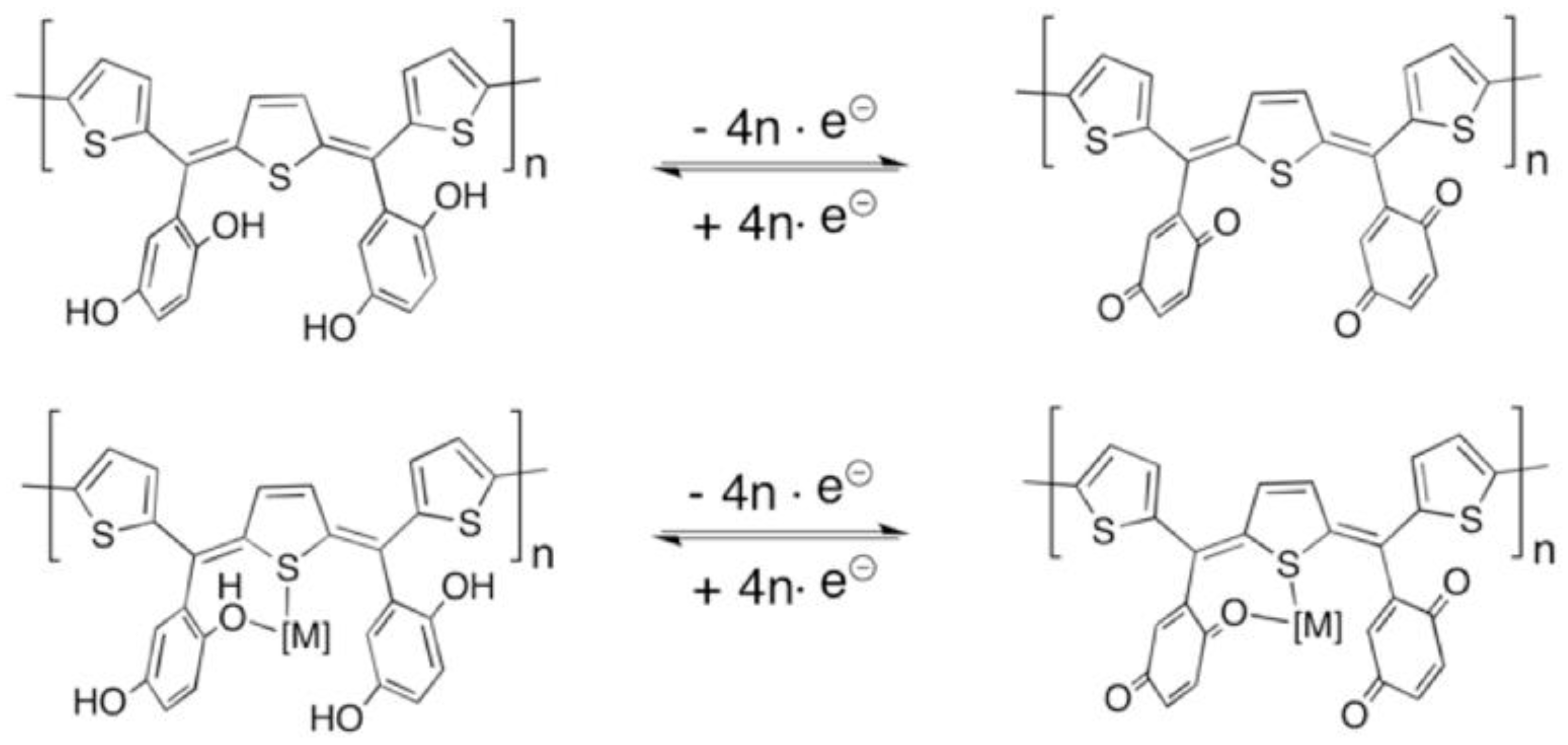
2.3. Metallopolymer Synthesis
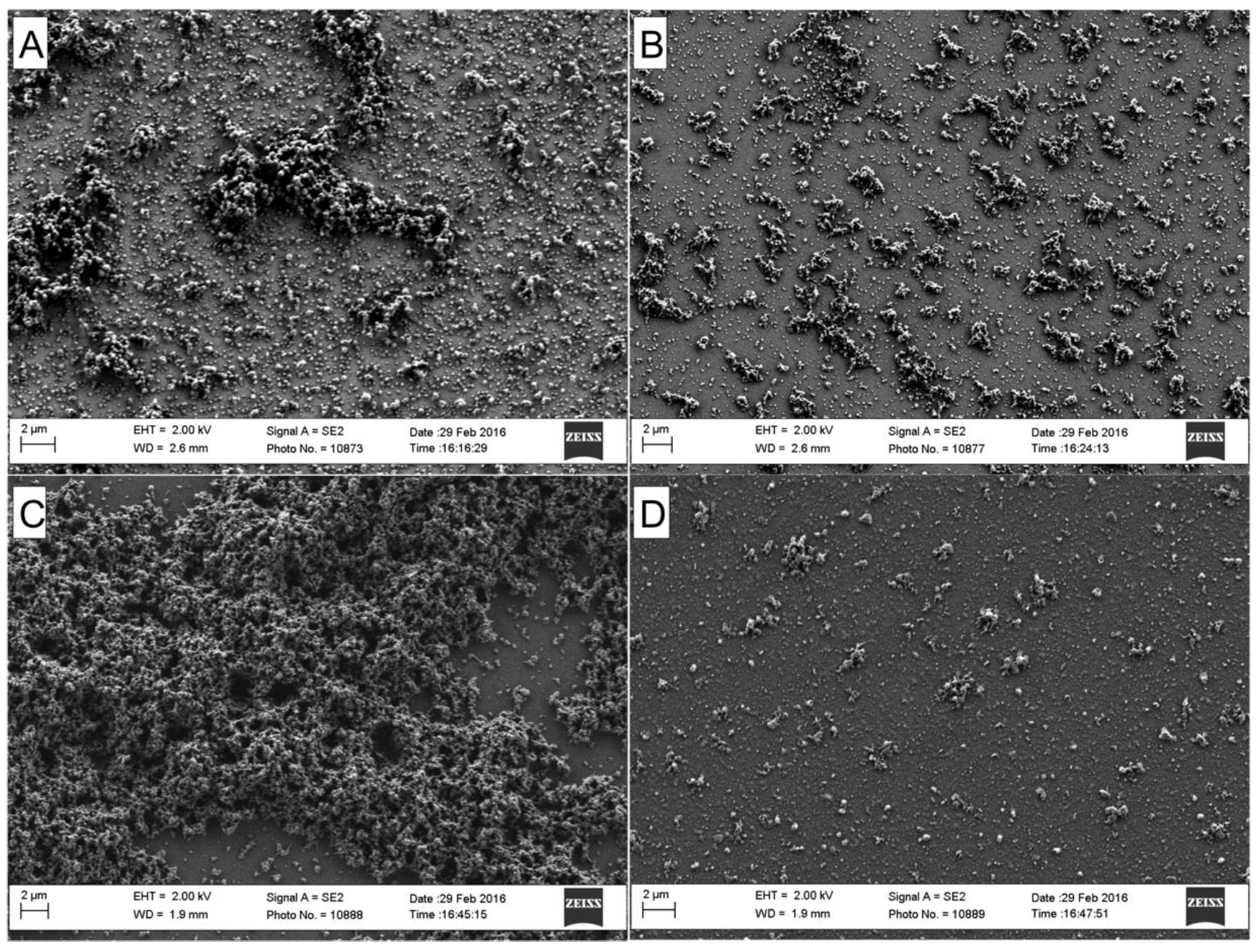
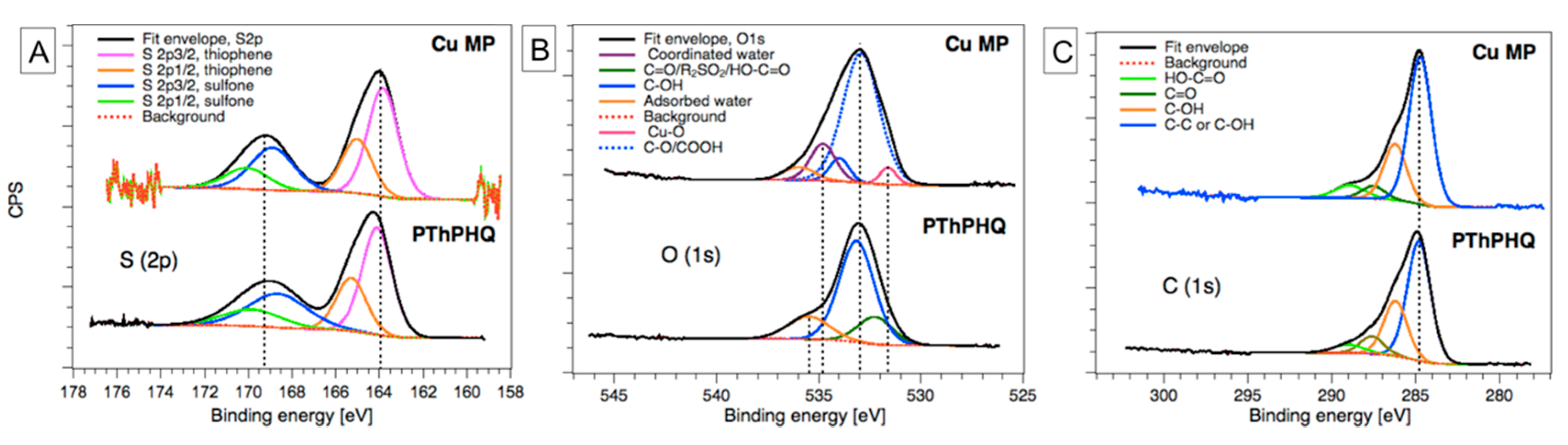
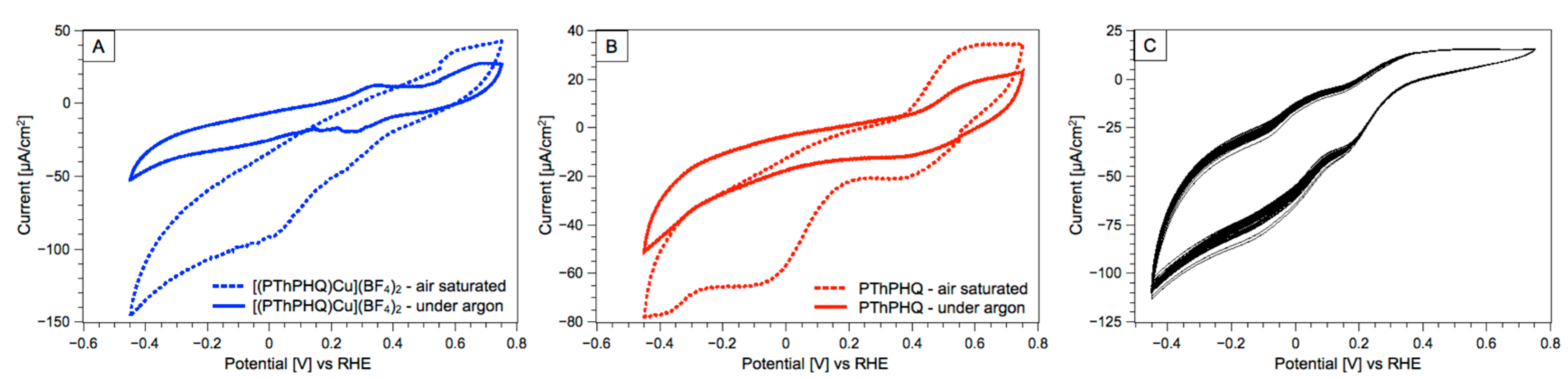
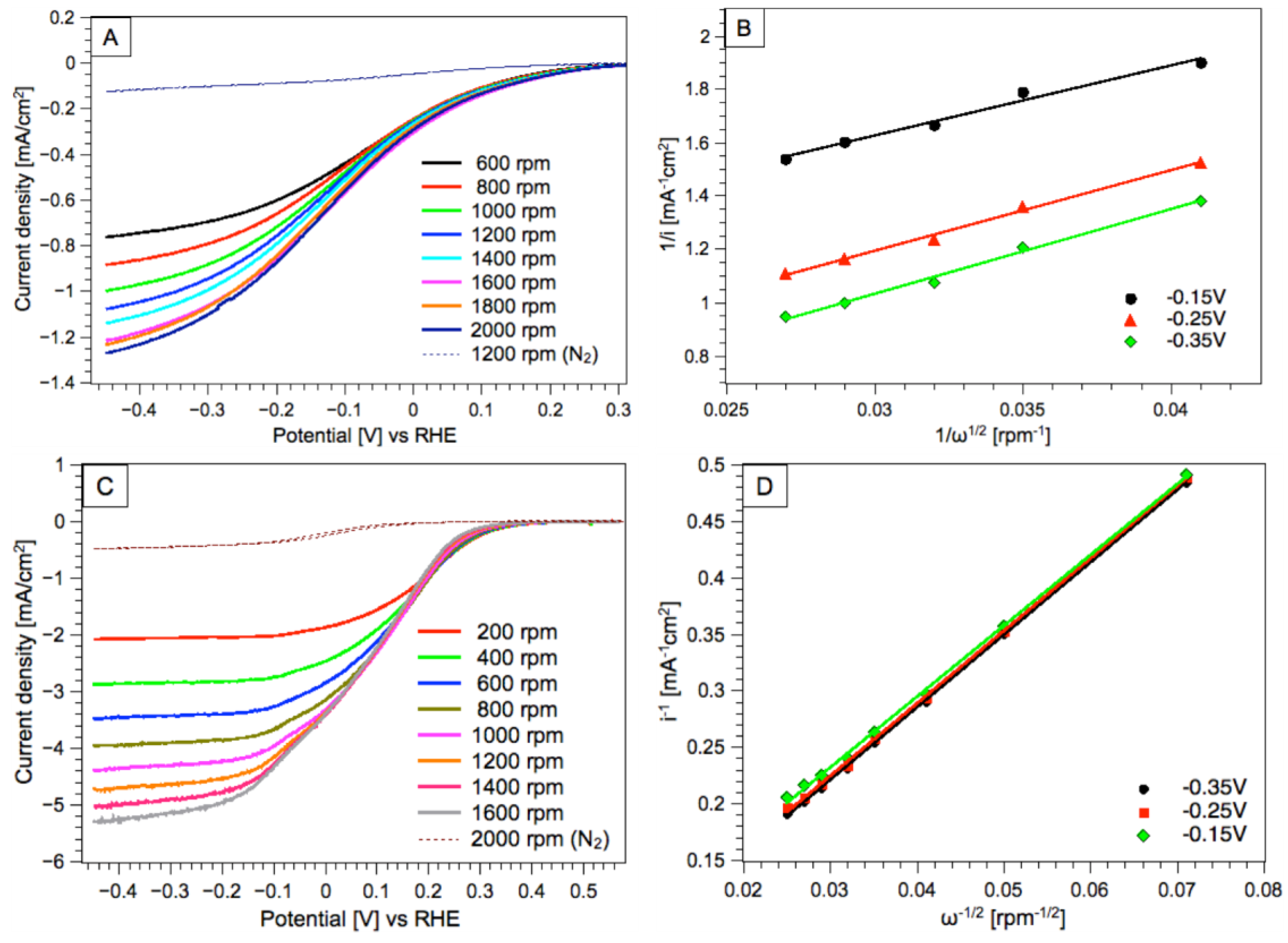
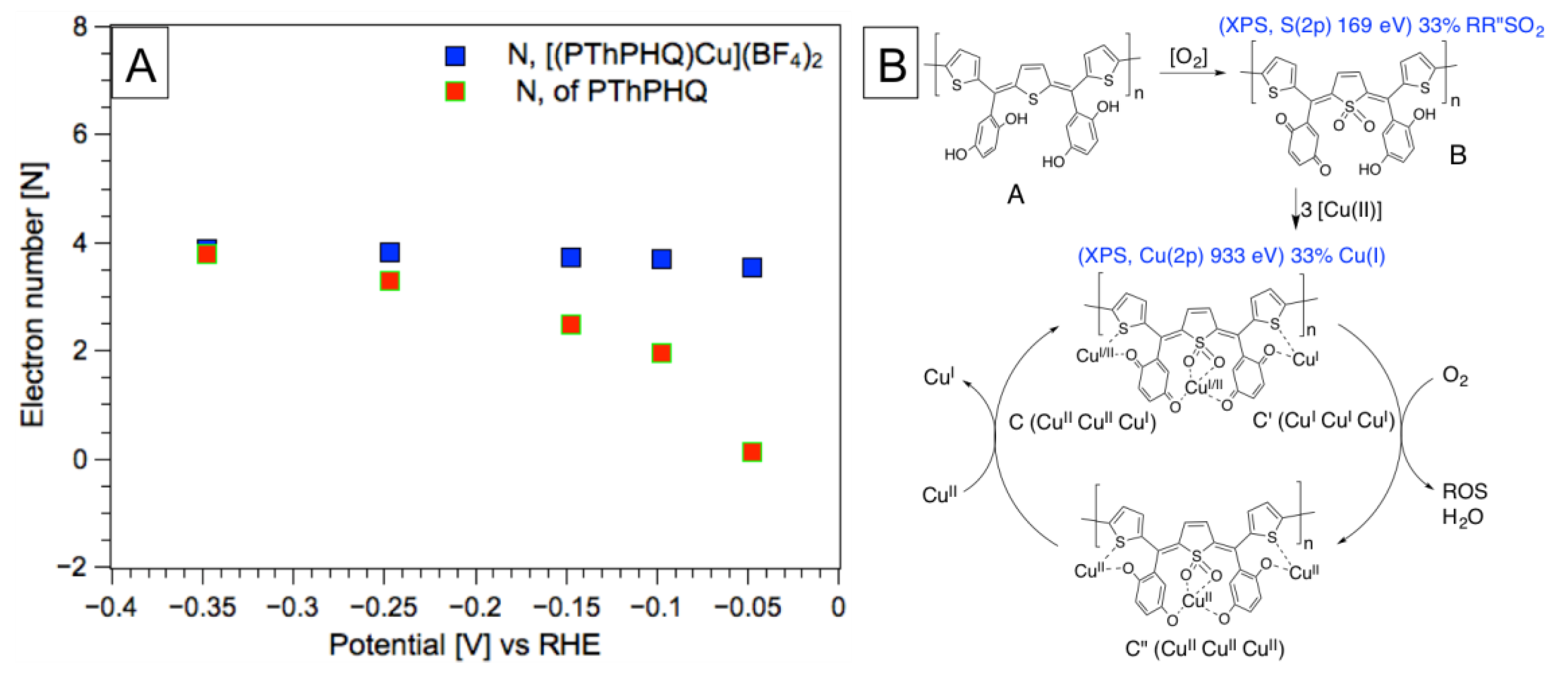
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Chen, C.S.; Zhang, Y. Hexaazatrinaphthylene-Based Porous Organic Polymers as Organic Cathode Materials for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 1772–1779. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.-G.; Hua, W.; Wang, J.; Nan, D.; Wei, C. Scale-up production of high-tap-density carbon/MnOx/carbon nanotube microcomposites for Li-ion batteries with ultrahigh volumetric capacity. Chem. Eng. J. 2018, 354, 220–227. [Google Scholar] [CrossRef]
- Wang, J.-G.; Sun, H.; Liu, H.; Jin, D.; Liu, X.; Li, X.; Kang, F. Triaxial Nanocables of Conducting Polypyrrole@SnS2@Carbon Nanofiber Enabling Significantly Enhanced Li-Ion Storage. ACS Appl. Mater. Interfaces 2018, 10, 13581–13587. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, S.; Li, D.; Sun, S.; Peng, Z.; Komarneni, S.; Yang, D. Direct Interfacial Growth of MnO2 Nanostructure on Hierarchically Porous Carbon for High-Performance Asymmetric Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 633–641. [Google Scholar] [CrossRef]
- Wang, J.-G.; Liu, H.; Liu, H.; Hua, W.; Shao, M. Interfacial Constructing Flexible V2O5 @Polypyrrole Core–Shell Nanowire Membrane with Superior Supercapacitive Performance. ACS Appl. Mater. Interfaces 2018, 10, 18816–18823. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-G.; Liu, H.; Sun, H.; Hua, W.; Wang, H.; Liu, X.; Wei, B. One-pot synthesis of nitrogen-doped ordered mesoporous carbon spheres for high-rate and long-cycle life supercapacitors. Carbon 2018, 127, 85–92. [Google Scholar] [CrossRef]
- Sahoo, S.; Shim, J.-J. Facile Synthesis of Three-Dimensional Ternary ZnCo2O4/Reduced Graphene Oxide/NiO Composite Film on Nickel Foam for Next Generation Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2017, 5, 241–251. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Garg, R.; Elmas, S.; Nann, T.; Andersson, M.R. Deposition Methods of Graphene as Electrode Material for Organic Solar Cells. Adv. Energy Mater. 2017, 7, 1601393. [Google Scholar] [CrossRef]
- Lin, X.-X.; Wang, A.-J.; Fang, K.-M.; Yuan, J.; Feng, J.-J. One-Pot Seedless Aqueous Synthesis of Reduced Graphene Oxide (rGO)-Supported Core–Shell Pt@Pd Nanoflowers as Advanced Catalysts for Oxygen Reduction and Hydrogen Evolution. ACS Sustain. Chem. Eng. 2017, 5, 8675–8683. [Google Scholar] [CrossRef]
- Hsu, P.-Y.; Hu, T.-Y.; Kumar, S.; Chang, C.-H.; Wu, K.; Tung, K.-L.; Lue, S. Highly Zeolite-Loaded Polyvinyl Alcohol Composite Membranes for Alkaline Fuel-Cell Electrolytes. Polymers 2018, 10, 102. [Google Scholar] [CrossRef]
- Brian, C.H. Steele Angelika Heinzel Materials for fuel cell technologies. Nat. Mater. 2001, 414, 345–351. [Google Scholar]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, E. Development of direct methanol alkaline fuel cells using anion exchange membranes. J. Power Sources 2004, 137, 248–256. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific Aspects of Polymer Electrolyte Fuel Cell Durability and Degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.; Barnett, S.; Gorte, R.J.; Irvine, J.T.S.; McEvoy, A.J.; Mogensen, M.; Singhal, S.C.; Vohs, J. Advanced anodes for high-temperature fuel cells. Nat. Mater. 2004, 3, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Sasaki, K.; Sutter, E.; Adzic, R.R. Stabilization of Platinum Oxygen-Reduction Electrocatalysts Using Gold Clusters. Science 2007, 315, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Iijima, Y.; Kondo, T.; Takahashi, Y.; Bando, Y.; Todoroki, N.; Wadayama, T. Oxygen Reduction Reaction Activities for Pt/Au(hkl) Bimetallic Surfaces Prepared by Molecular Beam Epitaxy. J. Electrochem. Soc. 2013, 160, F898–F904. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, Y.; Fu, G.; Duchesne, P.N.; Gu, L.; Zheng, Y.; Weng, X.; Chen, M.; Zhang, P.; Pao, C.-W.; et al. Interfacial Effects in Iron-Nickel Hydroxide-Platinum Nanoparticles Enhance Catalytic Oxidation. Science 2014, 344, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Markovic, N.M.; Stamenkovic, V.R. Advanced Platinum Alloy Electrocatalysts for the Oxygen Reduction Reaction. ACS Catal. 2012, 2, 891–898. [Google Scholar] [CrossRef]
- Gong, K.; Yu, P.; Su, L.; Xiong, S.; Mao, L. Polymer-Assisted Synthesis of Manganese Dioxide/Carbon Nanotube Nanocomposite with Excellent Electrocatalytic Activity toward Reduction of Oxygen. J. Phys. Chem. C 2007, 111, 1882–1887. [Google Scholar] [CrossRef]
- Donne, S.W. Redox Processes at the Manganese Dioxide Electrode. J. Electrochem. Soc. 1997, 144, 2954. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Kongkanand, A.; Kuwabata, S.; Girishkumar, G.; Kamat, P. Single-Wall Carbon Nanotubes Supported Platinum Nanoparticles with Improved Electrocatalytic Activity for Oxygen Reduction Reaction. Langmuir 2006, 22, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Guangli, C.; Brinda, B.L.; Ellen, R.F.; Charles, L. Martin Carbon nanotubule membranes for electrochemical energy storage and production. Nature 1998, 393, 346–349. [Google Scholar] [CrossRef]
- Coleman, E.J.; Chowdhury, M.H.; Co, A.C. Insights into the Oxygen Reduction Reaction Activity of Pt/C and PtCu/C Catalysts. ACS Catal. 2015, 5, 1245–1253. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.-L.; Boero, M.; Hou, Z.; Terakura, K.; Cheng, W. Indirect Four-Electron Oxygen Reduction Reaction on Carbon Materials Catalysts in Acidic Solutions. ACS Catal. 2017, 7, 7908–7916. [Google Scholar] [CrossRef]
- Gong, X.; Liu, S.; Ouyang, C.; Strasser, P.; Yang, R. Nitrogen- and Phosphorus-Doped Biocarbon with Enhanced Electrocatalytic Activity for Oxygen Reduction. ACS Catal. 2015, 5, 920–927. [Google Scholar] [CrossRef]
- Halime, Z.; Kotani, H.; Li, Y.; Fukuzumi, S.; Karlin, K.D. Homogeneous catalytic O2 reduction to water by a cytochrome c oxidase model with trapping of intermediates and mechanistic insights. Proc. Natl. Acad. Sci. USA 2011, 108, 13990–13994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collman, J.P.; Devaraj, N.K.; Decreau, R.A.; Yang, Y.; Yan, Y.-L.; Ebina, W.; Eberspacher, T.A.; Chidsey, C.E.D. A Cytochrome c Oxidase Model Catalyzes Oxygen to Water Reduction Under Rate-Limiting Electron Flux. Science 2007, 315, 1565–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parimi, N.S.; Umasankar, Y.; Atanassov, P.; Ramasamy, R.P. Kinetic and Mechanistic Parameters of Laccase Catalyzed Direct Electrochemical Oxygen Reduction Reaction. ACS Catal. 2012, 2, 38–44. [Google Scholar] [CrossRef]
- Winther-Jensen, B.; Winther-Jensen, O.; Forsyth, M.; MacFarlane, D.R. High Rates of Oxygen Reduction over a Vapor Phase-Polymerized PEDOT Electrode. Science 2008, 321, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Barsukov, V.; Chivikov, S. The “capacitor” concept of the current-producing process mechanism in polyaniline-type conducting polymers. Electrochim. Acta 1996, 41, 1773–1779. [Google Scholar] [CrossRef]
- Khomenko, V.G.; Barsukov, V.Z.; Katashinskii, A.S. The catalytic activity of conducting polymers toward oxygen reduction. Electrochim. Acta 2005, 50, 1675–1683. [Google Scholar] [CrossRef]
- Tylus, U.; Jia, Q.; Strickland, K.; Ramaswamy, N.; Serov, A.; Atanassov, P.; Mukerjee, S. Elucidating Oxygen Reduction Active Sites in Pyrolyzed Metal–Nitrogen Coordinated Non-Precious-Metal Electrocatalyst Systems. J. Phys. Chem. C 2014, 118, 8999–9008. [Google Scholar] [CrossRef] [PubMed]
- Bouwkamp-Wijnoltz, A.L.; Visscher, W.; van Veen, J.A.R.; Boellaard, E.; van der Kraan, A.M.; Tang, S.C. On Active-Site Heterogeneity in Pyrolyzed Carbon-Supported Iron Porphyrin Catalysts for the Electrochemical Reduction of Oxygen: An In Situ Mössbauer Study. J. Phys. Chem. B 2002, 106, 12993–13001. [Google Scholar] [CrossRef]
- Koslowski, U.I.; Abs-Wurmbach, I.; Fiechter, S.; Bogdanoff, P. Nature of the Catalytic Centers of Porphyrin-Based Electrocatalysts for the ORR: A Correlation of Kinetic Current Density with the Site Density of Fe−N 4 Centers. J. Phys. Chem. C 2008, 112, 15356–15366. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Wang, H.; Xie, L.; Liang, Y.; Wei, F.; Idrobo, J.-C.; Pennycook, S.J.; Dai, H. An oxygen reduction electrocatalyst based on carbon nanotube–graphene complexes. Nat. Nanotechnol. 2012, 7, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Genorio, B.; Strmcnik, D.; Subbaraman, R.; Tripkovic, D.; Karapetrov, G.; Stamenkovic, V.R.; Pejovnik, S.; Marković, N.M. Selective catalysts for the hydrogen oxidation and oxygen reduction reactions by patterning of platinum with calix[4]arene molecules. Nat. Mater. 2010, 9, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cheng, X.; Jao, T.-C.; Weng, F.-B.; Su, A.; Chiang, Y.-C. Degradation analyses of Ru85Se15 catalyst layer in proton exchange membrane fuel cells. J. Power Sources 2012, 218, 79–87. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Wu, S.; Merida, W. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 2008, 184, 104–119. [Google Scholar] [CrossRef]
- Kingsborough, R.P.; Swager, T.M. Electrocatalytic Conducting Polymers: Oxygen Reduction by a Polythiophene−Cobalt Salen Hybrid. Chem. Mater. 2000, 12, 872–874. [Google Scholar] [CrossRef]
- Elmas, S.; Beelders, W.; Bradley, S.J.; Kroon, R.; Laufersky, G.; Andersson, M.; Nann, T. Platinum Terpyridine Metallopolymer Electrode as Cost-Effective Replacement for Bulk Platinum Catalysts in Oxygen Reduction Reaction and Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2017, 5, 10206–10214. [Google Scholar] [CrossRef]
- Elmas, S.; Beelders, W.; Nash, J.; Macdonald, T.J.; Jasieniak, M.; Griesser, H.J.; Nann, T. Photo-doping of plasma-deposited polyaniline (PAni). RSC Adv. 2016, 6, 70691–70699. [Google Scholar] [CrossRef] [Green Version]
- Heeger, A.J. Semiconducting and Metallic Polymers: The Fourth Generation of Polymeric Materials (Nobel Lecture). Angew. Chem. Int. Ed. 2001, 40, 2591–2611. [Google Scholar] [CrossRef]
- Kingsborough, R.P.; Swager, T.M. Electroactivity Enhancement by Redox Matching in Cobalt Salen-Based Conducting Polymers. Adv. Mater. 1998, 10, 1100–1104. [Google Scholar] [CrossRef]
- Chen, W.-C.; Jenekhe, S.A. Small-Bandgap Conducting Polymers Based on Conjugated Poly(heteroarylene methines). 2. Synthesis, Structure, and Properties. Macromolecules 1995, 28, 465–480. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, J.; Liu, K.; Zhu, D.; Yang, M.; Ye, H.; Liu, X. Synthesis and characterization of novel low band gap polymers: Poly(heteroarylene methines). Synth. Met. 2006, 156, 804–808. [Google Scholar] [CrossRef]
- Yi, W.; Feng, W.; Cao, M.; Wu, H. Synthesis of third-order non-linear optical polymers based on conjugated poly(heteroarylene methines). Polym. Adv. Technol. 2004, 15, 431–438. [Google Scholar] [CrossRef]
- de Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.; Yousefi, A.; Elmas, S.; Lindén, J.B.; Nann, T.; Nydén, M. Electroactive Polyhydroquinone Coatings for Marine Fouling Prevention—A Rejected Dynamic pH Hypothesis and a Deceiving Artifact in Electrochemical Antifouling Testing. ACS Omega 2017, 2, 4751–4759. [Google Scholar] [CrossRef]
- Vonlanthen, D.; Lazarev, P.; See, K.A.; Wudl, F.; Heeger, A.J. A Stable Polyaniline-Benzoquinone-Hydroquinone Supercapacitor. Adv. Mater. 2014, 26, 5095–5100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, A.; Elmas, S.; Butsch, K. Oxido Pincer Ligands–Exploring the Coordination Chemistry of Bis(hydroxymethyl)pyridine Ligands for the Late Transition Metals. Eur. J. Inorg. Chem. 2009, 2009, 2271–2281. [Google Scholar] [CrossRef]
- Klein, A.; Butsch, K.; Elmas, S.; Biewer, C.; Heift, D.; Nitsche, S.; Schlipf, I.; Bertagnolli, H. Oxido-pincer complexes of copper(II)–An EXAFS and EPR study of mono- and binuclear [(pydotH2)CuCl2]n (n = 1 or 2). Polyhedron 2012, 31, 649–656. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, S.; Li, B.; Fan, D. A series of [Cu(N–N)(P–P)]BF4 complexes: Luminescence quenching caused by electron-configuration transformation in excited state. Inorg. Chim. Acta 2012, 384, 225–232. [Google Scholar] [CrossRef]
- Megiatto, J.D.; Schuster, D.I. Alternative Demetalation Method for Cu(I)-Phenanthroline-Based Catenanes and Rotaxanes. Org. Lett. 2011, 13, 1808–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, A.; Butsch, K.; Neudörfl, J. Electron transfer studies on Cu(II) complexes bearing phenoxy-pincer ligands. Inorg. Chim. Acta 2010, 363, 3282–3290. [Google Scholar] [CrossRef]
- Wavhal, D.S.; Fisher, E.R. Hydrophilic modification of polyethersulfone membranes by low temperature plasma-induced graft polymerization. J. Membr. Sci. 2002, 209, 255–269. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, F.; Li, M.; Peng, W.; Qu, J. Reactivity and Kinetics of Vinyl Sulfone-Functionalized Self-Assembled Monolayers for Bioactive Ligand Immobilization. Langmuir 2015, 31, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Tzorbatzoglou, F.; Brouzgou, A.; Tsiakaras, P. Electrocatalytic activity of Vulcan-XC-72 supported Pd, Rh and PdxRhy toward HOR and ORR. Appl. Catal. B Environ. 2015, 174–175, 203–211. [Google Scholar] [CrossRef]
- Jiang, L.; Hsu, A.; Chu, D.; Chen, R. Oxygen reduction on carbon supported Pt and PtRu catalysts in alkaline solutions. J. Electroanal. Chem. 2009, 629, 87–93. [Google Scholar] [CrossRef]
- Ikeuchi, H.; Hayafuji, M.; Aketagawa, Y.; Taki, J.; Sato, G.P. Diffusion coefficients of dioxygen in aqueous electrolyte solutions. J. Electroanal. Chem. 1995, 396, 553–556. [Google Scholar] [CrossRef]
- Tammeveski, K.; Kontturi, K.; Nichols, R.J.; Potter, R.J.; Schiffrin, D.J. Surface redox catalysis for O2 reduction on quinone-modified glassy carbon electrodes. J. Electroanal. Chem. 2001, 515, 101–112. [Google Scholar] [CrossRef]
- Yuan, X.; Pham, A.N.; Miller, C.J.; Waite, T.D. Copper-Catalyzed Hydroquinone Oxidation and Associated Redox Cycling of Copper under Conditions Typical of Natural Saline Waters. Environ. Sci. Technol. 2013, 47, 8355–8364. [Google Scholar] [CrossRef] [PubMed]
- Gubler, L.; Dockheer, S.M.; Koppenol, W.H. Radical (HO●, H● and HOO●) Formation and Ionomer Degradation in Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2011, 158, B755. [Google Scholar] [CrossRef]
- Chen, C.; Fuller, T.F. XPS Analysis of Polymer Membrane Degradation in PEMFCs. J. Electrochem. Soc. 2009, 156, B1218. [Google Scholar] [CrossRef]
- Shah, A.A.; Ralph, T.R.; Walsh, F.C. Modeling and Simulation of the Degradation of Perfluorinated Ion-Exchange Membranes in PEM Fuel Cells. J. Electrochem. Soc. 2009, 156, B465. [Google Scholar] [CrossRef]
- Song, A.; Cao, L.; Yang, W.; Li, Y.; Qin, X.; Shao, G. Uniform Multilayer Graphene-Coated Iron and Iron-Carbide as Oxygen Reduction Catalyst. ACS Sustain. Chem. Eng. 2018, 6, 4890–4898. [Google Scholar] [CrossRef]
- Pan, F.; Cao, Z.; Zhao, Q.; Liang, H.; Zhang, J. Nitrogen-doped porous carbon nanosheets made from biomass as highly active electrocatalyst for oxygen reduction reaction. J. Power Sources 2014, 272, 8–15. [Google Scholar] [CrossRef]
- Chen, X.; He, F.; Shen, Y.; Yang, Y.; Mei, H.; Liu, S.; Mori, T.; Zhang, Y. Effect of Carbon Supports on Enhancing Mass Kinetic Current Density of Fe-N/C Electrocatalysts. Chem. Eur. J. 2017, 23, 14597–14603. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Feng, X.; Wang, X.; Müllen, K. Graphene-Based Carbon Nitride Nanosheets as Efficient Metal-Free Electrocatalysts for Oxygen Reduction Reactions. Angew. Chem. Int. Ed. 2011, 50, 5339–5343. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, Y.; Chen, J.; Liu, J.; Liang, J.; Du, A.; Zhang, W.; Zhu, Z.; Smith, S.C.; Jaroniec, M.; et al. Nanoporous Graphitic-C 3 N 4 @Carbon Metal-Free Electrocatalysts for Highly Efficient Oxygen Reduction. J. Am. Chem. Soc. 2011, 133, 20116–20119. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, J.; Zhou, W.; Lin, J.; Shen, Z. Nitrogen-doped Graphene-Supported Transition-metals Carbide Electrocatalysts for Oxygen Reduction Reaction. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]

| Catalyst | Electrolyte | Ered,ons (V vs. RHE) (a) | Ired,ons (mA/cm2) | N | Lit. |
|---|---|---|---|---|---|
| Fe3C/Fe@G-800 | 0.1M KOH | 0.91 | n.r. (b) | 3.71 | [71] |
| NPCN | 0.1M KOH | 0.72 | 13.57 | 3.70 | [72] |
| Pt/C | 0.1M KOH | 0.72–0.8 | 9.28–14.00 | 4.00 | [64,72,73] |
| GC-N800 | 0.1M KOH | 0.58 | 7.30 | 4.00 | [74] |
| g-CN3@C | 0.1M KOH | 0.37 | 11.30 | 3.90 | [75] |
| FeMo-NG | 0.1M KOH | 0.17 | 3.50 | 3.90 | [76] |
| PdRh/C, Rh/C | 0.5M H2SO4 | 0.70 (c) | 0.89–5.20 | 4.00 | [63] |
| Cu MP | 0.1M KCl | −0.15 | 22.62–30.78 | 3.75–3.90 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmas, S.; Beelders, W.; Pan, X.; Nann, T. Conducting Copper(I/II)-Metallopolymer for the Electrocatalytic Oxygen Reduction Reaction (ORR) with High Kinetic Current Density. Polymers 2018, 10, 1002. https://doi.org/10.3390/polym10091002
Elmas S, Beelders W, Pan X, Nann T. Conducting Copper(I/II)-Metallopolymer for the Electrocatalytic Oxygen Reduction Reaction (ORR) with High Kinetic Current Density. Polymers. 2018; 10(9):1002. https://doi.org/10.3390/polym10091002
Chicago/Turabian StyleElmas, Sait, Wesley Beelders, Xun Pan, and Thomas Nann. 2018. "Conducting Copper(I/II)-Metallopolymer for the Electrocatalytic Oxygen Reduction Reaction (ORR) with High Kinetic Current Density" Polymers 10, no. 9: 1002. https://doi.org/10.3390/polym10091002