Effects of Sulfuric Acid on the Curing Behavior and Bonding Performance of Tannin–Sucrose Adhesive
Abstract
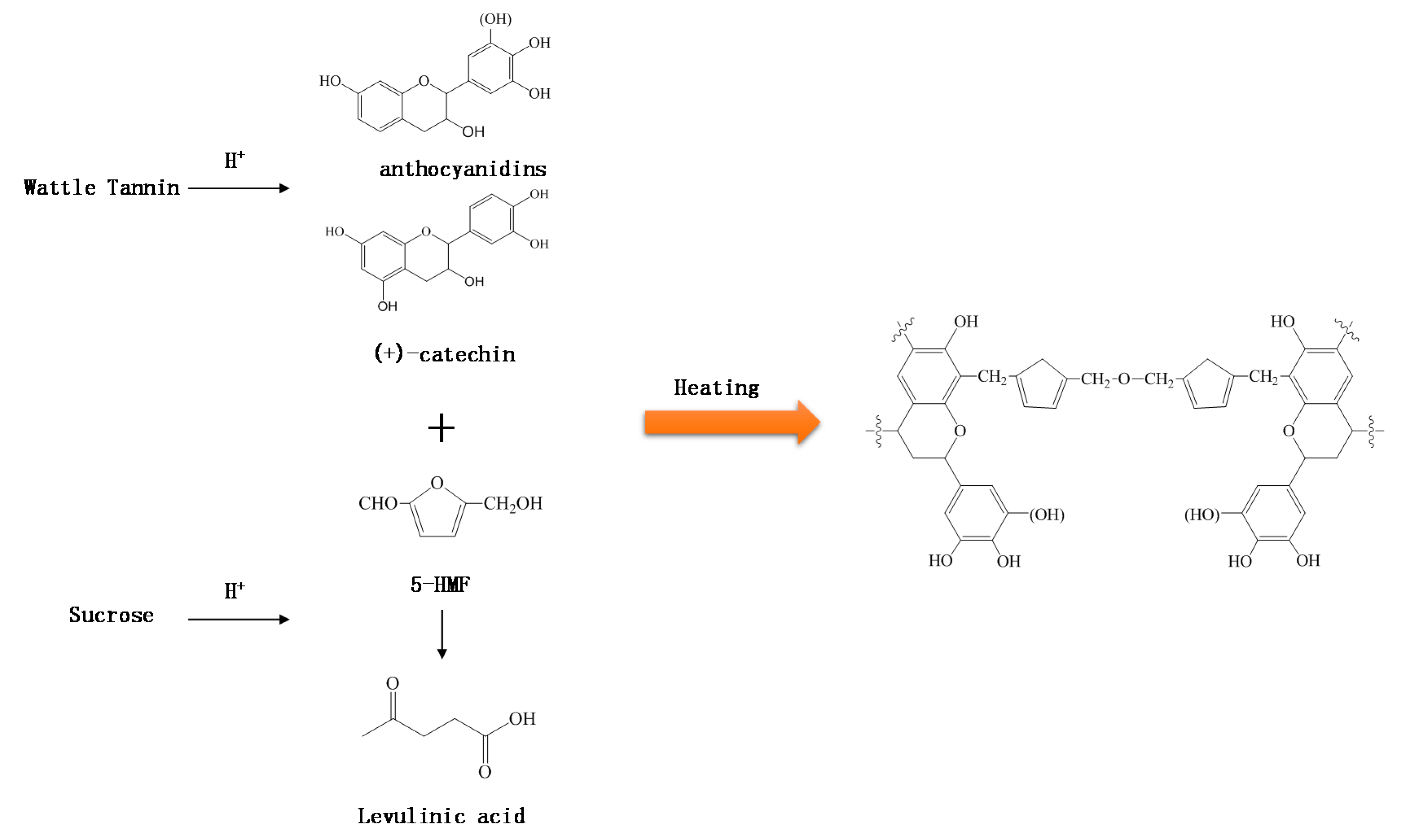
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. pH Value Adjustment of Adhesive Solution by Adding Sulfuric Acid
2.3. Thermal Analysis
2.4. Measurement of Insoluble Mass Proportion
2.5. Fourier Transform Infrared Spectra (FTIR)
2.6. 13C Cross Polarization-Magic Angle Spinning (CP-MAS) NMR
2.7. Manufacture of Particleboard
2.8. Evaluation of Particleboard Properties
3. Results and Discussion
3.1. Thermal Analysis
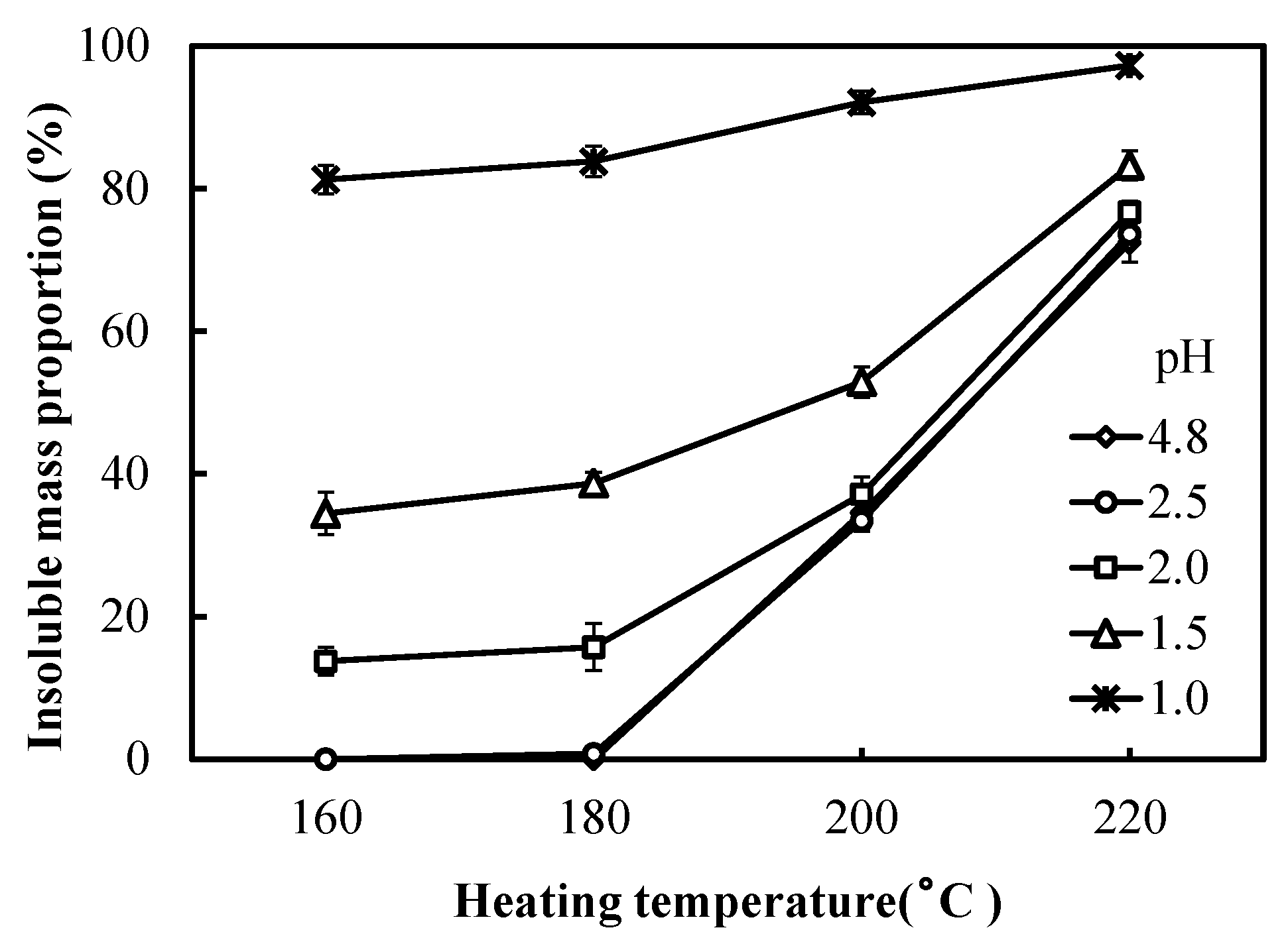
3.2. Insoluble Mass Proportion
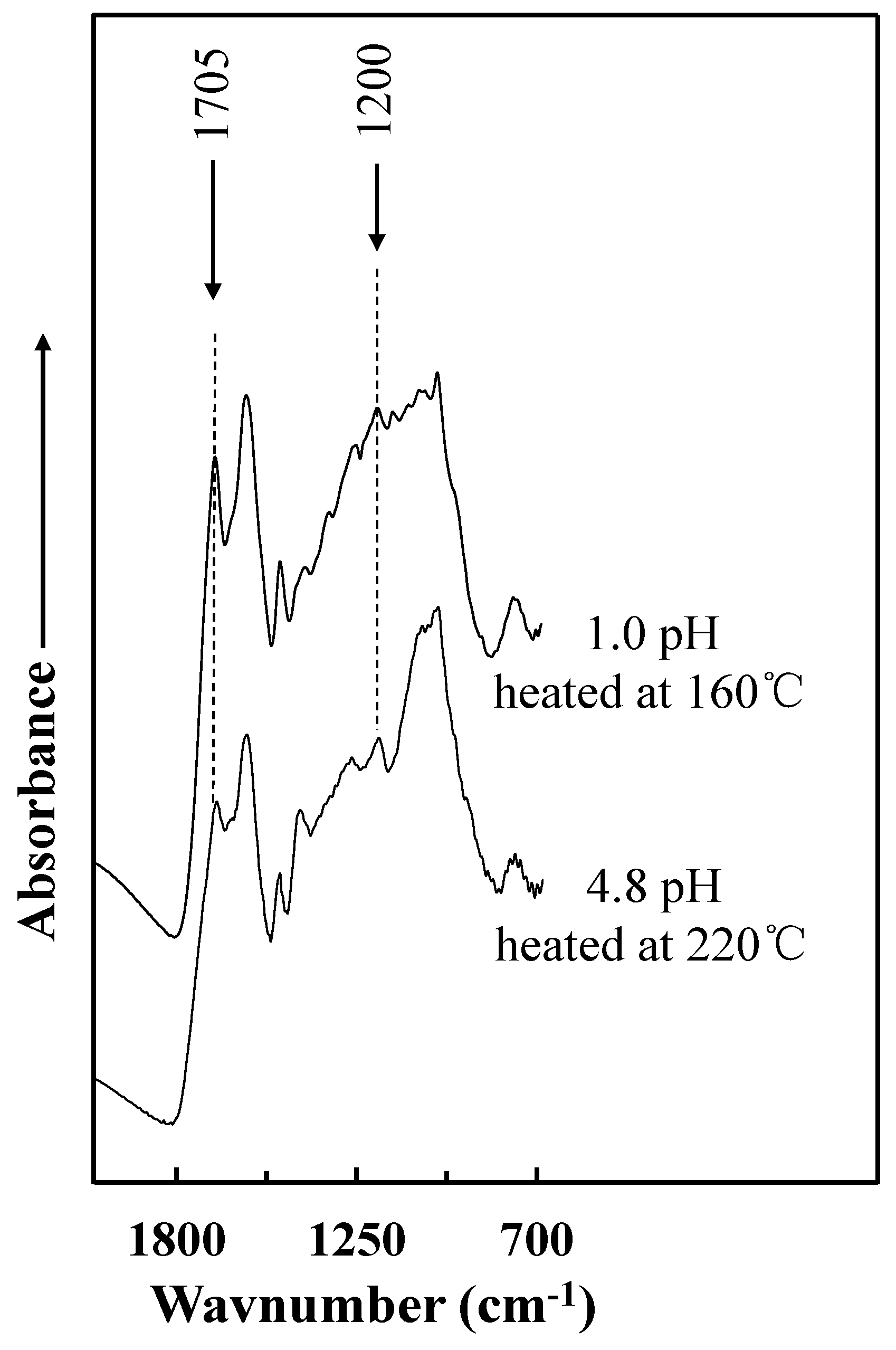
3.3. FT-IR Spectroscopic Analysis
3.4. 13C CP–MAS NMR Analysis
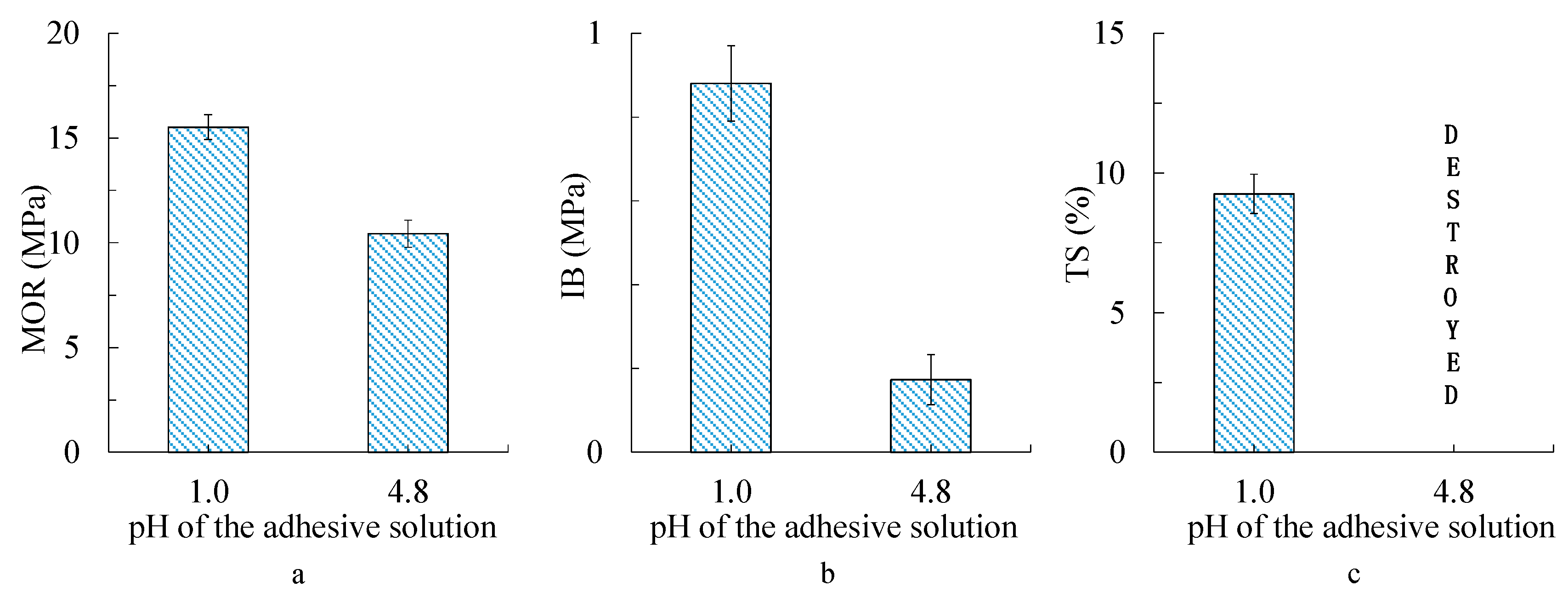
3.5. Bending, Internal Bond Strength, and Thickness Swelling of Particleboard
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guan, C.; Zhang, H.; Hunt, J.; Yan, H. Determining shear modulus of thin wood composite materials using a cantilever beam vibration method. Constr. Build. Mater. 2016, 121, 285–289. [Google Scholar] [CrossRef]
- Sellers, T.J. Wood adhesive innovations and applications in north america. For. Prod. J. 2001, 51, 12–22. [Google Scholar]
- Li, J.; Luo, J.; Li, X.; Yi, Z.; Gao, Q.; Li, J. Soybean meal-based wood adhesive enhanced by ethylene glycol diglycidyl ether and diethylenetriamine. Ind. Crops Prod. 2015, 74, 613–618. [Google Scholar] [CrossRef]
- Cheng, H.; Ford, C.; Dowd, M.; He, Z. Soy and cottonseed protein blends as wood adhesives. Ind. Crops Prod. 2016, 85, 324–330. [Google Scholar] [CrossRef]
- Aracri, E.; Blanco, D.; Tzanov, T. An enzymatic approach to develop a lignin-based adhesive for wool floor coverings. Green Chem. 2016, 16, 2597–2603. [Google Scholar] [CrossRef]
- Li, R.; Gutierrez, J.; Chung, Y.; Frank, C.; Billington, S.; Sattely, E. A lignin-expoxy resin derived from biomass as an alternative to formaldehyde-based wood adhesive. Green Chem. 2018, 20, 1459–1466. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, M.; Luo, J.; Li, X.; Gao, Q.; Li, J. A novel water-based process produces eco-friendly bio-adhesive made from green cross-linked soybean soluble polysacharide and soy protein. Carbohydr. Polym. 2017, 169, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Ferdosian, F.; Pan, Z.; Gao, G.; Zhao, B. Bio-based adhesives and evaluation for wood composites application. Polymers 2017, 9, 70. [Google Scholar] [CrossRef]
- Pizzi, A. Wood products and green chemistry. Ann. For. Sci. 2016, 73, 185–203. [Google Scholar] [CrossRef]
- Umemura, K.; Ueda, T.; Munawar, S.; Kawai, S. Application of citric acid as natural adhesive for wood. J. Appl. Polym. Sci. 2012, 123, 1991–1996. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Li, H.; Yuan, T.; Wang, Y.; Sun, R. Heat treatment of industrial alkaline lignin and its potential application as an adhesive for green wood-lignin composites. ACS Sustain. Chem. Eng. 2017, 5, 7269–7277. [Google Scholar] [CrossRef]
- Kusumah, S.; Umemura, K.; Yoshioka, K.; Miyafuji, H.; Kanayama, K. Utilization of sweet sorghum bagasse and sitric acid for manufacturing of particleboard I: Effects of pre-drying treatment and citric acid content on the board properties. Ind. Crops Prod. 2016, 84, 34–42. [Google Scholar] [CrossRef]
- Pizzi, A. Recent developments in eco-efficient bio-based adhesives for wood bon bonding: Opportunities and issues. J. Adhes. Sci. Technol. 2006, 20, 829–846. [Google Scholar] [CrossRef]
- Khanbabaee, K.; Van, T. Tannins: Classification and definition. Nat. Prod. Rep. 2011, 18, 641–649. [Google Scholar]
- Maier, M.; Oelbermann, A.; Renner, M.; Weidner, E. Screening of european medicinal herbs on their tannin content- new potenitial tanning agents for the leather industry. Ind. Crops Prod. 2017, 99, 19–26. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H. Curing behavior and viscoelastic properties of pine and wattle tannin-based adhesives studied by dynamic mechanical thermal analysis and FT-IR-ATR spectroscopy. J. Adhes. Sci. Technol. 2003, 17, 1369–1383. [Google Scholar] [CrossRef]
- Jeong, J.; Antonyraj, C.; Shin, S.; Kim, S.; Kim, B.; Lee, K.; Cho, J. Commercially attractive process for production of 5-hydroxymethyl-2-furfural from high fructose corn syrup. J. Ind. Eng. Chem. 2013, 19, 1106–1111. [Google Scholar] [CrossRef]
- Lee, J.; Thomas, L.; Jerrell, J.; Feng, H.; Cadwallader, K.; Schmidt, S. Investigation of thermal decomopsition as the kinetic process that causes the loss of crystalline structure in sucrose using a chemical analysis approach. J. Agric. Food Chem. 2011, 59, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Umemura, K. Investigation of a new natural particleboard adhesive composed of tannin and sucrose. J. Wood Sci. 2014, 60, 269–277. [Google Scholar] [CrossRef]
- Zhao, Z.; Umemura, K. Investigation of a new natural particleboard adhesive composed of tannin and sucrose. 2. effect of pressing temperature and time on board properties, and characterization of adhesive. Bioresources 2015, 10, 2444–2460. [Google Scholar] [CrossRef]
- Wigins, L. The utilization of sucrose. Adv. Carbohydr. Chem. 1949, 4, 293–336. [Google Scholar]
- Girisuta, B.; Danon, B.; Manurung, R.; Janssen, L.; Heeres, H. Experimental and kinetic modelling studies on the acid-catalysed hydrolysis of the water hyacinth plant to levulinic acid. Bioresour. Technol. 2008, 99, 8367–8375. [Google Scholar] [CrossRef] [PubMed]
- Tarabanko, V.; Chernyak, M.; Aralova, S.; Kuznetsov, B. Kinetisc of levulinic acid formation from carbohydrates at moderate temperatures. React. Kinet. Catal. Lett. 2002, 75, 117–126. [Google Scholar] [CrossRef]
- Arbenz, A.; Avérous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef] [Green Version]
- Tan-Soetedjo, J.; Bovenkamp, H.; Abdilla, R.; Rasrendra, C.; Ginkel, J.; Heeres, H. Experimental and kinetic modeling studies on the conversion of sucrose to levulinic acid and 5-HMF using sulfuric acid in water. Ind. Eng. Chem. Res. 2017, 56, 13228–13239. [Google Scholar] [CrossRef] [PubMed]
- Dalluge, D.; Daugaard, T.; Johnston, P.; Kuzhiyil, N.; Wright, M.; Brown, R. Continuous production of sugars from pyrolysis of acid-infused lignocellulosic biomass. Green Chem. 2014, 16, 4144–4155. [Google Scholar] [CrossRef]
- Ajandouz, E.; Puigserver, A. Nonenzymatic browning reaction of essential amino acids: Effect of pH on caramelization and maillard reaction kinetics. J. Agric. Food Chem. 1999, 47, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman characterization of non-cellulosic polysaccharides fractions isolated from plant cell wall. Carbohydr. Polym. 2016, 154, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Sritham, E.; Gunasekaran, S. FTIR spectroscopic evaluation of sucrose-maltodextrin-sodium citrate bioglass. Food Hydrocoll. 2017, 70, 371–382. [Google Scholar] [CrossRef]
- Jing, L.; Zong, S.; Li, J.; Surhio, M.; Ye, M. Purification, structural features and inhibition activity on α-glucosidase of a novel polysaccharide from Lachnum YM406. Process Biochem. 2016, 51, 1706–1713. [Google Scholar] [CrossRef]
- Xu, G.; Chang, C.; Fang, S.; Ma, X. Cellulose reactivity in ethanol at elevate temperature and the kinetics of one-pot preparation of ethyl levulinate from cellulose. Renew. Energy 2015, 78, 583–589. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, H.; Liu, Y.; Sun, X.; Zhang, D.; Xue, D. Hydrophobic precipitation of carbonaceous spheres from fructose by a hydrothermal process. Carbon 2012, 50, 2155–2161. [Google Scholar] [CrossRef]
- Alakhras, F.; Holze, R. In situ UV-vis-and FT-IR-spectroscopy of electrochemically synthesized furan–thiophene copolymers. Synth. Met. 2007, 157, 109–119. [Google Scholar] [CrossRef]
- Vaz, P.; Ribeiro-Claro, P. C–H···O hydrogen bonds in liquid cyclohexanone revealed by the vC=O splitting and the vC–H blue shift. J. Raman Spectrosc. 2003, 34, 863–867. [Google Scholar] [CrossRef]
- Gandini, A.; Belgacem, M. Furans in polymer chemistry. Prog. Polym. Sci. 1997, 22, 1203–1379. [Google Scholar] [CrossRef]
- Pizzi, A.; Scharfetter, H. The chemistry and development of tannin-based adhesives for exterior plywood. J. Appl. Polym. Sci. 1978, 22, 1745–1761. [Google Scholar] [CrossRef]
- Kiatgrajai, P.; Wellons, J.; Gollob, L.; White, J. Kinetics of polymerization of (+)-catechin with formaldehyde. J. Org. Chem. 1982, 47, 2913–2917. [Google Scholar] [CrossRef]
- Grenier-Loustalot, M.; Larroque, S.; Grenier, P.; Bedel, D. Phenolic resins: 4. Self-condensation of methylolphenols in formaldehyde-free media. Polymer 1996, 37, 955–964. [Google Scholar] [CrossRef]
- Rego, R.; Adriaensens, P.; Carleer, R.; Gelan, J. Fully quantitative carbon-13 NMR characterization of resol phenol–formaldehyde prepolymer resins. Polymer 2004, 45, 33–38. [Google Scholar] [CrossRef]
- Tondi, G. Tannin-based copolymer resins: Synthesis and characterization by solid state 13C NMR and FT-IR spectroscopy. Polymers 2017, 9, 223. [Google Scholar] [CrossRef]
- Saeman, J. Kinetics of wood saccharification-hydrolysis of cellulose and decomposition of sugars in dilute acid at high temperature. Ind. Eng. Chem. Res. 1945, 37, 43–52. [Google Scholar] [CrossRef]




| Addition of Sulfuric Acid Solution (g) | Mixture Ratio of Tannin–Sucrose–Sulfuric Acid | Concentration (wt %) | Viscosity at 20 °C (mPa·s) | pH |
|---|---|---|---|---|
| 0 | 25:75:0 | 40 | 51.3 | 4.8 |
| 0.9 | 25:75:0.36 | 51.0 | 2.5 | |
| 1.25 | 25:75:0.50 | 50.4 | 2.0 | |
| 2.4 | 25:75:0.96 | 49.5 | 1.5 | |
| 10.4 | 25:75:4.16 | 47.3 | 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Miao, Y.; Yang, Z.; Wang, H.; Sang, R.; Fu, Y.; Huang, C.; Wu, Z.; Zhang, M.; Sun, S.; et al. Effects of Sulfuric Acid on the Curing Behavior and Bonding Performance of Tannin–Sucrose Adhesive. Polymers 2018, 10, 651. https://doi.org/10.3390/polym10060651
Zhao Z, Miao Y, Yang Z, Wang H, Sang R, Fu Y, Huang C, Wu Z, Zhang M, Sun S, et al. Effects of Sulfuric Acid on the Curing Behavior and Bonding Performance of Tannin–Sucrose Adhesive. Polymers. 2018; 10(6):651. https://doi.org/10.3390/polym10060651
Chicago/Turabian StyleZhao, Zhongyuan, Yanfeng Miao, Ziqian Yang, Hua Wang, Ruijuan Sang, Yanchun Fu, Caoxing Huang, Zhihui Wu, Min Zhang, Shijing Sun, and et al. 2018. "Effects of Sulfuric Acid on the Curing Behavior and Bonding Performance of Tannin–Sucrose Adhesive" Polymers 10, no. 6: 651. https://doi.org/10.3390/polym10060651






