1. Introduction
Typical linear polyurethane (PUR) is built with (i) a soft segment (based on oligomeric polyester- or polyetherdiol), which gives flexibility and softness of polymer and (ii) a hard segment (synthetized with diisocyanate and low molecular chain extender) the aim of which is to increase stiffness, hardness changes, etc. Immiscibility of soft and hard segments facilitates their reorganization into domains. Further ordering of chains in domains can lead to formation of crystallites. The polar nature of the urethane group influences their tendency to hydrogen bonding. Creation of hydrogen bonds between N–H group and C=O in urethanes facilitates their ordering and phase separation. Whereas hydrogen bonds of the urethane group with oxygen of the soft segment can reduce chains mobility and their ordering [
1]. Generally, hydrogen bonds create physical reinforcement of the PUR network which causes increased strength and stiffness [
2].
Thermodynamic incompatibility of hard and soft segments and connected with this, their insolubility, cause microphase separation in segmented PURs [
3]. Ordering of chains in both separated domains of segments can lead to formation of crystallites.
Branched polymers are a kind of macromolecules, whose architecture is neither linear nor cross-linked. In addition to polymers with long or short side-chains, this group of polymers also includes densely branched structures with a large number of functional end groups (star like and hyperbranched polymers, and dendrimers).
The architecture of branched polymers causes an intermediate structure between cross-linked and linear polymers. The presence of side chains in their structure significantly influences the properties of polymers.
Branched PURs are generally characterized by good solubility in many organic solvents and compatibility with different materials, low viscosity (both in the molten state and in the solution) and they have free spaces inside the network. In consequence, they can be used as drug carriers, catalysts, chemical sensors, coatings, binders, elastomers, sorption mats, etc. [
2,
4].
In branched polymers linear side-chains diverge (uniformly or randomly) from branching points of the linear flexible chain (backbone). In this case, introducing trifunctional compound into the PUR structure reduces a possibility of chains to order and form soft and hard domains. However, local aggregation of soft or hard segments and their microphase separation can often still occur even in cross-linked PURs [
1]. The presence of branching points generally introduces irregularities in the polymer structure and consequently leads to lowered crystallinity and creation of smaller crystals with the lower melting point in comparison to linear polymers [
5]. Tendency of urethane groups to create hydrogen bonds is strongly affected by side-chains. Both short and long side-chains can influence a possibility of interaction between polymer chains.
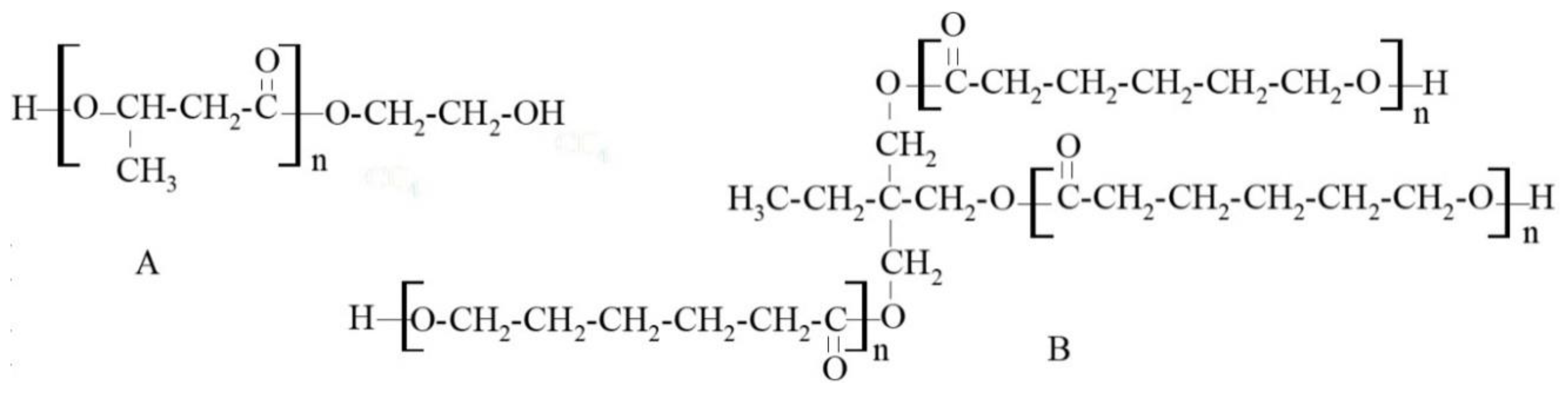
Polyhydroxybutyrate (PHB) is a polymer that naturally exists in prokaryote and eukaryote cells. Biosynthesized PHB is high crystalline, stiff and brittle material, which makes it difficult to use. Therefore, it is valuable to us its synthetic analogue, which can be obtained as an amorphous, elastic polymer.
Synthetic PHB can be obtained via ring-opening polymerization (ROP) of β-butyrolactone to isotactic, atactic and syndiotactic poly(3-hydroxybutyrate) [
6]. Atactic (R,S)-PHB is an amorphous polymer with low glass transition temperature and it maintains elastomer properties at room temperature. Because of the secondary hydroxyl group in the PHB
diol structure methyl side-groups are present in its chains (
Figure 1A).
Through the right combination of polycaprolactonetriol (PCL
triol) (
Figure 1B) with polycaprolactonediol (PCL
diol), branched PURs with the highest possible molecular weight without reaching gelation and formed into foils could be obtained [
5]. Knowing that introduction of amorphous R,S-PHB influences thermal, sorptive and surface [
7] properties of linear and cross-linking PUR materials and their degradability profile [
8], such effects are also expected in branched PURs.
In this paper branched PURs, as potential sorptive materials and their linear analogues were comparatively studied. The influence of PCLtriol and R,S-PHB presence in the soft segments structure on thermal and sorptive properties of obtained PURs was estimated.
3. Results and Discussion
The chemical structure of synthetized PURs was confirmed with FTIR and Raman spectroscopy.
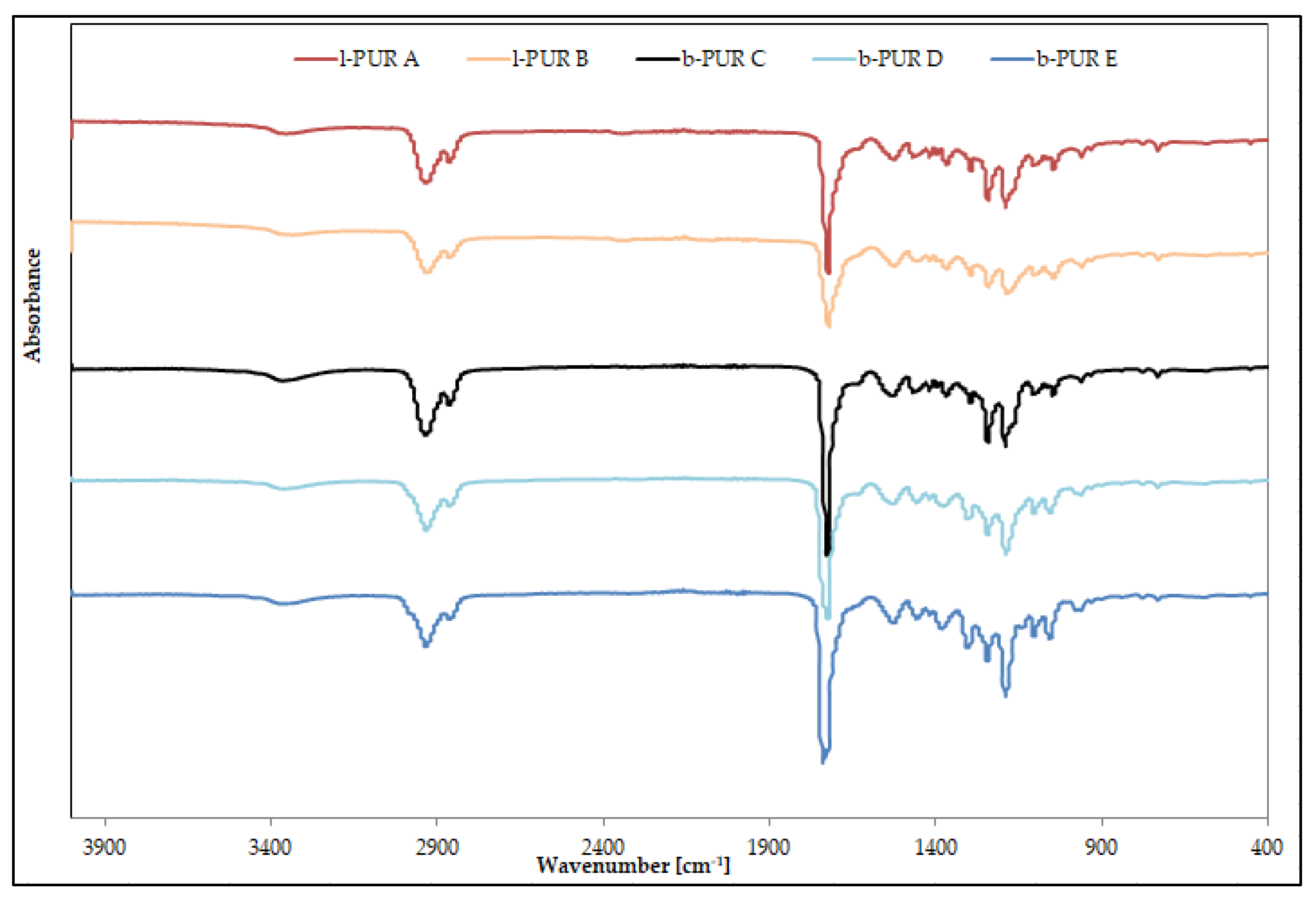
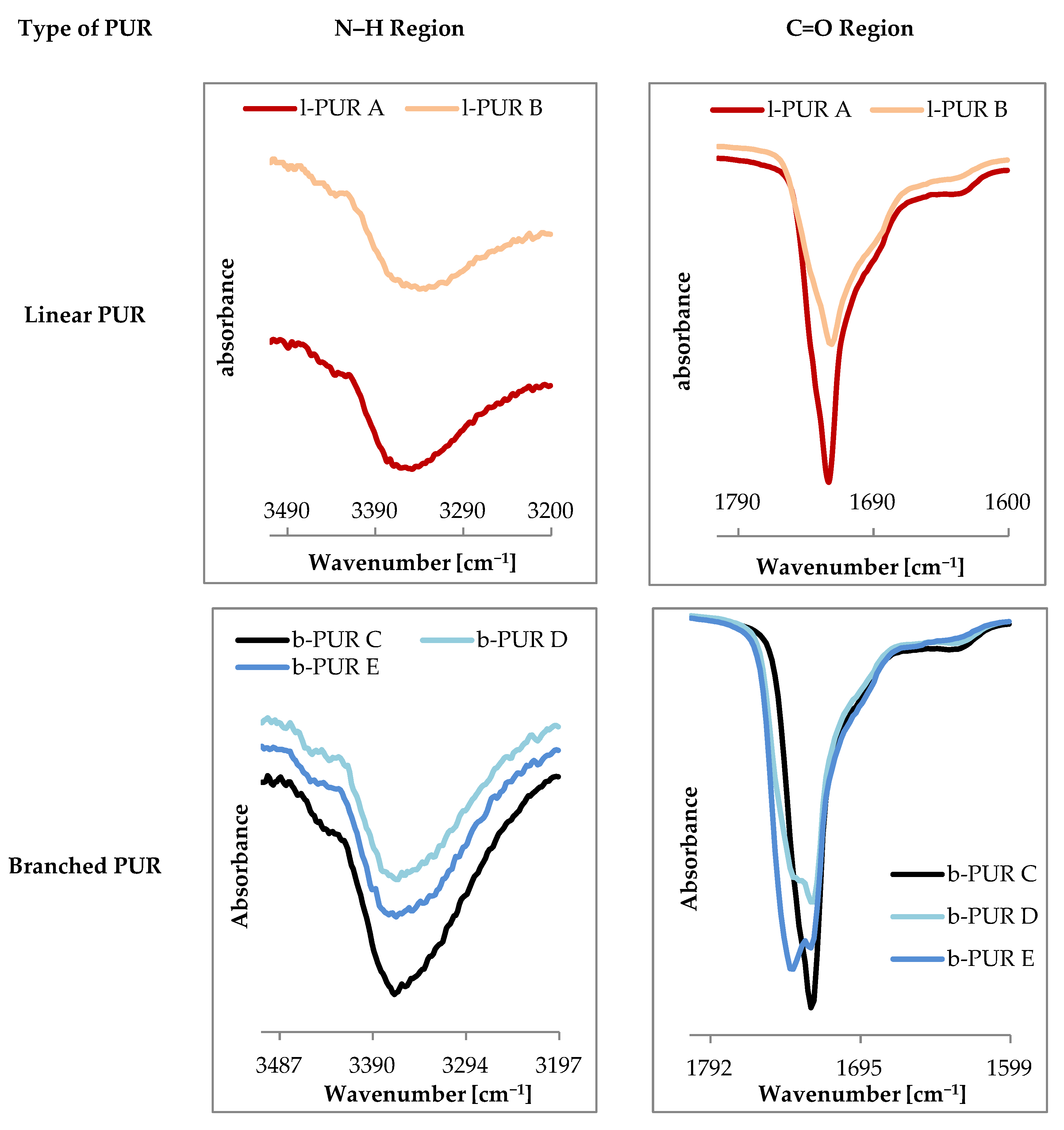
Figure 3 shows FTIR spectra of linear and branched PURs.
Infrared spectra of linear and branched PURs were very similar and showed characteristic bands typical for PURs. The absence of bands ascribing functional groups of starting substrates on FTIR spectra indicated completion of the polyaddition reaction.
The region between 3410 and 3200 cm
−1 was ascribed to N–H stretching vibration, whereas the region between 2990 and 2840 cm
−1 to C–H stretching vibration (of both soft and hard segments). Infrared absorptions between 1780 and 1620 cm
−1 indicated the presence of the carbonyl group (amide I), whereas bands 1550–1510 cm
−1 ascribed deformation vibrations of N–H groups (amide II) and 1242–1240 cm
−1 C–O stretching of the carbonate group. Bands corresponding to asymmetric and symmetric C–H stretching vibrations of aliphatic –CH
2 groups were observed at 2905 and 2860 cm
–1 respectively (
Figure 3).
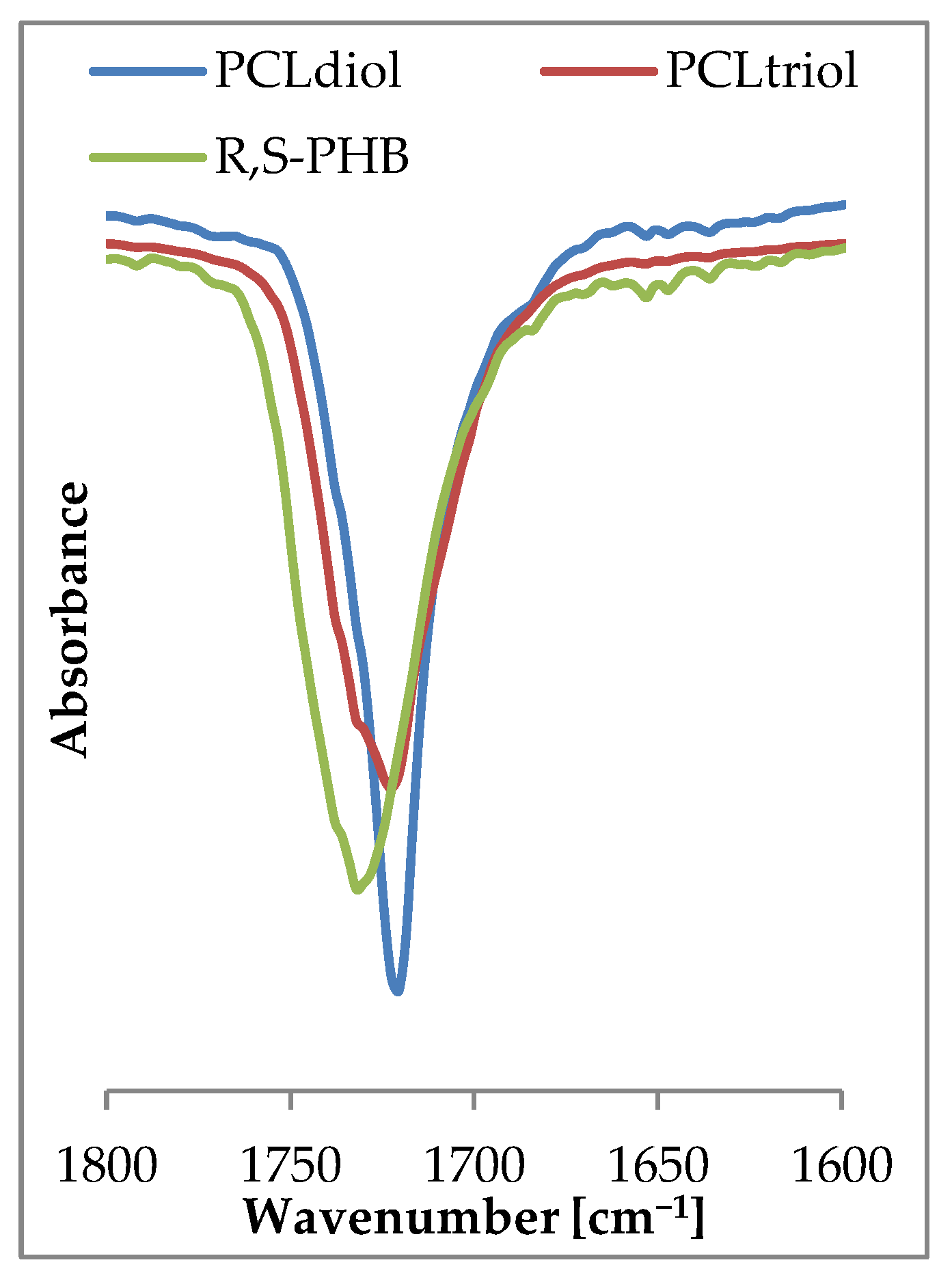
Spectra of carbonyl moiety in PURs can be highly complex due to numerous origins of carbonyl and hydrogen bond interactions within the polymer network. The observed on FTIR spectrum of PCL
diol (
Figure 4) carbonyl peak at 1720 cm
−1 was narrow and slender, which suggested homogeneous nature of this moiety. The presence of branch nodes in PCL
triol and the lateral methyl group in chains of R,S-PHB (only with CH
2 and CH groups between ester moiety in comparison to five CH
2 in PCL) changed molecular interaction, causing in these cases the carbonyl peak to be wider and less uniform than for PCL
diol. Moreover, the peak appeared at a higher wavenumber (1730 cm
−1).
On FTIR spectra observed carbonyl bands were a result of overlapping of the peaks of carbonyl moieties from oligomeroles and urethane groups. In the case of linear l-PUR A with soft segments built with only PCL
diol frequency (1720 cm
−1) and shape of the carbonyl stretching peak were identical as in pure PCL
diol (
Figure 5 compared to
Figure 4). Only a small shoulder at right sight of the peak was observed. It was the overlapping band of carbonyl groups from urethane (with wavenumber 1690 cm
−1). After introducing the R,S-PHB into soft segments the band in the stretching carbonyl region became wider and less sharp. The overlapped urethane C=O peak was also observed on l-PUR B FTIR spectra as a shoulder at lower frequencies (at 1690 cm
−1) in relation to that of the ester group. This band was almost no discernible at 1720 cm
−1.
Using PCL
triol to build soft segments caused that shape of the carbonyl band to be more complicated (
Figure 5). Besides two mentioned peaks at 1740 cm
−1 another one appeared, which could be attributed to free C=O groups. On FTIR spectra of linear PURs no bands were detected above 3500 cm
−1 (
Figure 5), which suggested that all N–H groups were engaged in formation of hydrogen bonds. However, at 3430–3460 cm
−1 on FTIR spectra of branched PURs (
Figure 5), a little intensity shoulder was observed. It was often described as the overtone of the band at 1720 cm
−1 [
12]. Taking into consideration that small intensity bands indicating the presence of non-bonded C=O stretching of the urethane and carbonate groups, were visible at 1740 cm
−1 (
Figure 5), it was concluded that this peak (at 3430–3460 cm
−1) ascribed non-associated N-H groups. Moreover, increasing R,S-PHB in the structure of branched PURs inhibited a tendency of urethane groups to hydrogen bonding. In b-PUR E intensity of bands ascribed free C=O ester groups were larger than those bound by hydrogen bonding. Probably, the presence of the side-chain methyl group in the R,S-PHB chain hindered formation of hydrogen bonds. As it was said, the band attributed to stretching vibrations of C=O in the urethane group strongly overlapped with these of the ester group, so no quantitative information about relations between free and bonded ester and urethane groups could be obtained.
The peak observed at 1381 cm
−1 on FTIR spectra of R,S-PHB (
Figure S1) was ascribed as banding deformation vibration of CH
3 according to Eldessouki et al. [
13]. Its presence was noted on FTIR spectra of branched PURs based on R,S-PHB at 1380 cm
−1. Intensity of the peak was the highest in the case of b-PUR E, because of the high amount of R,S-PHB (
Figure 3). On spectra of l-PUR B (obtained with the smaller amount R,S-PHB in comparison to branched PURs) this peak overlapped with C–H bending vibration (
Figure 3).
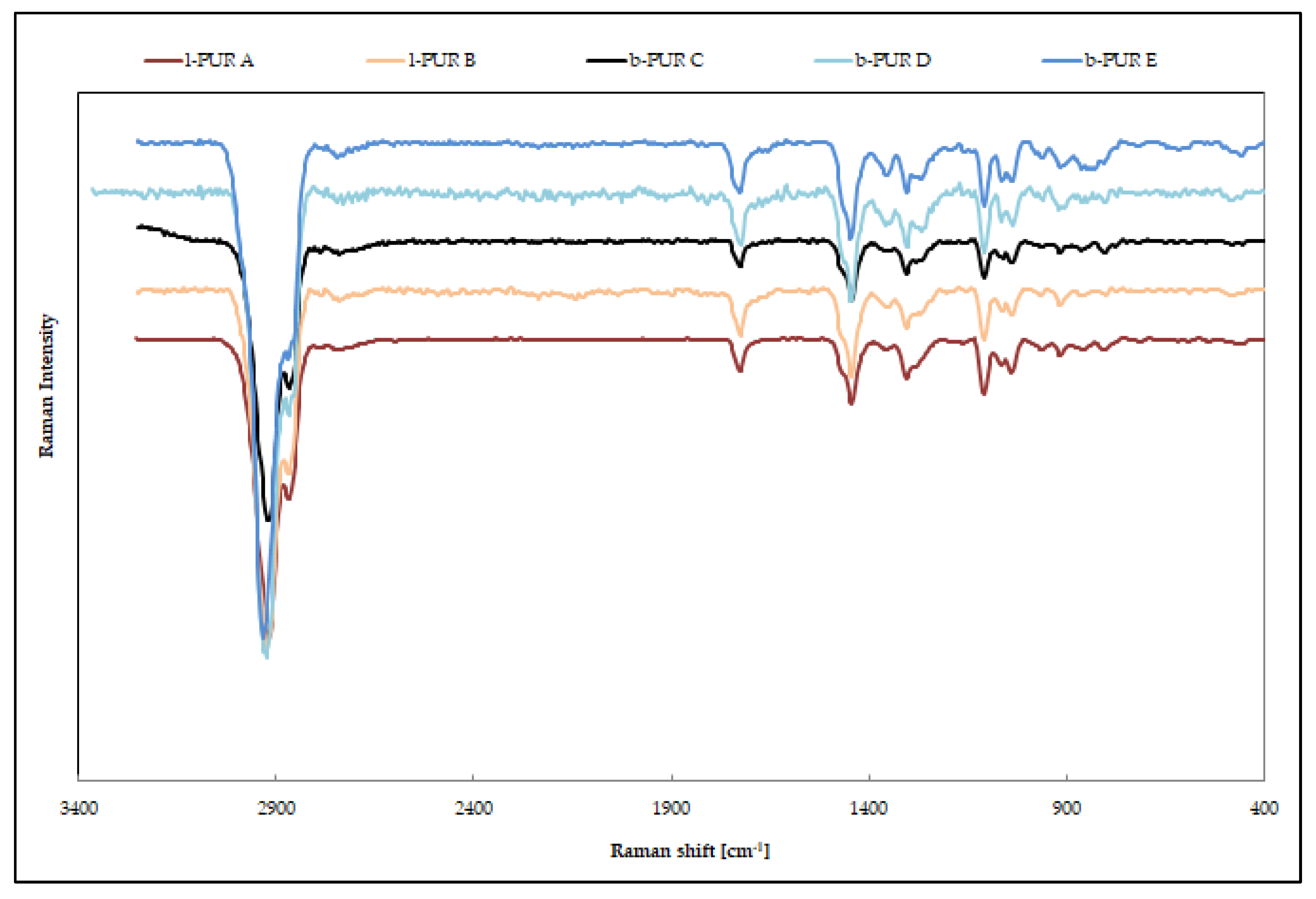
Raman spectroscopy, which is a complementary technique to infrared spectroscopy, also measures vibrational energy levels [
14].
The very strong FTIR spectra band at about 1740–1720 cm
−1, ascribed the free C=O ester groups, was quite weak in Raman spectra of both types of PURs (
Figure 6). In addition. because of polarity of urethane, the N–H presence on Raman spectra was invisible. The largest bands were asymmetric at 2930–2917 cm
−1 and symmetric at 2890–2863 cm
−1, with CH
2 stretching vibration and CH
2 bending vibration at ≈1448 cm
−1.
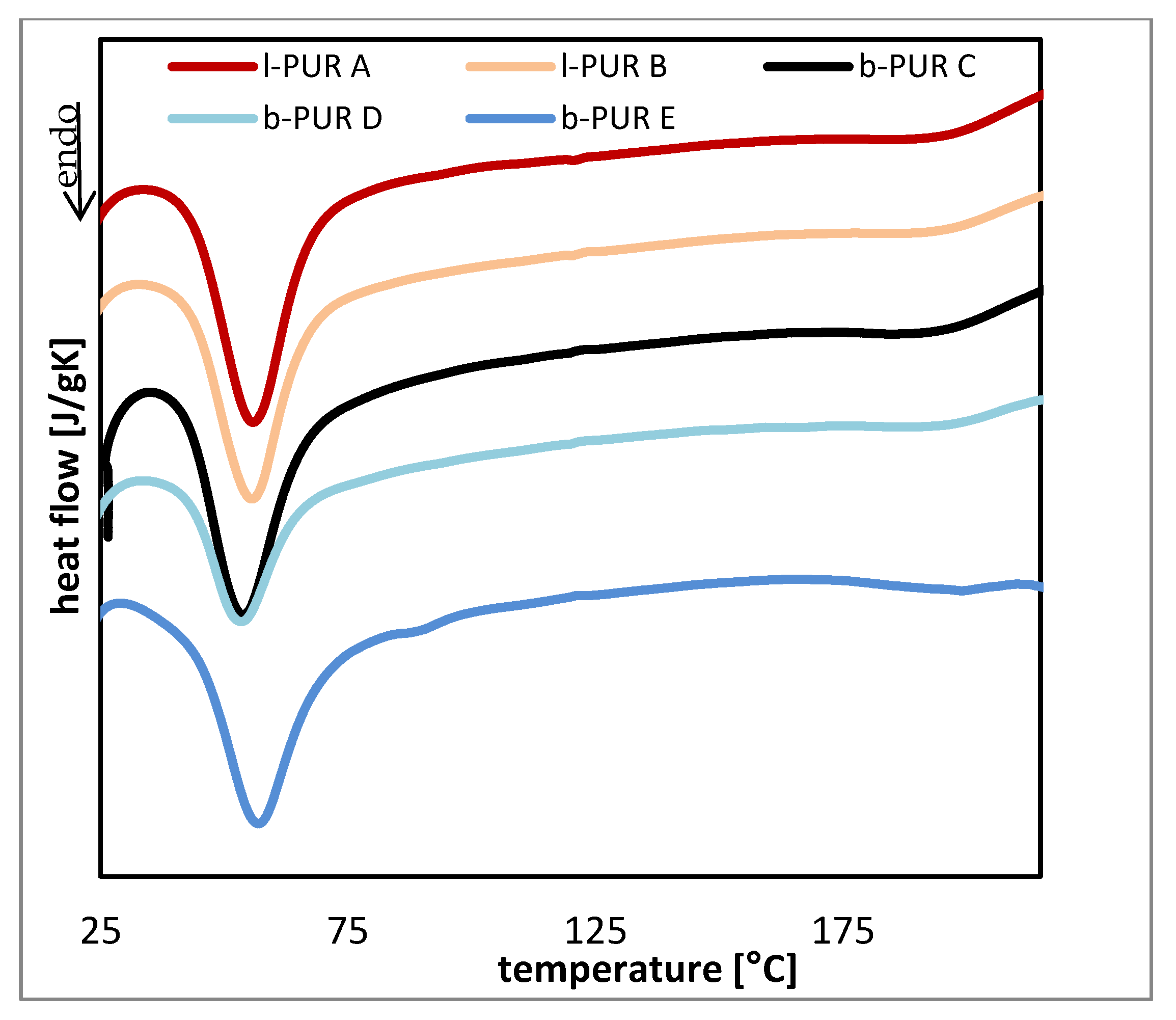
DSC thermograms of investigated samples were typical for segmented PURs. Two endotherms, ascribed to the melting crystalline phase of soft (with melting temperature
Tm1) and hard (with melting temperature
Tm2) segments were visible on DSC diagrams of linear and branched PURs (
Figure 7). The data read from thermograms are shown in
Table 2.
Using semicrystalline PCL to build soft segments caused obtained PURs to be characterized by partial crystallinity of soft segment domains.
Total melting enthalpies (ΔH) of both soft and hard segments of linear PURs were higher than branched PURs, which suggested that linear PURs were more crystalline than branched ones, as it was expected.
Melting temperatures of soft and hard segments depended on segments separation. In case of linear PURs (l-PUR A and l-PUR B) and branched b-PUR E (with low amount of PCLtriol) Tm1 was closer to melting temperature of PCLdiol (Tm = 60.1 °C) than for b-PUR C and b-PUR D, suggesting the good separation of soft segment chains. However, the endothermic peak ascribing melting of crystalline phase of soft segments on DSC thermograms of b-PUR E was very wide and was integrated to baseline, including a small peak about 88 °C. It was concluded that this broad peak represents melting of all kinds of spherulites with different volume that formed in range of soft segments. This was a reason of high ΔH1 b-PUR E.
Mobility of long aliphatic chains of PUR in its structure facilitated re-organization of PCL and, in consequence, formation of crystals. The presence of amorphous R,S-PHB did not restrict a tendency of PCL to crystallize. A different situation was observed for branched PURs. In the case of reduction of chain mobility via branch nodes, partial replacement of PCL
diol chains by amorphous R,S-PHB reduced crystallinity of soft segments. In b-PUR E with a very small amount of trifunctional PCL
triol the tendency was the same as in linear samples. This dependence of crystallinity with the number of branch was also observed by Mahapatra et al. in hyperbranched PURs [
15].
What was interesting was the presence of R,S-PHB in the PURs structure reduced melting enthalpy of hard segments. It was supposed that the presence of the lateral methyl group in R,S-PHB hindered ordering of the PUR chain. As mentioned before the presence of non-bonded C=O in PURs containing R,S-PHB was confirmed by FTIR and contribution of C=O urethane groups in formation of hydrogen bonds could not be determined. However, reduced crystallinity of hard segments (lowered ΔH2) suggested the absence of hydrogen bonds between urethane groups and connected with it chains ordering.
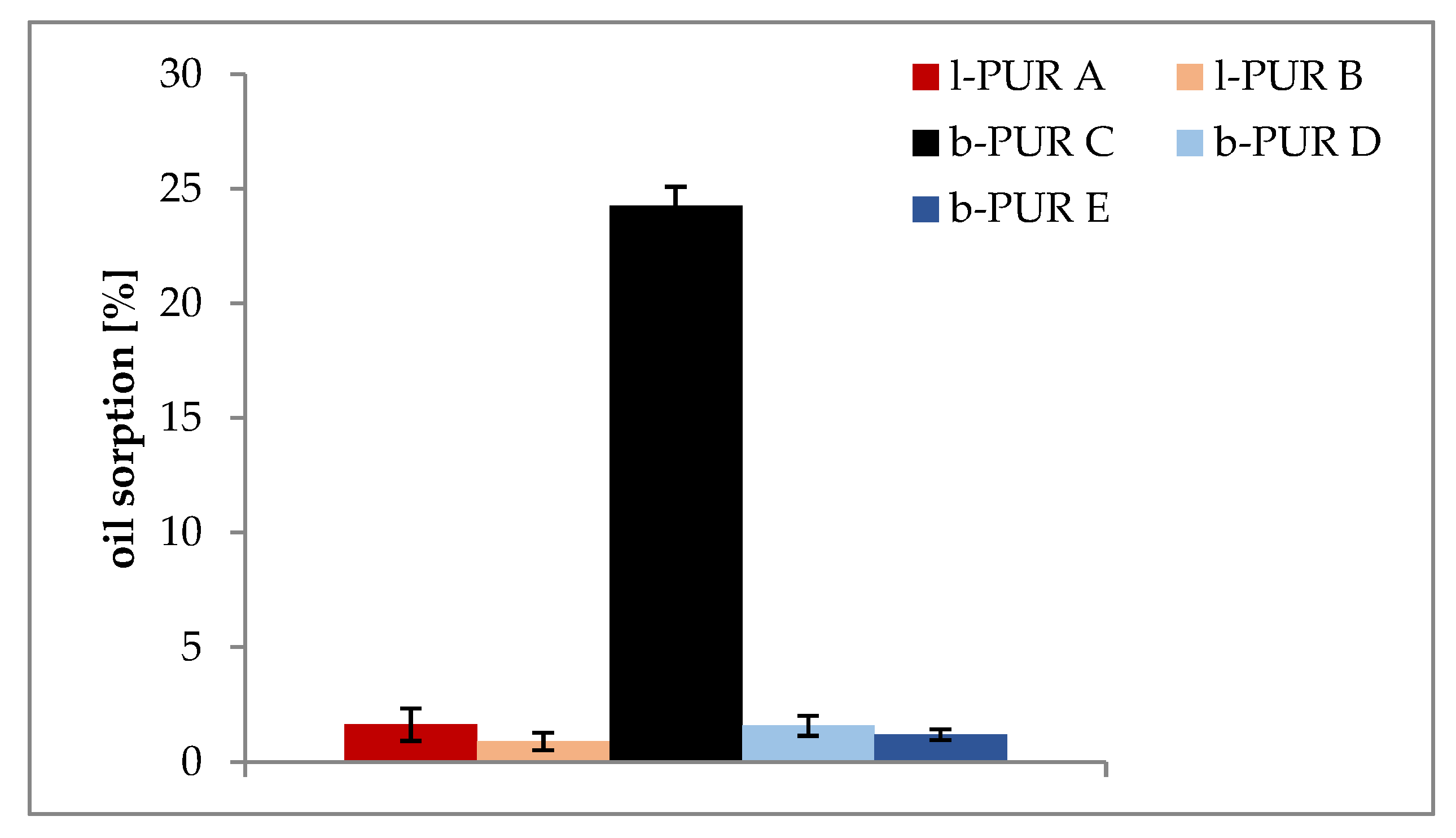
Architecture of PURs structure and their hydrophilicity also influenced tendency to oil sorption. The data presented in
Figure 8 indicated that branched PUR without R,S-PHB absorbed more vegetable oil than linear l-PUR A. The presence of free spaces in the polymer network of branched PURs, which were a result of the presence of branching points, ought to facilitate migration of oil molecules. However, the ability of investigated PURs to sorb oil was also connected with their soft segment building. Soft segments, both linear and branched PURs, were mainly built with chains of hydrophobic polycaprolactone. Oil sorptions of l-PUR A and b-PUR C were higher than appropriate PURs containing R,S-PHB in their structure. Increasing PURs hydrophilicity after using R,S-PHB to build soft segments was observed earlier [
7]. In consequence, branched b-PUR C with soft segments built only with polycaprolactone chains and characterized by the lowest density absorbed highest amount of lipid among all investigated PURs.
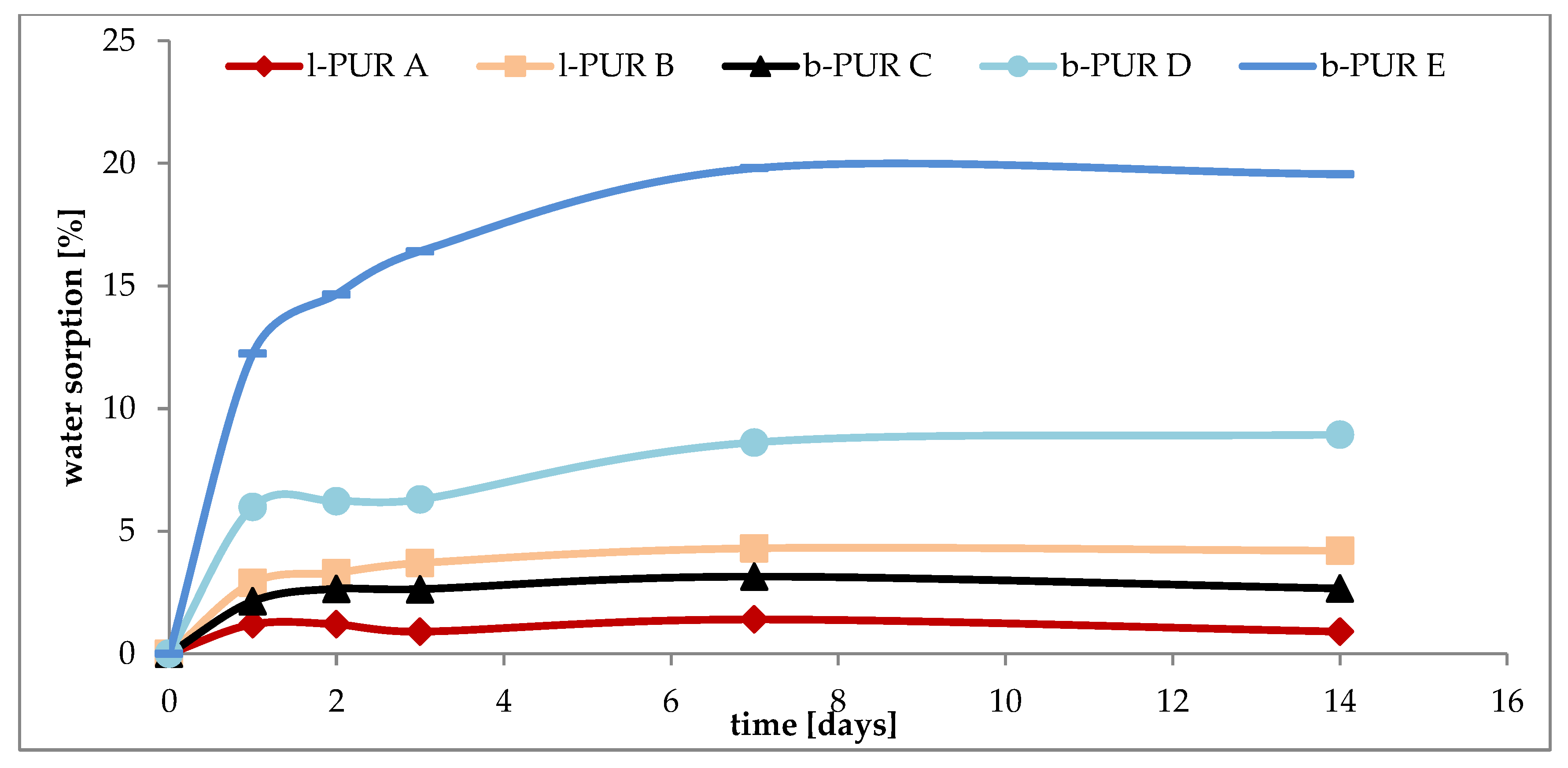
The water sorption is a synergistic effect of crystallinity and the chemical structure of chains in PURs. Introducing R,S-PHB into the PURs (both linear and branched) structure, instead of hydrophobic polycaprolactone chains, increased their hydrophilicity (
Figure 9), as it was observed earlier [
16]. Crystallinity of samples and the presence of free spaces in polymer network were very important for water sorption. Comparing the structure of l-PUR A and b-PUR C, it could be said that their chemical building was very similar to each other. They differed only in architecture, i.e., the presence of long, side chains in the branched PUR structure resulting from the introduction of tri-functional PCL
triol (
Figure 1). In consequence, the free spaces in the b-PUR C structure were formed, which facilitated migration of water molecules into polymer bulk. However, as it was said before, more important was using amorphous, hydrophilic R,S-PHB as a part of soft segments. Its presence significantly increased the ability of investigated PURs to absorb water. Branched b-PUR E with the high amount of R,S-PHB and with very low crystallinity of hard segments absorbed the most water (
Figure 9).