Synthesis and Characterization of the Conducting Polymer Micro-Helix Based on the Spirulina Template
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cultivation and Preparations of Spirulina platensis
2.2.2. Fabrication of the Spirulina-Templated PPy Helical Microstructure
2.2.3. Fabrication of the Spirulina-Templated PANI Helical Microstructure
2.2.4. Fabrication of the Spirulina-Templated PEDOT Helical Microstructure
2.2.5. Preparations of PPy, PANI, and PEDOT Helix Modified Electrodes
2.3. Characterizations
2.3.1. Morphology Analysis
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.3. Raman Spectroscopy
2.3.4. X-ray Diffraction (XRD)
2.3.5. Electrochemical Characterization
3. Results and Discussion
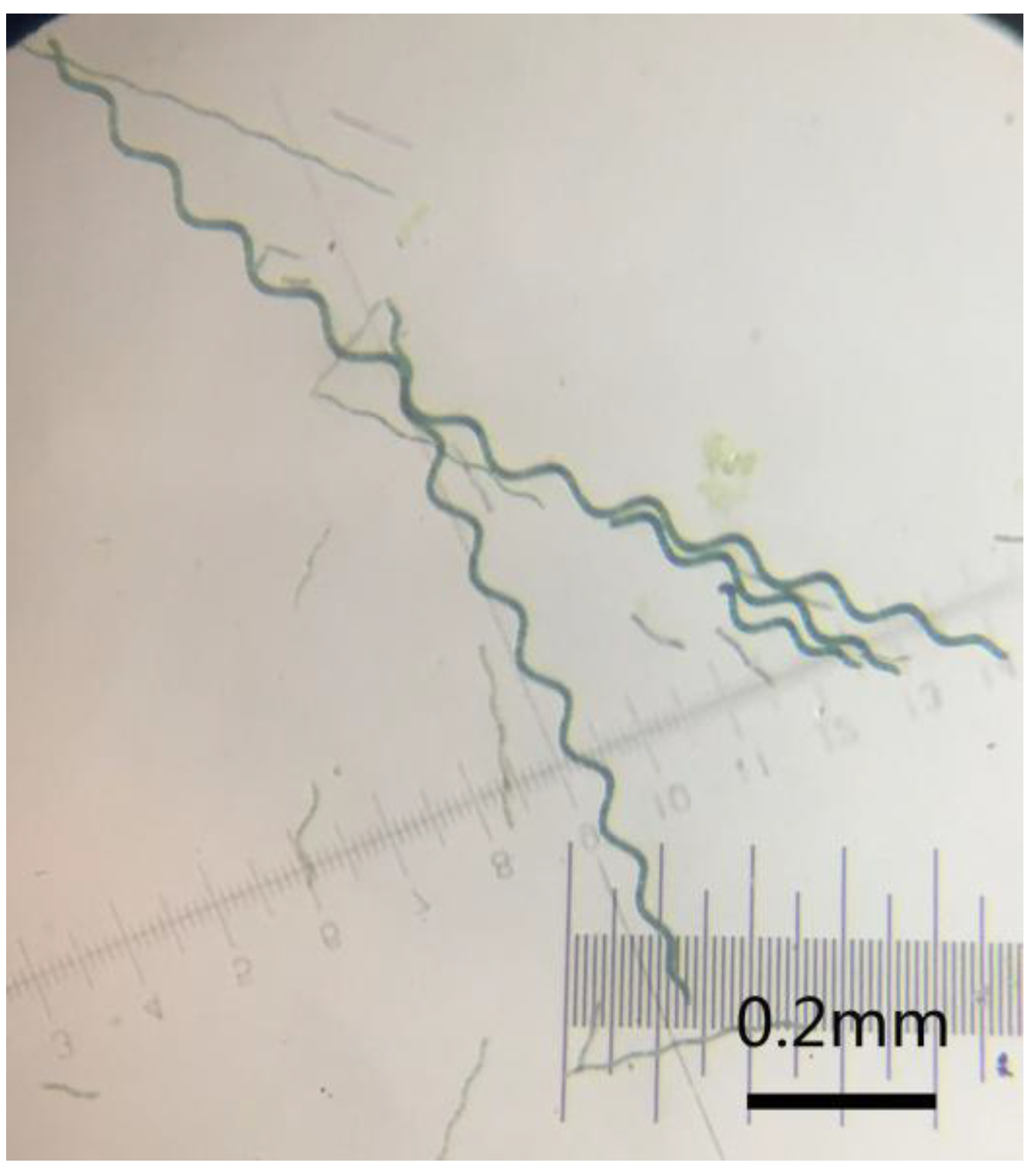
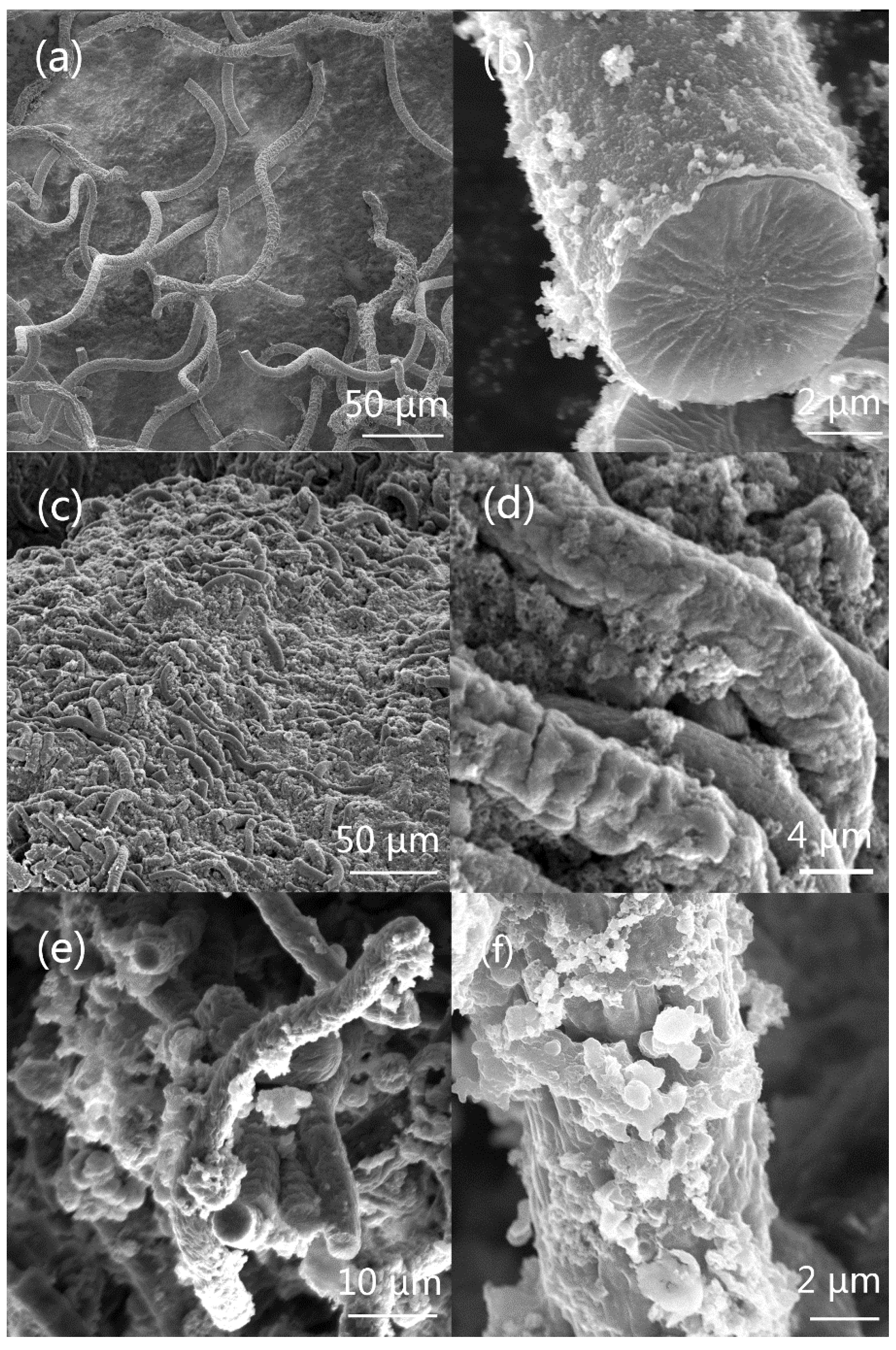
3.1. Morphologies of PPy, PANI, and PEDOT Micro-Helices
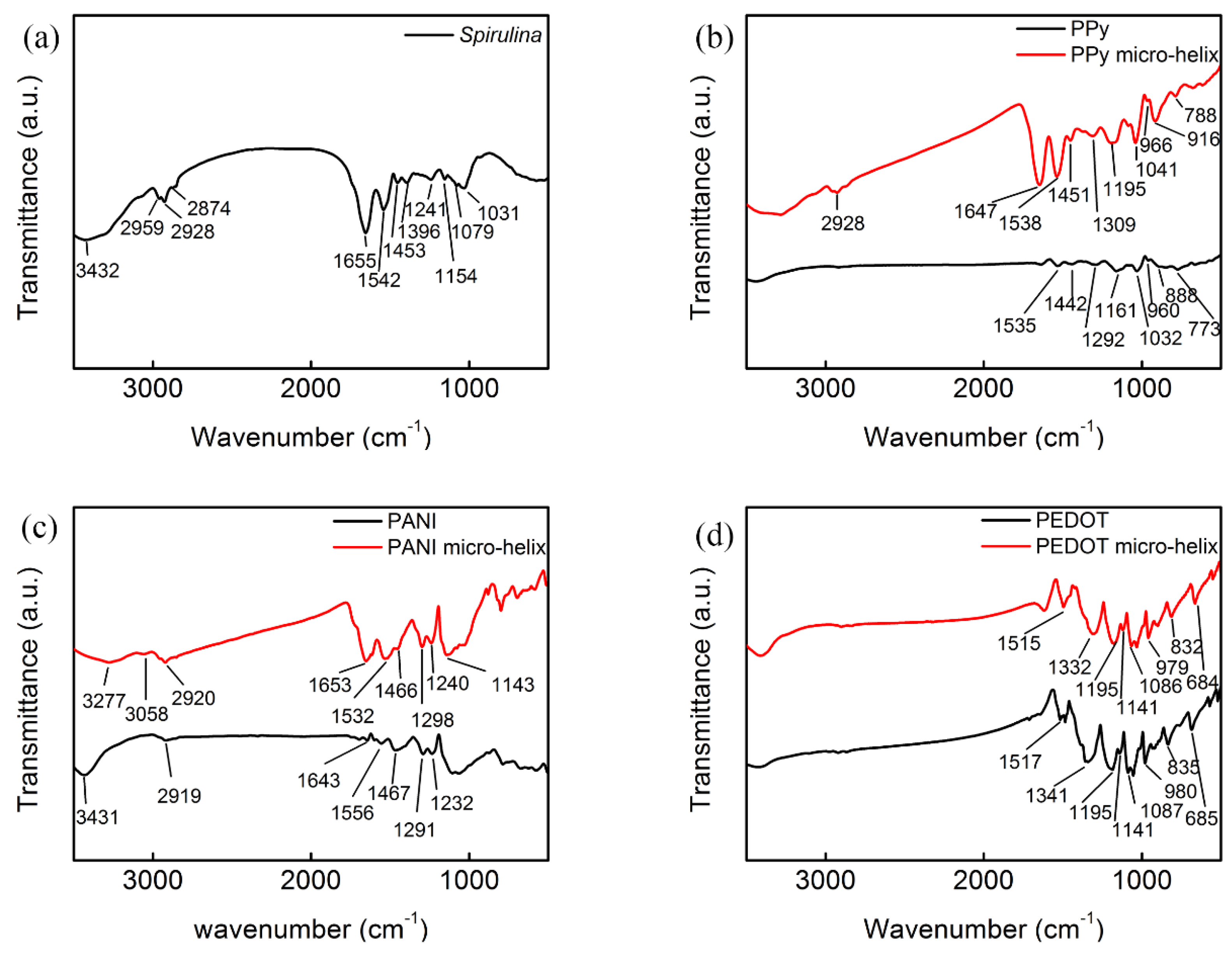
3.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.3. Raman Spectroscopy Analysis
3.4. X-ray Diffraction Analysis
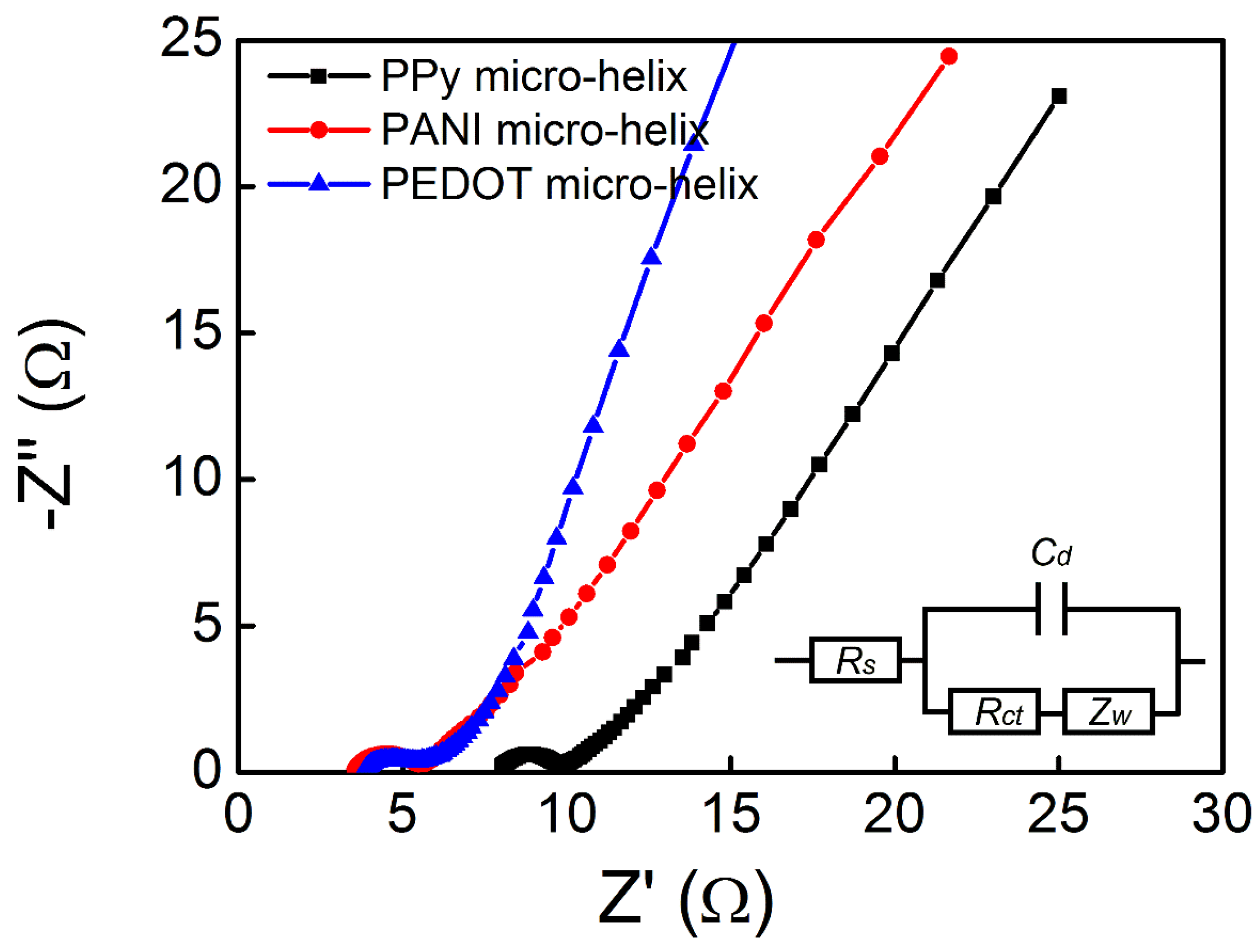
3.5. Electrical and Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, P.; Xie, R.; Liu, Y.; Luo, G.; Ding, M.; Liang, Q. Bioinspired microfibers with embedded perfusable helical channels. Adv. Mater. 2017, 29, 1701664. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-W.; Sakar, M.S.; Petruska, A.J.; Pane, S.; Nelson, B.J. Soft micromachines with programmable motility and morphology. Nat. Commun. 2016, 7, 12263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tottori, S.; Zhang, L.; Qiu, F.; Krawczyk, K.K.; Franco-Obregon, A.; Nelson, B.J. Magnetic helical micromachines: Fabrication, controlled swimming, and cargo transport. Adv. Mater. 2012, 24, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhou, Q.; Yu, J.; Xu, T.; Deng, Y.; Tang, T.; Feng, Q.; Bian, L.; Zhang, Y.; Ferreira, A.; et al. Magnetite nanostructured porous hollow helical microswimmers for targeted delivery. Adv. Funct. Mater. 2015, 25, 5333–5342. [Google Scholar] [CrossRef]
- Sengupta, S.; Ibele, M.E.; Sen, A. Fantastic voyage: Designing self-powered nanorobots. Angew. Chem. Int. Ed. 2012, 51, 8434–8445. [Google Scholar] [CrossRef] [PubMed]
- Peyer, K.E.; Zhang, L.; Nelson, B.J. Bio-inspired magnetic swimming microrobots for biomedical applications. Nanoscale 2013, 5, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sattayasamitsathit, S.; Dong, R.; Gao, W.; Tam, R.; Feng, X.; Ai, S.; Wang, J. Template electrosynthesis of tailored-made helical nanoswimmers. Nanoscale 2014, 6, 9415–9420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.; Zhou, Q.; Vincent, M.; Deng, Y.; Yu, J.; Xu, J.; Xu, T.; Tang, T.; Bian, L.; Wang, Y.X.J. Multifunctional biohybrid magnetite microrobots for imaging-guided therapy. Sci. Robot. 2017, 2, 1155. [Google Scholar] [CrossRef]
- Gansel, J.K.; Thiel, M.; Rill, M.S.; Decker, M.; Bade, K.; Saile, V.; von Freymann, G.; Linden, S.; Wegener, M. Gold helix photonic metamaterial as broadband circular polarizer. Science 2009, 325, 1513–1515. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, G.; Wang, J.; Yu, Y.; Wu, X.; Cui, X.; Mei, Y. Superelastic metal microsprings as fluidic sensors and actuators. Lab Chip 2012, 12, 2322–2328. [Google Scholar] [CrossRef] [PubMed]
- Kesapragada, S.V.; Victor, P.; Nalamasu, O.; Gall, D. Nanospring pressure sensors grown by glancing angle doposition. Nano Lett. 2006, 6, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Fonseca, L.F. Wet-chemical approaches to porous nanowires with linear, spiral, and meshy topologies. Nano Lett. 2013, 13, 5642–5646. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Liu, D.L.; Ye, D.X.; Picu, R.C.; Lu, T.M.; Wang, G.C. Metal-coated si springs: Nanoelectromechanical actuators. Appl. Phys. Lett. 2004, 84, 3657–3659. [Google Scholar] [CrossRef]
- Gong, J.; Lin, H.; Dunlop, J.W.C.; Yuan, J. Hierarchically arranged helical fiber actuators derived from commercial cloth. Adv. Mater. 2017, 29, 1605103. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Fan, Q.; Yang, F.; Cai, L.; Zhang, N.; Zhou, W.; Zhou, W.; Xie, S. Hydro-actuation of hybrid carbon nanotube yarn muscles. Nanoscale 2016, 8, 17881–17886. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kwon, C.H.; Park, K.; Mun, T.J.; Lepro, X.; Baughman, R.H.; Spinks, G.M.; Kim, S.J. Bio-inspired, moisture-powered hybrid carbon nanotube yarn muscles. Sci. Rep. 2016, 6, 23016. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, B.; Zhang, Q.; Yim, W.-l.; Chen, L. Catalytic oxygen activation on helical gold nanowires. J. Phys. Chem. C 2012, 116, 11189–11194. [Google Scholar] [CrossRef]
- Fan, Z.; Govorov, A.O. Chiral nanocrystals: Plasmonic spectra and circular dichroism. Nano Lett. 2012, 12, 3283–3289. [Google Scholar] [CrossRef] [PubMed]
- Droulias, S.; Yannopapas, V. Broad-band giant circular dichroism in metamaterials of twisted chains of metallic nanoparticles. J. Phys. Chem. C 2013, 117, 1130–1135. [Google Scholar] [CrossRef]
- Lu, Z.; Zhao, M.; Xie, P.; Wu, L.; Yu, Y.; Zhang, P.; Yang, Z. Reflection properties of metallic helical metamaterials. J. Lightwave Technol. 2012, 30, 3050–3054. [Google Scholar] [CrossRef]
- Kessick, R.; Tepper, G. Microscale polymeric helical structures produced by electrospinning. Appl. Phys. Lett. 2004, 84, 4807–4809. [Google Scholar] [CrossRef]
- Kaschke, J.; Wegener, M. Gold triple-helix mid-infrared metamaterial by sted-inspired laser lithography. Opt. Lett. 2015, 40, 3986–3989. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Wang, C.; He, X.; Li, J.; Peng, Q.; Shi, E.; Wang, R.; Du, S.; Cao, A.; Li, Y. Self-stretchable, helical carbon nanotube yarn supercapacitors with stable performance under extreme deformation conditions. Nano Energy 2015, 12, 401–409. [Google Scholar] [CrossRef]
- Yu, Y.; Fu, F.; Shang, L.; Cheng, Y.; Gu, Z.; Zhao, Y. Bioinspired helical microfibers from microfluidics. Adv. Mater. 2017, 29, 1605765. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.G.; Gibbs, J.G.; Lee, T.-C.; Fischer, P. Hybrid nanocolloids with programmed three-dimensional shape and material composition. Nat. Mater. 2013, 12, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Peng, X.; Pei, A.; Kane, C.R.; Tam, R.; Hennessy, C.; Wang, J. Bioinspired helical microswimmers based on vascular plants. Nano Lett. 2014, 14, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fan, T.; Zhang, D. Biotemplated materials for sustainable energy and environment: Current status and challenges. ChemSusChem 2011, 4, 1344–1387. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Suzuki, S.; Ohtsuka, M.; Nakagawa, M.; Iyoda, T.; Yamada, A. Fabrication of left-handed metal microcoil from spiral vessel of vascular plant. Adv. Mater. 2011, 23, 5509–5513. [Google Scholar] [CrossRef] [PubMed]
- Zafaralla, M.T.; Barril, C.R.; Vidal, L.R. Protein from spirulina. NSTA Technol. J. 1985, 10, 18–26. [Google Scholar]
- Zhang, D.; Zhang, W.; Cai, J. Magnetization of microorganism cells by thermal decomposition method. Sci. China-Technol. Sci. 2011, 54, 1275–1280. [Google Scholar] [CrossRef]
- Courchesne, N.-M.D.; Steiner, S.A., III; Cantu, V.J.; Hammond, P.T.; Belcher, A.M. Biotemplated silica and silicon materials as building blocks for micro- to nanostructures. Chem. Mater. 2015, 27, 5361–5370. [Google Scholar] [CrossRef]
- Kamata, K.; Piao, Z.; Suzuki, S.; Fujimori, T.; Tajiri, W.; Nagai, K.; Iyoda, T.; Yamada, A.; Hayakawa, T.; Ishiwara, M.; et al. Spirulina-templated metal microcoils with controlled helical structures for thz electromagnetic responses. Sci. Rep. 2014, 4, 4919. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Li, S.; Yu, M. Preparation and characterization of metallic Cu spiral based on spirulina bioscaffold. Mater. Lett. 2012, 77, 51–53. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, M.; Liu, J.; Li, S. Bioinspired synthesis of a hollow metallic microspiral based on a spirulina bioscaffold. Langmuir 2012, 28, 3690–3694. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Cai, J.; Yuan, L.; Xu, Y.; Zhang, D. Fabrication and electromagnetic properties of soft-core functional particles by way of electroless Ni-Fe-P alloy plating on helical microorganism cells. Surf. Coat. Tech. 2013, 216, 152–157. [Google Scholar] [CrossRef]
- Cai, J.; Lan, M.; Zhang, D.; Zhang, W. Electrical resistivity and dielectric properties of helical microorganism cells coated with silver by electroless plating. Appl. Surf. Sci. 2012, 258, 8769–8774. [Google Scholar] [CrossRef]
- Olsson, H.; Nystrom, G.; Stromme, M.; Sjodin, M.; Nyholm, L. Cycling stability and self-protective properties of a paper-based polypyrrole energy storage device. Electrochem. Commun. 2011, 13, 869–871. [Google Scholar] [CrossRef]
- Das, C.; Krishnamoorthy, K. Flexible microsupercapacitors using silk and cotton substrates. ACS Appl. Mater. Interfaces 2016, 8, 29504–29510. [Google Scholar] [CrossRef] [PubMed]
- Kizling, M.; Stolarczyk, K.; Kiat, J.S.S.; Tammela, P.; Wang, Z.; Nyholm, L.; Bilewicz, R. Pseudocapacitive polypyrrole-nanocellulose composite for sugar-air enzymatic fuel cells. Electrochem. Commun. 2015, 50, 55–59. [Google Scholar] [CrossRef]
- Spinks, G.M.; Mottaghitalab, V.; Bahrami-Saniani, M.; Whitten, P.G.; Wallace, G.G. Carbon-nanotube-reinforced polyaniline fibers for high-strength artificial muscles. Adv. Mater. 2006, 18, 637–640. [Google Scholar] [CrossRef]
- Hardy, J.G.; Lee, J.Y.; Schmidt, C.E. Biomimetic conducting polymer-based tissue scaffolds. Curr. Opin. Biotechnol. 2013, 24, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.T.; Kurup, S.; Larsson, K.C.; Hori, R.; Tybrandt, K.; Goiny, M.; Jager, E.H.; Berggren, M.; Canlon, B.; Richter-Dahlfors, A. Organic electronics for precise delivery of neurotransmitters to modulate mammalian sensory function. Nat. Mater. 2009, 8, 742–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Gao, C. Graphene in macroscopic order: Liquid crystals and wet-spun fibers. Accounts Chem. Res. 2014, 47, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, Q.; Chen, X.; Yang, W.; Wu, Z.; Xu, X.; Jiang, M.; Zhou, Z. Enhanced performance of core-shell structured polyaniline at helical carbon nanotube hybrids for ammonia gas sensor. Appl. Phys. Lett. 2014, 105, 203109. [Google Scholar] [CrossRef]
- Backlund, F.G.; Elfwing, A.; Musumeci, C.; Ajjan, F.; Babenko, V.; Dzwolak, W.; Solin, N.; Inganas, O. Conducting microhelices from self-assembly of protein fibrils. Soft Matter 2017, 13, 4412–4417. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, J.C.; Mascaro, S.A. Sequential growth and monitoring of a polypyrrole actuator system. In Proceedings of the Electroactive Polymer Actuators and Devices, San Diego, CA, USA, 9–13 March 2014; Volume 9056, p. 90563. [Google Scholar]
- Tadesse, Y.; Grange, R.W.; Priya, S. Synthesis and cyclic force characterization of helical polypyrrole actuators for artificial facial muscles. Smart Mater. Struct. 2009, 18, 085008. [Google Scholar] [CrossRef]
- Cheng, M.Y.; Tsao, C.M.; Lai, Y.Z.; Yang, Y.J. The development of a highly twistable tactile sensing array with stretchable helical electrodes. Sens. Actuator A Phys. 2011, 166, 226–233. [Google Scholar] [CrossRef]
- Chen, W.; Li, C.M.; Chen, P.; Sun, C.Q. Electrosynthesis and characterization of polypyrrole/au nanocomposite. Electrochim. Acta 2007, 52, 2845–2849. [Google Scholar] [CrossRef]
- Beveridge, T.J.; Graham, L.L. Surface layers of bacteria. Microbiol. Rev. 1991, 55, 684–705. [Google Scholar] [PubMed]
- Weckesser, J.; Jügrens, U.J. Cell walls and external layers. In Method. Enzymol.; Academic Press: Cambridge, MA, USA, 1988; Volume 167, pp. 173–188. [Google Scholar]
- Hoiczyk, E.; Hansel, A. Cyanobacterial cell walls: News from an unusual prokaryotic envelope. J. Bacteriol. 2000, 182, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.H.; Moraes, J.; Borrmann, T. Conducting polymers on paper fibres. Synth. Met. 2005, 153, 65–68. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Ye, Y. Synthesis and characterization of polyaniline microbelt via cotton template. J. Thermoplast. Compos. Mater. 2011, 24, 389–398. [Google Scholar] [CrossRef]
- Gagrai, M.K.; Das, C.; Golder, A.K. Non-ideal metal binding model for Cr(iii) sorption using Spirulina platensis biomass: Experimental and theoretical approach. Can. J. Chem. Eng. 2013, 91, 1904–1912. [Google Scholar] [CrossRef]
- Koduru, H.K.; Marino, L.; Vallivedu, J.; Choi, C.-J.; Scaramuzza, N. Microstructural, wetting, and dielectric properties of plasma polymerized polypyrrole thin films. J. Appl. Polym. Sci. 2016, 133, 43982. [Google Scholar] [CrossRef]
- Liu, H.J.; Xu, C.H.; Zhou, Q.; Wang, F.; Li, W.M.; Ha, Y.M.; Sun, S.Q. Analysis and identification of irradiated spirulina powder by a three-step infrared macro-fingerprint spectroscopy. Radiat. Phys. Chem. 2013, 85, 210–217. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.S.; Park, J.M. Studies on hexavalent chromium biosorption by chemically-treated biomass of ecklonia sp. Chemosphere 2005, 60, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Arief, V.O.; Trilestari, K.; Sunarso, J.; Indraswati, N.; Ismadji, S. Recent progress on biosorption of heavy metals from liquids using low cost biosorbents: Characterization, biosorption parameters and mechanism studies. Clean-Soil Air Water 2008, 36, 937–962. [Google Scholar] [CrossRef]
- Hebeish, A.; Farag, S.; Sharaf, S.; Shaheen, T.I. Advancement in conductive cotton fabrics through in situ polymerization of polypyrrole-nanocellulose composites. Carbohydr. Polym. 2016, 151, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Rambo, C.R.; Porto, L.M.; Schreiner, W.H.; Barra, G.M.O. Structure and properties of polypyrrole/bacterial cellulose nanocomposites. Carbohydr. Polym. 2013, 94, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Omastova, M.; Trchova, M.; Kovarova, J.; Stejskal, J. Synthesis and structural study of polypyrroles prepared in the presence of surfactants. Synth. Met. 2003, 138, 447–455. [Google Scholar] [CrossRef]
- Blinova, N.V.; Stejskal, J.; Trchova, M.; Prokes, J.; Omastova, M. Polyaniline and polypyrrole: A comparative study of the preparation. Eur. Polym. J. 2007, 43, 2331–2341. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S. One-step synthesis of superparamagnetic Fe3O4@PANI nanocomposites. J. Chem. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Trchova, M.; Stejskal, J. Polyaniline: The infrared spectroscopy of conducting polymer nanotubes (iupac technical report). Pure Appl. Chem. 2011, 83, 1803–1817. [Google Scholar] [CrossRef]
- Muller, D.; Mandelli, J.S.; Marins, J.A.; Soares, B.G.; Porto, L.M.; Rambo, C.R.; Barra, G.M.O. Electrically conducting nanocomposites: Preparation and properties of polyaniline (PAni)-coated bacterial cellulose nanofibers (BC). Cellulose 2012, 19, 1645–1654. [Google Scholar] [CrossRef]
- Chal, P.; Shit, A.; Nandi, A.K. Dye-sensitized solar cell from a new organic n-type semiconductor/polyaniline composite: Insight from impedance spectroscopy. J. Mater. Chem. C 2016, 4, 272–285. [Google Scholar] [CrossRef]
- Saini, P.; Choudhary, V.; Singh, B.P.; Mathur, R.B.; Dhawan, S.K. Polyaniline-mwcnt nanocomposites for microwave absorption and emi shielding. Mater. Chem. Phys. 2009, 113, 919–926. [Google Scholar] [CrossRef]
- Trchova, M.; Sedenkova, I.; Stejskal, J. In-situ polymerized polyaniline films 6. Ftir spectroscopic study of aniline polymerization. Synth. Met. 2005, 154, 1–4. [Google Scholar] [CrossRef]
- Ohlan, A.; Singh, K.; Chandra, A.; Dhawan, S.K. Microwave absorption behavior of core-shell structured poly(3,4-ethylenedioxy thiophene)-barium ferrite nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Park, W.; Sin, B.C.; Noh, J.; Lee, Y. Synthesis of electrically conductive and superparamagnetic monodispersed iron oxide-conjugated polymer composite nanoparticles by in situ chemical oxidative polymerization. J. Colloid Interface Sci. 2009, 335, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Xu, B.; Su, Y.; Luo, Y. Ultralight conducting polymer/carbon nanotube composite aerogels. Carbon 2011, 49, 1884–1893. [Google Scholar] [CrossRef]
- Shin, S.; Jang, J. Thiol containing polymer encapsulated magnetic nanoparticles as reusable and efficiently separable adsorbent for heavy metal ions. Chem. Commun. 2007, 4230–4232. [Google Scholar] [CrossRef]
- Han, G.; Yuan, J.; Shi, G.; Wei, F. Electrodeposition of polypyrrole/multiwalled carbon nanotube composite films. Thin Solid Films 2005, 474, 64–69. [Google Scholar] [CrossRef]
- Duchet, J.; Legras, R.; Demoustier-Champagne, S. Chemical synthesis of polypyrrole: Structure–properties relationship. Synth. Met. 1998, 98, 113–122. [Google Scholar] [CrossRef]
- Chen, F.e.; Shi, G.; Fu, M.; Qu, L.; Hong, X. Raman spectroscopic evidence of thickness dependence of the doping level of electrochemically deposited polypyrrole film. Synth. Met. 2003, 132, 125–132. [Google Scholar] [CrossRef]
- Cong, H.-P.; Ren, X.-C.; Wang, P.; Yu, S.-H. Flexible graphene-polyaniline composite paper for high-performance supercapacitor. Energy Environ. Sci. 2013, 6, 1185–1191. [Google Scholar] [CrossRef]
- Yan, X.; Chen, J.; Yang, J.; Xue, Q.; Miele, P. Fabrication of free-standing, electrochemically active, and biocompatible graphene oxide-polyaniline and graphene-polyaniline hybrid papers. ACS Appl. Mater. Interfaces 2010, 2, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, T.; Ivaska, A. Raman based ph measurements with polyaniline. J. Electroanal. Chem. 2005, 580, 320–329. [Google Scholar] [CrossRef]
- Sun, Y.; Shao, D.; Chen, C.; Yang, S.; Wang, X. Highly efficient enrichment of radionuclides on graphene oxide-supported polyaniline. Environ. Sci. Technol. 2013, 47, 9904–9910. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.C.; Hugot-Le Goff, A. Quantitative characterization of polyaniline films using raman spectroscopy: I: Polaron lattice and bipolaron. Electrochim. Acta 2006, 52, 595–603. [Google Scholar] [CrossRef]
- Jain, M.; Annapoorni, S. Raman study of polyaniline nanofibers prepared by interfacial polymerization. Synth. Met. 2010, 160, 1727–1732. [Google Scholar] [CrossRef]
- Bernard, M.C.; Hugot-Le Goff, A.; Arkoub, H.; Saïdani, B. Characterization of substituted polyaniline films using raman spectroscopy: Iii. Study of a methoxylated polymer: Poma. Electrochim. Acta 2007, 52, 5030–5038. [Google Scholar] [CrossRef]
- Pang, F.-F.; Li, S.; Sun, W.-Q.; Han, G.-Z. Reversible conductivity modulation of pedot:Pss based on ph. Mater. Chem. Phys. 2017, 186, 246–250. [Google Scholar] [CrossRef]
- Wang, X.J.; Wong, K.Y. Effects of a base coating used for electropolymerization of poly(3,4-ethylenedioxythiophene) on indium tin oxide electrode. Thin Solid Films 2006, 515, 1573–1578. [Google Scholar] [CrossRef]
- Back, J.-W.; Lee, S.; Hwang, C.-R.; Chi, C.-S.; Kim, J.-Y. Fabrication of conducting pedot nanotubes using vapor deposition polymerization. Macromol. Res. 2011, 19, 33–37. [Google Scholar] [CrossRef]
- Garcia-Barbera, A.; Culebras, M.; Roig-Sanchez, S.; Gomez, C.M.; Cantarero, A. Three dimensional pedot nanowires network. Synth. Met. 2016, 220, 208–212. [Google Scholar] [CrossRef]
- He, C.; Yang, C.H.; Li, Y.F. Chemical synthesis of coral-like nanowires and nanowire networks of conducting polypyrrole. Synth. Met. 2003, 139, 539–545. [Google Scholar] [CrossRef]
- Hazarika, J.; Kumar, A. Swift heavy ion irradiation effects on structural, optical properties and ac conductivity of polypyrrole nanofibers. Radiat. Eff. Defects Solids 2016, 171, 978–998. [Google Scholar] [CrossRef]
- Huang, H.; Feng, X.; Zhu, J.-J. Synthesis, characterization and application in electrocatalysis of polyaniline/au composite nanotubes. Nanotechnology 2008, 19, 145607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Waterhouse, G.I.N.; Zhang, L. Coaxially aligned polyaniline nanofibers doped with 3-thiopheneacetic acid through interfacial polymerization. J. Nanomater. 2011, 2011, 7. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Xu, Q.; Zhang, Q.; Chen, D. Facile preparation and enhanced capacitance of the polyaniline/sodium alginate nanofiber network for supercapacitors. Langmuir 2011, 27, 6458–6463. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wang, T.; Qiu, X.; Zhao, D.; Liu, D.; Deng, Y. Conductivity enhancement of poly(3,4-ethylenedioxythiophene)/lignosulfonate acid complexes via pickering emulsion polymerization. ACS Sustain. Chem. Eng. 2016, 4, 7193–7199. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, W.; Li, G.; Wang, J.; Lu, Y. Graphene/poly(3,4-ethylenedioxythiophene) hydrogel with excellent mechanical performance and high conductivity. Carbon 2013, 59, 495–502. [Google Scholar] [CrossRef]
- Du, X.-S.; Zhou, C.-F.; Mai, Y.-W. Novel synthesis of poly(3,4-ethylenedioxythiophene) nanotubes and hollow micro-spheres. Mater. Lett. 2009, 63, 1590–1593. [Google Scholar] [CrossRef]
- Choi, J.W.; Han, M.G.; Kim, S.Y.; Oh, S.G.; Im, S.S. Poly(3,4-ethylenedioxythiophene) nanoparticles prepared in aqueous dbsa solutions. Synth. Met. 2004, 141, 293–299. [Google Scholar] [CrossRef]
- Liu, C.; Cai, Z.; Zhao, Y.; Zhao, H.; Ge, F. Potentiostatically synthesized flexible polypyrrole/multi-wall carbon nanotube/cotton fabric electrodes for supercapacitors. Cellulose 2016, 23, 637–648. [Google Scholar] [CrossRef]
- Bidgoli, M.M.; Mohsennia, M.; Boroumand, F.A. Low driving voltage characteristics of polyaniline–silica nanocomposites as hole-injection material of organic electroluminescent devices. Mater. Res. Bull. 2015, 72, 29–34. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Q.; Gu, Y. Effects of inorganic acid in dbsa-pani polymerization on transparent pani-sio2 hybrid conducting films. J. Alloys Compd. 2010, 501, 313–316. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y. In-situ synthesis of transparent conductive pedot coating on pet foil by liquid phase depositional polymerization of edot. Synth. Met. 2016, 217, 185–188. [Google Scholar] [CrossRef]
- Babu, K.F.; Subramanian, S.P.S.; Kulandainathan, M.A. Functionalisation of fabrics with conducting polymer for tuning capacitance and fabrication of supercapacitor. Carbohydr. Polym. 2013, 94, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.J.; Looney, M.G.; Pandolfo, A.G. Enhanced capacitance textile fibres for supercapacitors via an interfacial molecular templating process. Synth. Met. 2010, 160, 655–663. [Google Scholar] [CrossRef]
- Hepowit, L.R.; Kim, K.M.; Kim, S.H.; Ryu, K.S.; Lee, Y.M.; Ko, J.M. Supercapacitive properties of electrodeposited polypyrrole on acrylonitrile-butadiene rubber as a flexible current collector. Polym. Bull. 2012, 69, 873–880. [Google Scholar] [CrossRef]
- Prasanna, B.P.; Avadhani, D.N.; Raghu, M.S.; Kumar, Y.K. Synthesis of polyaniline/alpha-fe2o3 nanocomposite electrode material for supercapacitor applications. Mater. Today Commun. 2017, 12, 72–78. [Google Scholar]
- Lota, K.; Khomenko, V.; Frackowiak, E. Capacitance properties of poly(3,4-ethylenedioxythiophene)/carbon nanotubes composites. J. Phys. Chem. Solids 2004, 65, 295–301. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Zhang, Y.; Yang, L.; Yao, J. Preparation and enhanced electrochemical properties of ag/polypyrrole composites electrode materials. J. Appl. Polym. Sci. 2013, 129, 3787–3792. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, P.; Wang, C.; Pei, L.; Han, Z.; Fang, C. In situ synthesis and characterization of polypyrrole/graphene conductive nanocomposites via electrochemical polymerization and chemical reduction. J. Macromol. Sci. Part B Phys. 2014, 53, 1116–1127. [Google Scholar] [CrossRef]
- Saranya, S.; Selvan, R.K.; Priyadharsini, N. Synthesis and characterization of polyaniline/mnwo4 nanocomposites as electrodes for pseudocapacitors. Appl. Surf. Sci. 2012, 258, 4881–4887. [Google Scholar] [CrossRef]
- Lupu, S.; Javier del Campo, F.; Xavier Munoz, F. Sinusoidal voltage electrodeposition and characterization of conducting polymers on gold microelectrode arrays. J. Electroanal. Chem. 2012, 687, 71–78. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.-Y.; Ouyang, J.; Liu, G.-C.; Gao, M.-J.; Song, L.-B.; Zang, J.; Chen, W. Synthesis and Characterization of the Conducting Polymer Micro-Helix Based on the Spirulina Template. Polymers 2018, 10, 882. https://doi.org/10.3390/polym10080882
Hu X-Y, Ouyang J, Liu G-C, Gao M-J, Song L-B, Zang J, Chen W. Synthesis and Characterization of the Conducting Polymer Micro-Helix Based on the Spirulina Template. Polymers. 2018; 10(8):882. https://doi.org/10.3390/polym10080882
Chicago/Turabian StyleHu, Xiao-Yu, Jun Ouyang, Guo-Chang Liu, Meng-Juan Gao, Lai-Bo Song, Jianfeng Zang, and Wei Chen. 2018. "Synthesis and Characterization of the Conducting Polymer Micro-Helix Based on the Spirulina Template" Polymers 10, no. 8: 882. https://doi.org/10.3390/polym10080882






