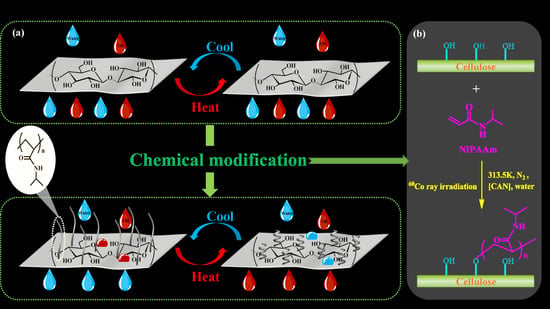
Thermo-Responsive Cellulose-Based Material with Switchable Wettability for Controllable Oil/Water Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Thermo-Responsive Cellulose-Based Material
2.3. Oil/Water Adsorption and Desorption Experiment
2.4. Oil/Water Separation Experiment
2.5. Physical and Chemical Characterization
3. Results and Discussion
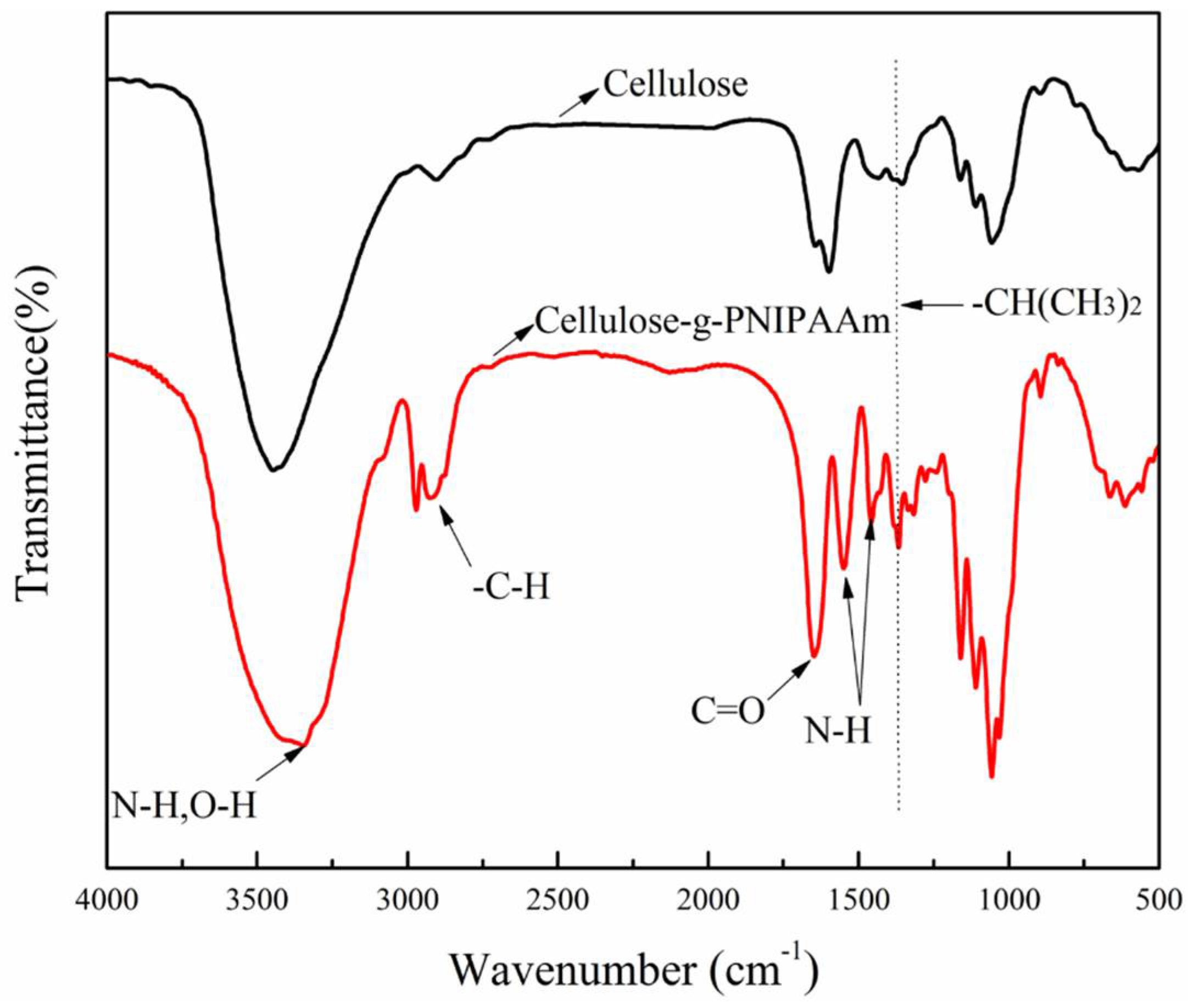
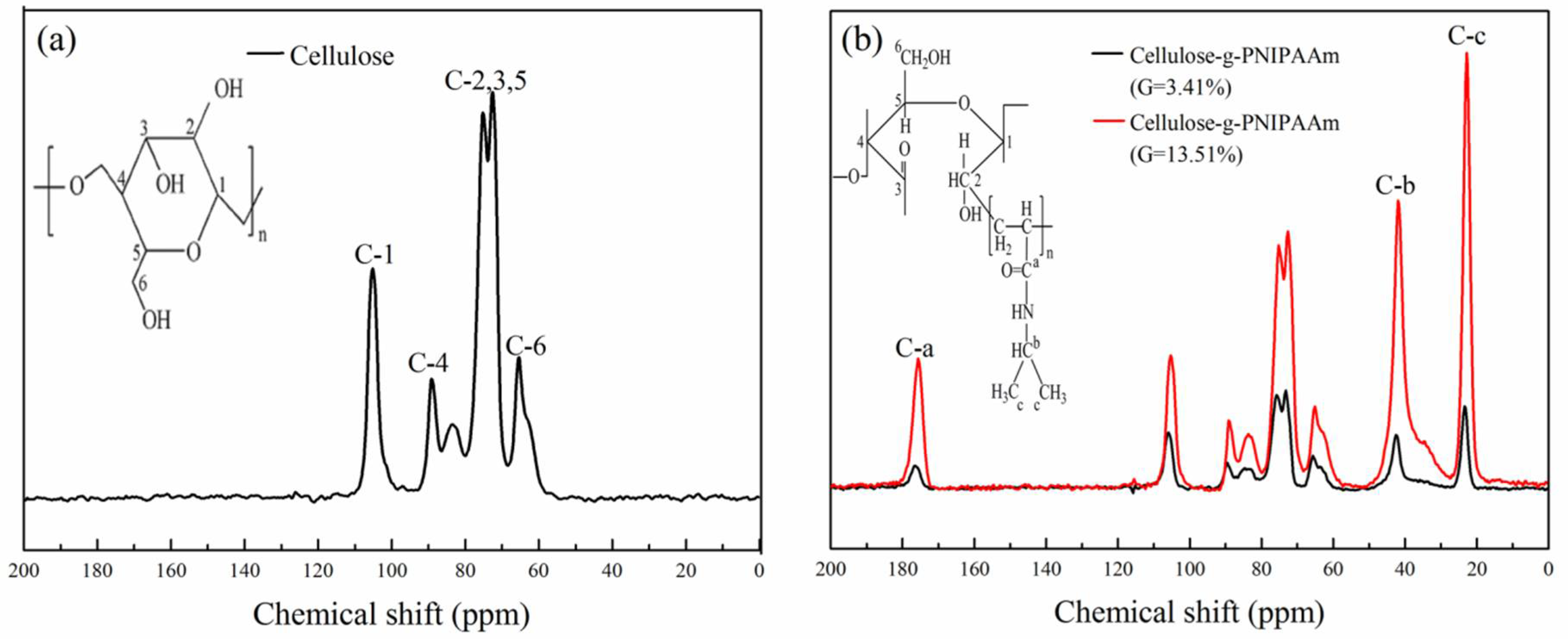
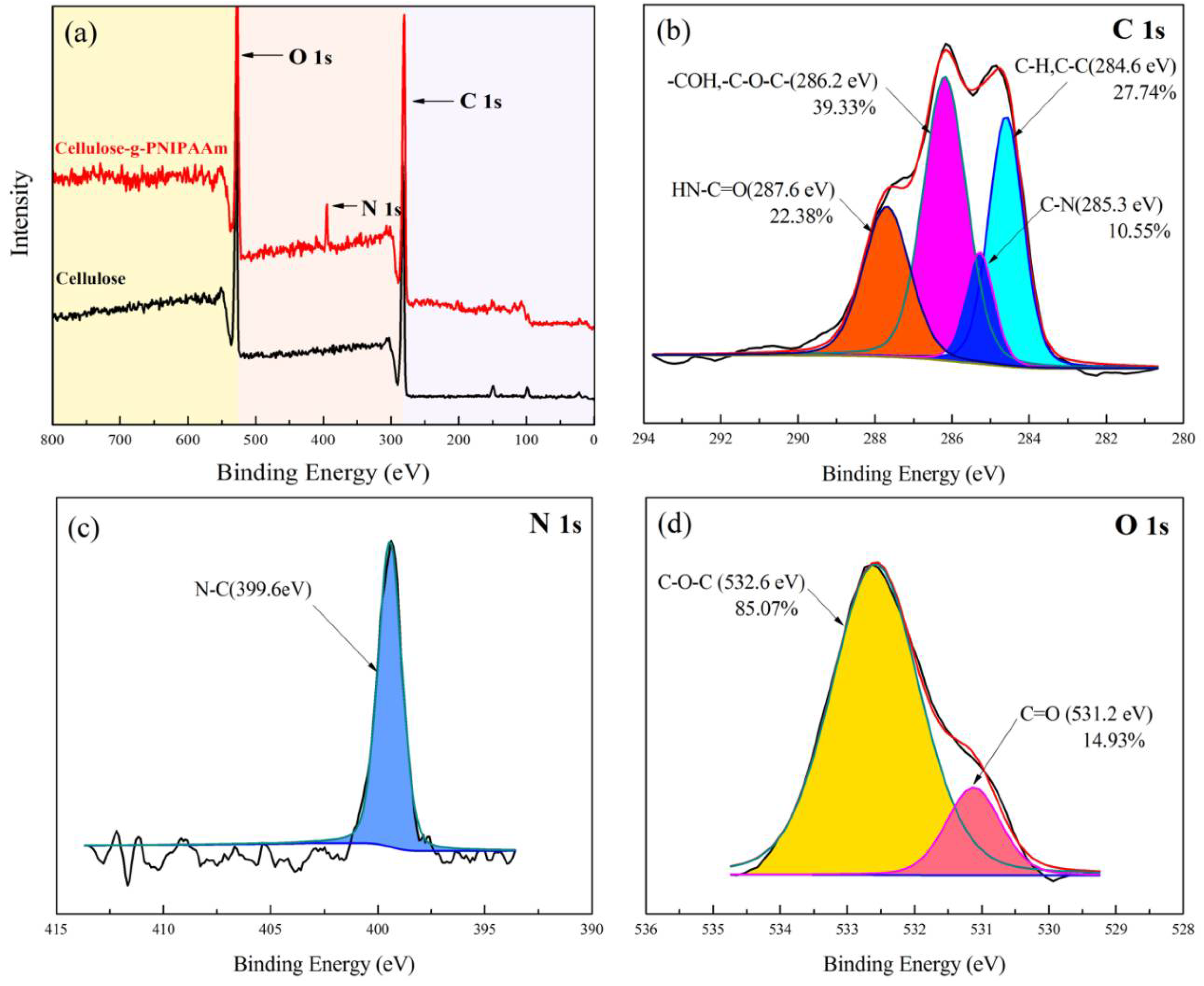
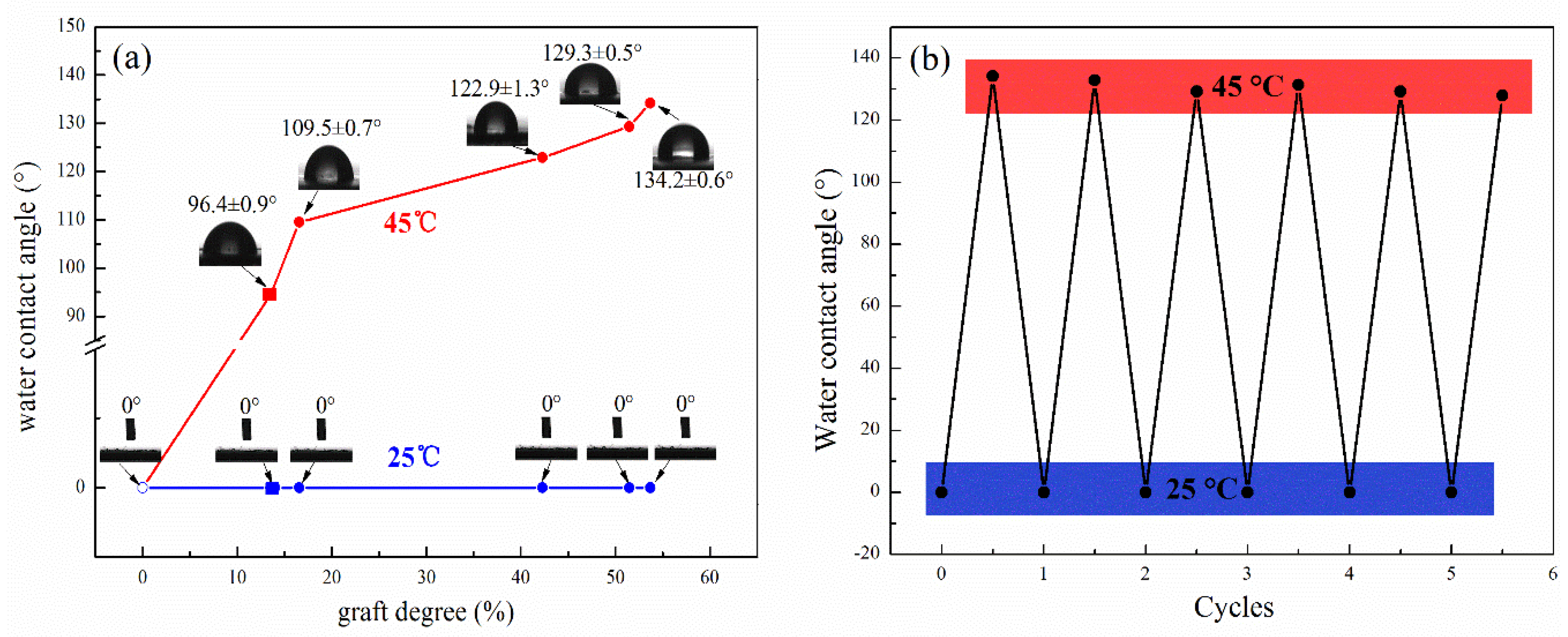
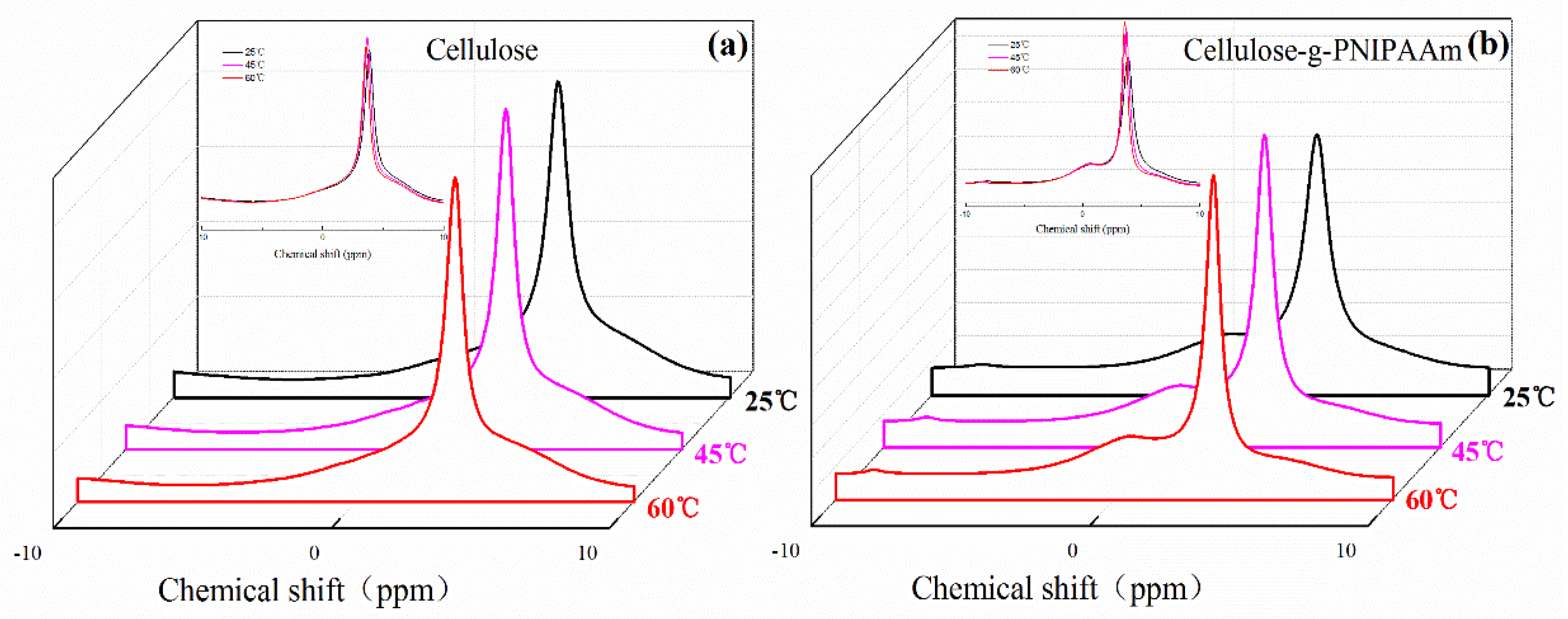
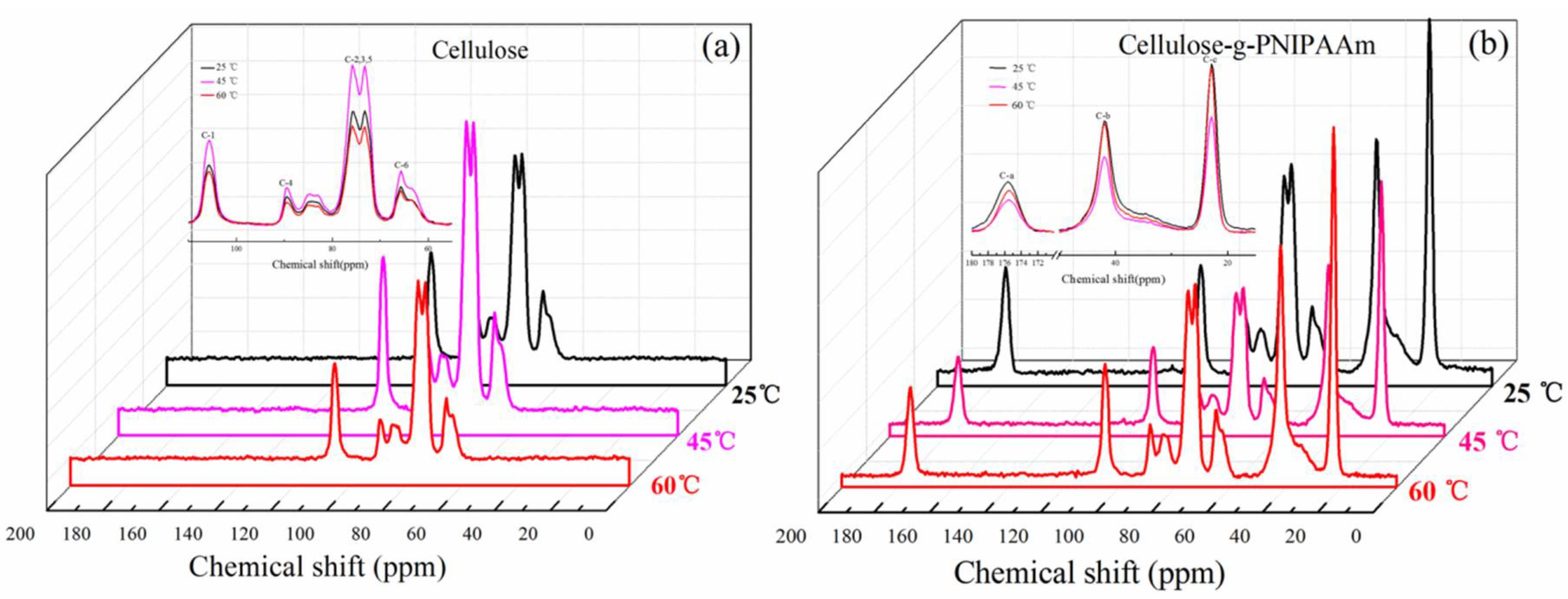
3.1. Chemical Structure and Thermal Stability of Cellulose-g-PNIPAAm
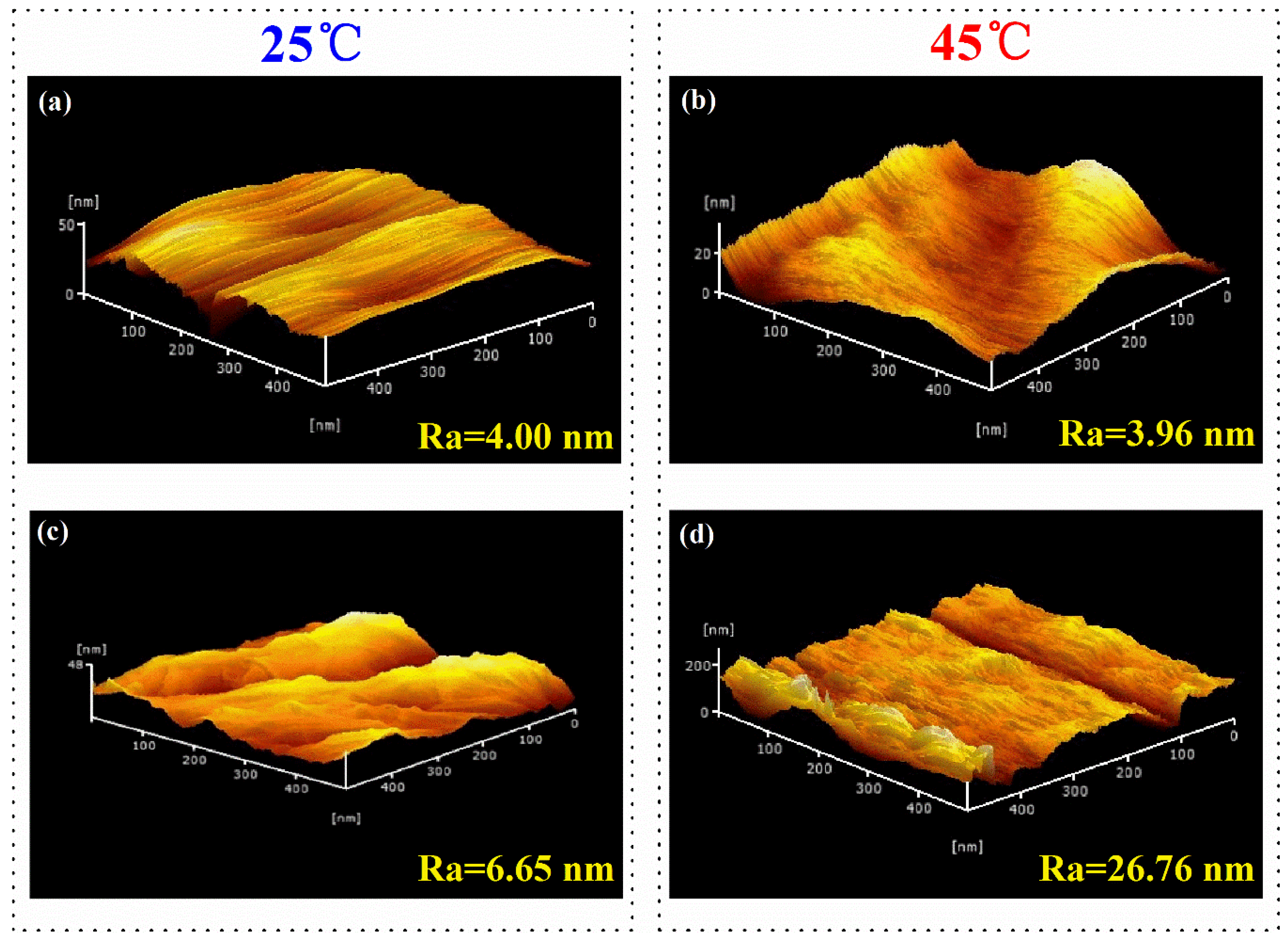
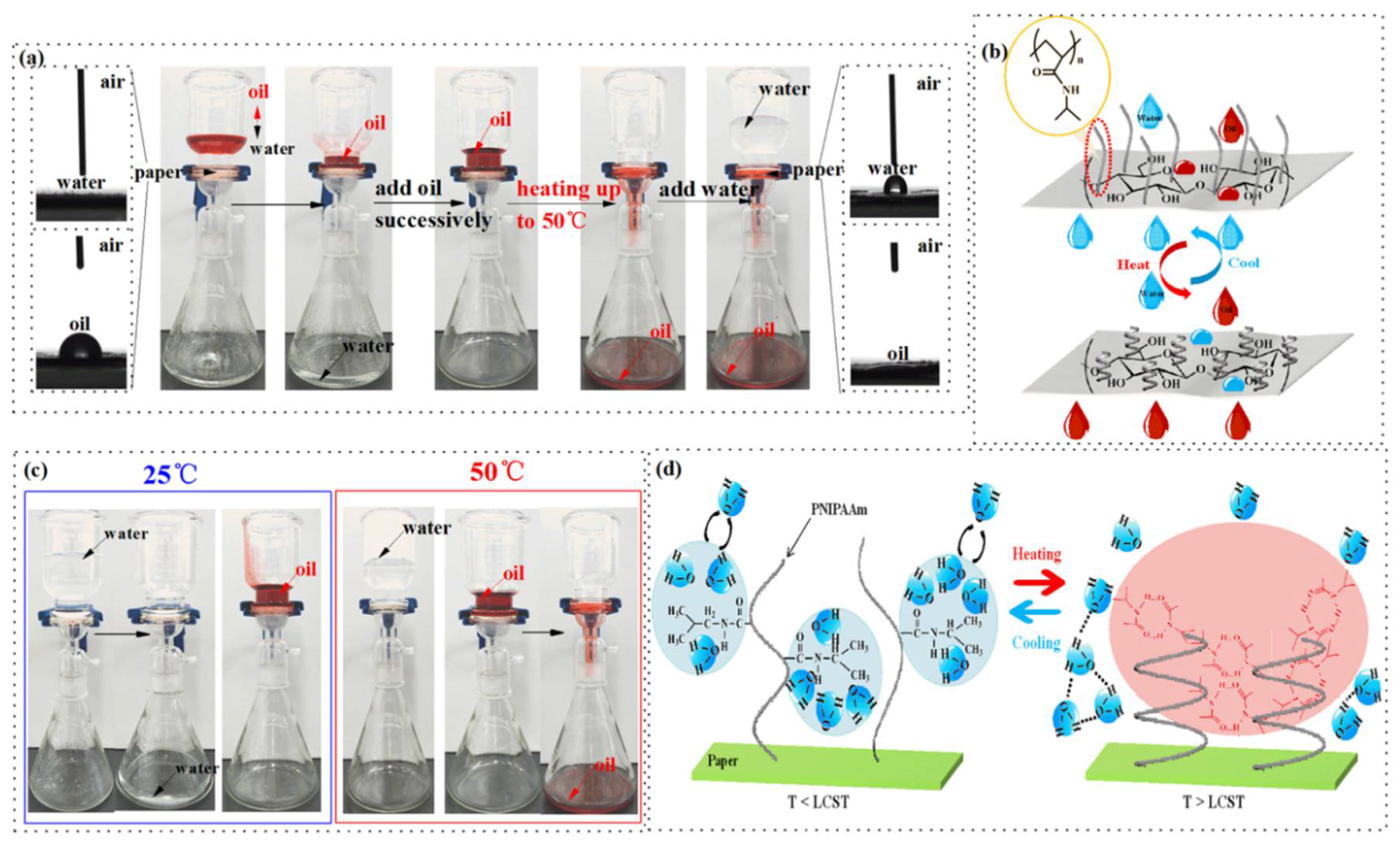
3.2. Thermo-Responsive Mechanism of Cellulose-g-PNIPAAm
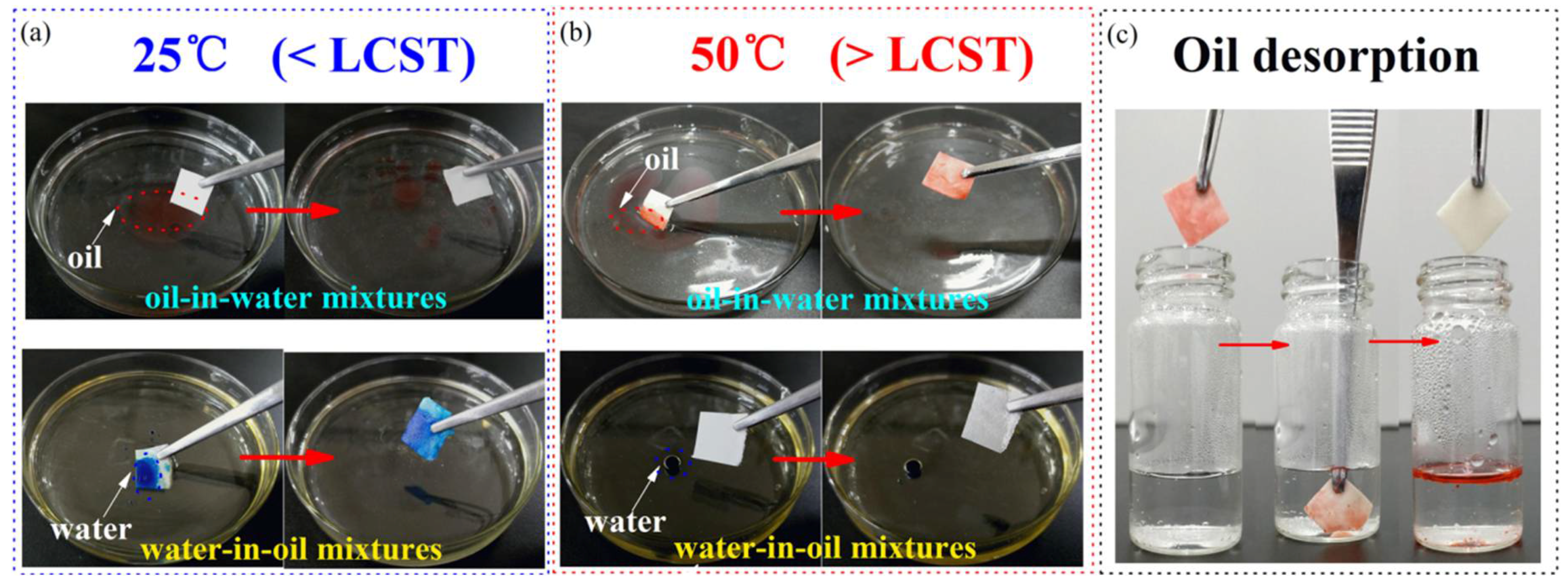
3.3. Controllable Oil/Water Adsorption Experiment
3.4. Controllable Oil/Water Separation Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Sun, Z.; Li, K.; Chen, Y.; Cao, Y.; Zhang, S.; Feng, L. Integrated oil separation and water purification by a double-layer tio 2-based mesh. Energy Environ. Sci. 2013, 6, 1147–1151. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Tai, N.-H.; Lee, S.-B.; Kuo, W.-S. Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ. Sci. 2012, 5, 7908–7912. [Google Scholar] [CrossRef]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef] [PubMed]
- Ou, R.; Wei, J.; Jiang, L.; Simon, G.P.; Wang, H. Robust thermoresponsive polymer composite membrane with switchable superhydrophilicity and superhydrophobicity for efficient oil–water separation. Environ. Sci. Technol. 2016, 50, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, C.; Zhang, Y.; Wang, R.; Zha, F.; She, H. Robust superhydrophobic attapulgite coated polyurethane sponge for efficient immiscible oil/water mixture and emulsion separation. J. Mater. Chem. A 2016, 4, 15546–15553. [Google Scholar] [CrossRef]
- Matsubayashi, T.; Tenjimbayashi, M.; Komine, M.; Manabe, K.; Shiratori, S. Bioinspired hydrogel-coated mesh with superhydrophilicity and underwater superoleophobicity for efficient and ultrafast oil/water separation in harsh environments. Ind. Eng. Chem. Res. 2017, 56, 7080–7085. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Dual wet and dry resilient cellulose II fibrous aerogel for hydrocarbon–water separation and energy storage applications. ACS Omega 2018, 3, 3530–3539. [Google Scholar] [CrossRef]
- Xue, Z.; Cao, Y.; Liu, N.; Feng, L.; Jiang, L. Special wettable materials for oil/water separation. J. Mater. Chem. A 2014, 2, 2445–2460. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, P.; Chen, P.; Zhang, L.; Wang, H.; Dai, L.; Song, L.; Huang, Y.; Zhang, J.; Chen, T. Functionalization of biodegradable pla nonwoven fabric as superoleophilic and superhydrophobic material for efficient oil absorption and oil/water separation. ACS Appl. Mater. Interfaces 2017, 9, 5968–5973. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Sharifnia, S. Fabrication of robust and durable superhydrophobic fiberglass fabrics for oil–water separation based on self-assembly of novel N-Tespo and N-TESPS reagents. J. Mater. Chem. A 2017, 5, 680–688. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, J.; Wang, F.; Li, Z.; Yu, J.; Ding, B. Superhydrophilic and underwater superoleophobic nanofibrous membrane with hierarchical structured skin for effective oil-in-water emulsion separation. J. Mater. Chem. A 2017, 5, 497–502. [Google Scholar] [CrossRef]
- Luo, Z.-Y.; Lyu, S.-S.; Wang, Y.-Q.; Mo, D.-C. Fluorine-induced superhydrophilic ti foam with surface nanocavities for effective oil-in-water emulsion separation. Ind. Eng. Chem. Res. 2017, 56, 699–707. [Google Scholar] [CrossRef]
- Guo, F.; Guo, Z. Inspired smart materials with external stimuli responsive wettability: A review. RSC Adv. 2016, 6, 36623–36641. [Google Scholar] [CrossRef]
- Chen, F.; Song, J.; Liu, Z.; Liu, J.; Zheng, H.; Huang, S.; Sun, J.; Xu, W.; Liu, X. Atmospheric pressure plasma functionalized polymer mesh: An environmentally friendly and efficient tool for oil/water separation. ACS Sustain. Chem. Eng. 2016, 4, 6828–6837. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Beija, M.; Marty, J.-D.; Destarac, M. Thermoresponsive poly(N-vinyl caprolactam)-coated gold nanoparticles: Sharp reversible response and easy tunability. Chem. Commun. 2011, 47, 2826–2828. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zhang, G.; Deng, Y.; Wang, C. Thermoresponsive melamine sponges with switchable wettability by interface-initiated atom transfer radical polymerization for oil/water separation. ACS App. Mater. Interfaces 2017, 9, 8967–8974. [Google Scholar] [CrossRef] [PubMed]
- Riskin, M.; Basnar, B.; Huang, Y.; Willner, I. Magnetoswitchable charge transport and bioelectrocatalysis using maghemite-au core-shell nanoparticle/polyaniline composites. Adv. Mater. 2007, 19, 2691–2695. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, S.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A robust absorbent material based on light-responsive superhydrophobic melamine sponge for oil recovery. Adv. Mater. Interfaces 2016, 3. [Google Scholar] [CrossRef]
- Zhou, X.; He, C. Tailoring the surface chemistry and morphology of glass fiber membranes for robust oil/water separation using poly(dimethylsiloxanes) as hydrophobic molecular binders. J. Mater. Chem. A 2018, 6, 607–615. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, G.; Hu, J.; Lu, X.; Li, J. Temperature, ionic strength and ph induced electrochemical switching of smart polymer interfaces. Chem. Commun. 2006, 4820–4822. [Google Scholar] [CrossRef]
- Ulijn, R.V. Enzyme-responsive materials: A new class of smart biomaterials. J. Mater. Chem. 2006, 16, 2217–2225. [Google Scholar] [CrossRef]
- Miyata, T.; Asami, N.; Uragami, T. A reversibly antigen-responsive hydrogel. Nature 1999, 399, 766. [Google Scholar] [CrossRef] [PubMed]
- Hemraz, U.D.; Lu, A.; Sunasee, R.; Boluk, Y. Structure of poly(N-isopropylacrylamide) brushes and steric stability of their grafted cellulose nanocrystal dispersions. J. Colloid Interface Sci. 2014, 430, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Hufendiek, A.; Trouillet, V.; Meier, M.A.; Barner-Kowollik, C. Temperature responsive cellulose-graft-copolymers via cellulose functionalization in an ionic liquid and raft polymerization. Biomacromolecules 2014, 15, 2563–2572. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Ponce-Vargas, S.M.; Licea-Claverie, A.; Steinbach, C. Chitosan and mixtures with aqueous biocompatible temperature sensitive polymer as flocculants. Colloids Surf. A Physicochem. Eng. Asp. 2012, 413, 7–12. [Google Scholar] [CrossRef]
- Ning, W.; Shang, P.; Wu, J.; Shi, X.; Liu, S. Novel amphiphilic, biodegradable, biocompatible, thermo-responsive aba triblock copolymers based on pcl and peg analogues via a combination of rop and raft: Synthesis, characterization, and sustained drug release from self-assembled micelles. Polymers 2018, 10, 214. [Google Scholar] [CrossRef]
- Gao, L.; Kong, T.; Huo, Y. Dual thermoresponsive and ph-responsive poly(vinyl alcohol) derivatives: Synthesis, phase transition study, and functional applications. Macromolecules 2016, 49, 7478–7489. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Don, T.-M.; Lu, K.-Y.; Lin, L.-J.; Hsu, C.-H.; Wu, J.-Y.; Mi, F.-L. Temperature/pH/Enzyme triple-responsive cationic protein/PAA-b-PNIPAAm nanogels for controlled anticancer drug and photosensitizer delivery against multidrug resistant breast cancer cells. Mol. Pharm. 2017, 14, 4648–4660. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Grailer, J.J.; Steeber, D.A.; Gong, S. Stimuli-responsive chitosan-graft-poly(N-vinylcaprolactam) as a promising material for controlled hydrophobic drug delivery. Macromol. Biosci. 2008, 8, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Zubik, K.; Singhsa, P.; Wang, Y.; Manuspiya, H.; Narain, R. Thermo-responsive poly(N-isopropylacrylamide)-cellulose nanocrystals hybrid hydrogels for wound dressing. Polymers 2017, 9, 119. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/water separation with selective superantiwetting/superwetting surface materials. Angew. Chem. Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-N.; Li, J.-J.; Luo, Z.-H. Photoatrp-based fluorinated thermosensitive block copolymer for controllable water/oil separation. Ind. Eng. Chem. Res. 2015, 54, 10714–10722. [Google Scholar] [CrossRef]
- Song, W.; Xia, F.; Bai, Y.; Liu, F.; Sun, T.; Jiang, L. Controllable water permeation on a poly(N-isopropylacrylamide)-modified nanostructured copper mesh film. Langmuir 2007, 23, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Liu, R.; Huang, Y. Graft modification of cellulose: Methods, properties and applications. Polymer 2015, 70, A1–A16. [Google Scholar] [CrossRef]
- Wang, B.; Yang, D.; Zhang, H.-R.; Huang, C.; Xiong, L.; Luo, J.; Chen, X.-D. Preparation of esterified bacterial cellulose for improved mechanical properties and the microstructure of isotactic polypropylene/bacterial cellulose composites. Polymers 2016, 8, 129. [Google Scholar] [CrossRef]
- Alosmanov, R.; Wolski, K.; Zapotoczny, S. Grafting of thermosensitive poly(N-isopropylacrylamide) from wet bacterial cellulose sheets to improve its swelling-drying ability. Cellulose 2017, 24, 285–293. [Google Scholar] [CrossRef]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef]
- Garcia-Valdez, O.; Champagne, P.; Cunningham, M.F. Graft modification of natural polysaccharides via reversible deactivation radical polymerization. Prog. Polym. Sci. 2017. [CrossRef]
- Gupta, K.; Khandekar, K. Temperature-responsive cellulose by ceric (iv) ion-initiated graft copolymerization of N-isopropylacrylamide. Biomacromolecules 2003, 4, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xue, F.; Ding, E. Cellulose nanocrystals grafted with polyacrylamide assisted by macromolecular raft agents. Cellulose 2016, 23, 3717–3735. [Google Scholar] [CrossRef]
- Liu, R.; Fraylich, M.; Saunders, B.R. Thermoresponsive copolymers: From fundamental studies to applications. Colloid Polym. Sci. 2009, 287, 627–643. [Google Scholar] [CrossRef]
- Tian, D.; Li, T.; Zhang, R.; Wu, Q.; Chen, T.; Sun, P.; Ramamoorthy, A. Conformations and intermolecular interactions in cellulose/silk fibroin blend films: A solid-state nmr perspective. J. Phys. Chem. B 2017, 121, 6108–6116. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xiao, T.; Fu, X.-B.; Jiang, T.-T.; Chen, Y.; Yao, Y.-F. Thermoresponsive hyperbranched polymers with spatially isomerized groups: Nmr implication to their thermoresponsive behaviors. Macromolecules 2017, 50, 9647–9655. [Google Scholar] [CrossRef]
- Yang, H.; Esteves, A.C.C.; Zhu, H.; Wang, D.; Xin, J.H. In-situ study of the structure and dynamics of thermo-responsive pnipaam grafted on a cotton fabric. Polymer 2012, 53, 3577–3586. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; He, H.; Zhu, H.; Cheng, M.; Li, Y.; Wang, S. Thermo-Responsive Cellulose-Based Material with Switchable Wettability for Controllable Oil/Water Separation. Polymers 2018, 10, 592. https://doi.org/10.3390/polym10060592
Chen W, He H, Zhu H, Cheng M, Li Y, Wang S. Thermo-Responsive Cellulose-Based Material with Switchable Wettability for Controllable Oil/Water Separation. Polymers. 2018; 10(6):592. https://doi.org/10.3390/polym10060592
Chicago/Turabian StyleChen, Wenbo, Hui He, Hongxiang Zhu, Meixiao Cheng, Yunhua Li, and Shuangfei Wang. 2018. "Thermo-Responsive Cellulose-Based Material with Switchable Wettability for Controllable Oil/Water Separation" Polymers 10, no. 6: 592. https://doi.org/10.3390/polym10060592