Novel Bacterial Cellulose/Gelatin Hydrogels as 3D Scaffolds for Tumor Cell Culture
Abstract
1. Introduction
2. Experiment, Materials, and Methods
2.1. Materials
2.2. Preparation of BC and BC/Gelatin Scaffolds
2.3. Characterization of Materials
2.4. Cell Study
2.5. Statistical Analysis
3. Results and Discussion
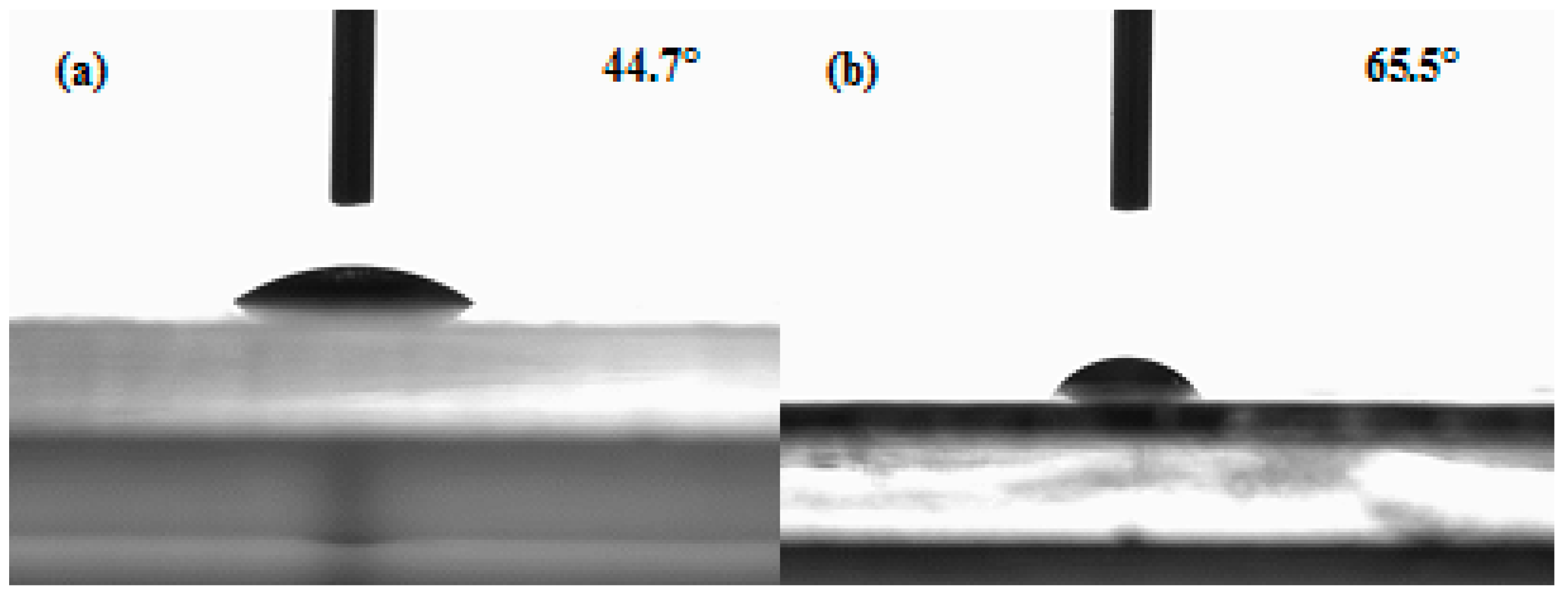
3.1. Morphology of BC and BC/Gelatin Hydrogel
3.2. Chemical and Surface Structure of BC and BC/Gelatin Hydrogel
3.3. Mechanical Properties of BC and BC/Gelatin Samples
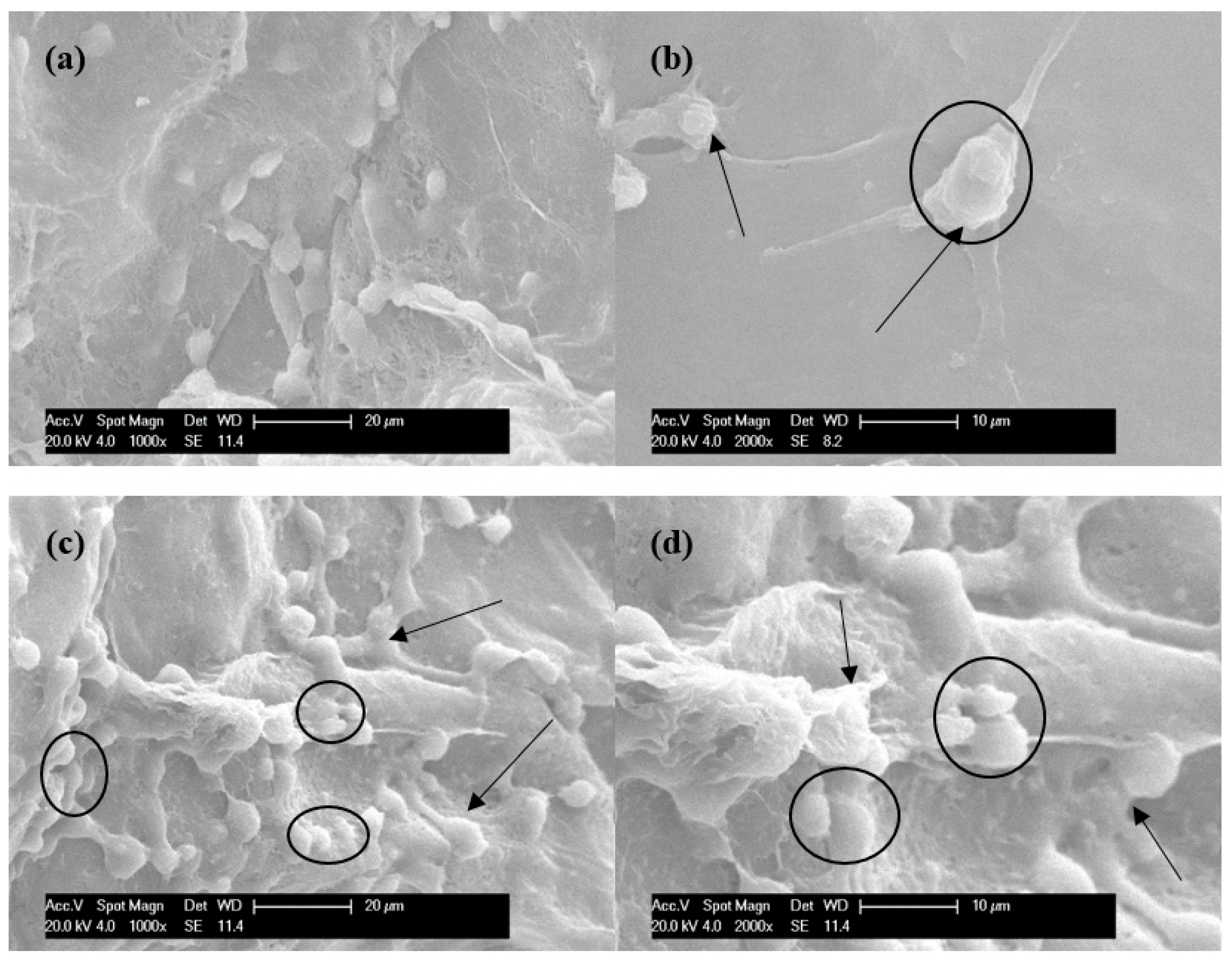
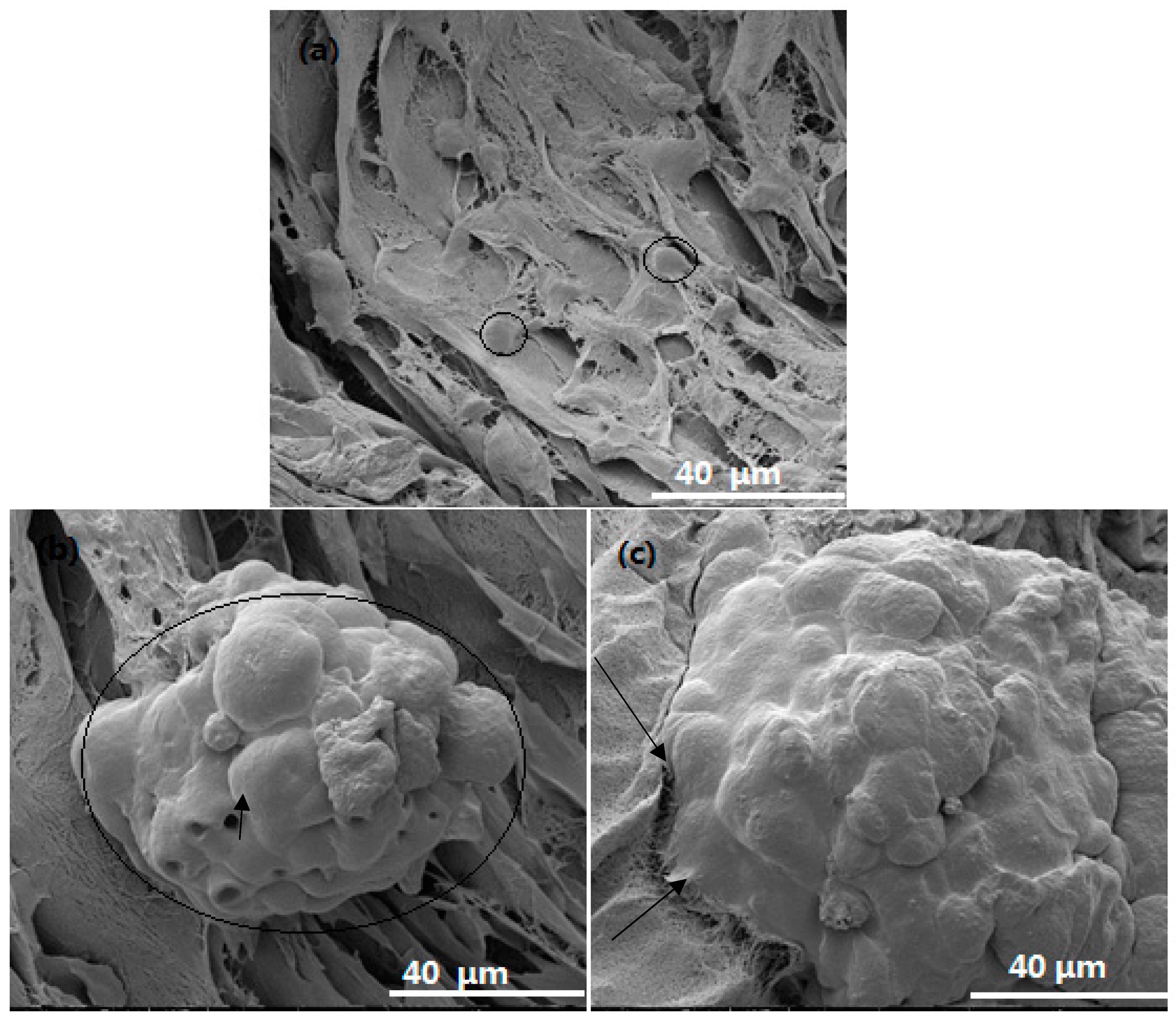
3.4. Cell Viability, Proliferation and Morphology in Scaffolds
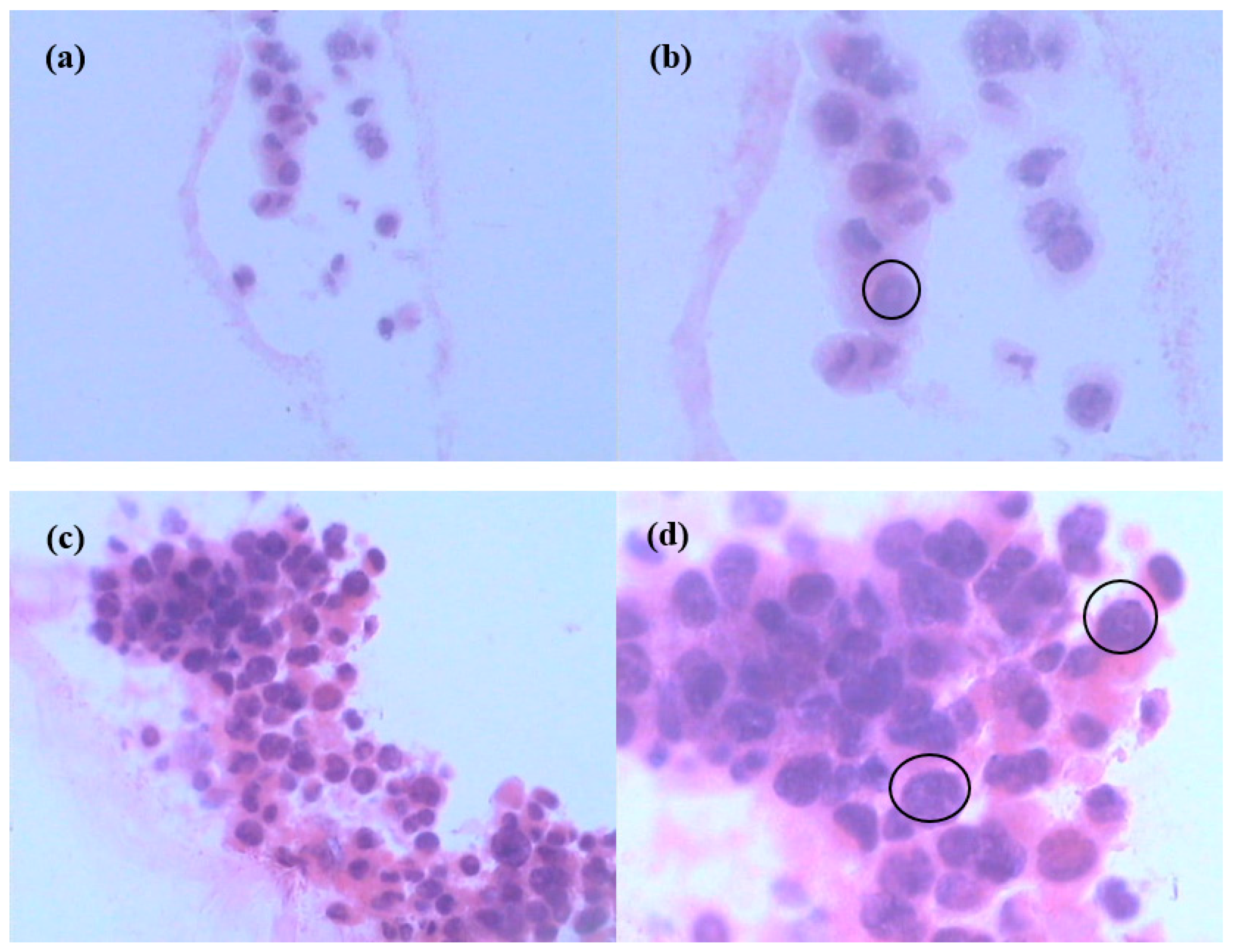
3.6. Hematoxylin-Eosin (H&E) Staining of Cells in Scaffolds
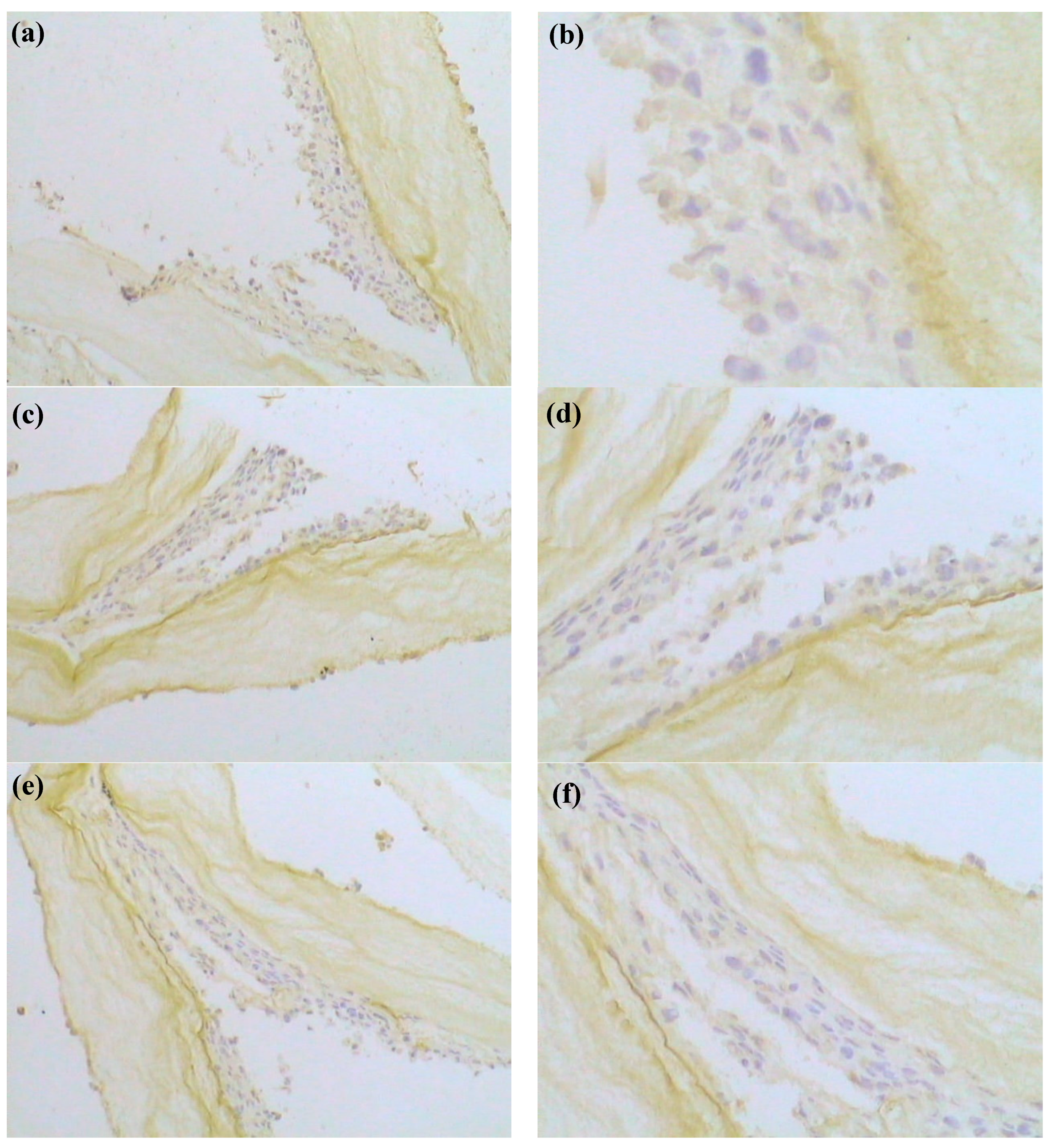
3.7. Immunohistochemistry (IHC) of Cells in Scaffold
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Phillips, T.M.; McBride, W.H.; Pajonk, F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J. Nanl. Cancer Inst. 2006, 98, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Kamangar, F.; Dores, G.M.; Anderson, W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006, 24, 2137–2150. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell. Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Horning, J.L.; Sahoo, S.K.; Vijayaraghavalu, S.; Dimitrijevic, S.; Vasir, J.K.; Jain, T.K.; Panda, A.K.; Labhasetwar, V. 3-D tumor model for in vitro evaluation of anticancer drugs. Mol. Pharmaceut. 2008, 5, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Mandal, M.; Hutmacher, D.W.; Russell, P.J.; Soekmadji, C.; Kundu, S.C. Engineered silk fibroin protein 3D matrices for in vitro tumor model. Biomaterials 2011, 32, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.L.; Wen, F.; Gao, S.J.; Ang, M.Y.; Pallathadka, P.K.; Liu, L.H.; Yu, H. Preparation of three-dimensional interconnected macroporous cellulosic hydrogels for soft tissue engineering. Biomaterials 2010, 31, 8141–8152. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Kenny, P.A.; Lee, E.H.; Bissell, M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 2007, 4, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiao, Z.; Meng, Y.; Zhao, Y.; Han, J.; Su, G.; Chen, B.; Dai, J. The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials 2012, 33, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.H.; Gazda, L.S.; Conn, B.L.; Jain, K.; Asina, S.; Levine, D.M.; Parker, T.S.; Laramore, M.A.; Martis, P.C.; Vinerean, H.V.; et al. Three-dimensional culture of mouse renal carcinoma cells in agarose macrobeads selects for a subpopulation of cells with cancer stem cell or cancer progenitor properties. Cancer Res. 2011, 71, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Panda, A.K.; Labhasetwar, V. Characterization of porous PLGA/PLA microparticles as a scaffold for three dimensional growth of breast cancer cells. Biomacromolecules 2005, 6, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Szot, C.S.; Buchanan, C.F.; Gatenholm, P.; Rylander, M.N.; Freeman, J.W. Investigation of cancer cell behavior on nanofibrous scaffolds. Mater. Sci. Eng. C 2011, 31, 37–42. [Google Scholar] [CrossRef]
- Gatenholm, P.; Klemm, D. Bacterial nanocellulose as a renewable material for biomedical applications. MRS Bull. 2010, 35, 208–213. [Google Scholar] [CrossRef]
- Hanif, U.; Fazli, W.; Helder, AS.; Taous, K. Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohyd. Polym. 2016, 150, 330–352. [Google Scholar]
- Helida Gomes, O.B.; Robson Rosa, S.; Hernane, S.B.; Agnieszka, T.; Junkal, G.; Wilton, R.L.; Junior, O.B.O.; Ribeiro, S.J.L. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohyd. Polym. 2016, 153, 406–420. [Google Scholar]
- Foresti, M.L.; Vazquez, A.; Boury, B. Applications of bacterial cellulose as precursor of carbon and composites with metal oxide, metal sulfide and metal nanoparticles: A review of recent advances. Carbohyd. Polym. 2017, 157, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Brackmann, C.; Bodin, A.; Keson, M.; Gatenholm, P.; Enejder, A. Visualization of the cellulose biosynthesis and cell integration into cellulose scaffolds. Biomacromolecules 2010, 11, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.; Faxalv, L.; Molnar, G.F.; Drotz, K.; Risberg, B.; Lindahl, T.L.; Sellborn, A. Real-time measurements of coagulation on bacterial cellulose and conventional vascular graft materials. Acta Biomater. 2010, 6, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Zaborowska, M.; Bodin, A.; Bakdahl, H.; Popp, J.; Goldstein, A.; Gatenholm, P. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010, 6, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Catchmark, J.H.; Zhu, Y.J.; Abidi, N.; Zhou, X.; Wang, J.H.; Liang, N.Y. Engineering of porous bacterial cellulose toward human fibroblasts ingrowth for tissue engineering for tissue engineering. J. Mater. Res. 2014, 29, 2682–2693. [Google Scholar] [CrossRef]
- Bakdahl, H.; Esguerra, M.; Delbro, D.; Risberg, B.; Gatenholm, P. Engineering microporosity in bacterial cellulose scaffolds. J. Tissue Eng. Regen. Med. 2008, 2, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.Y.; Luo, H.L.; Gu, F.; Zhang, J.; Hu, D. A novel in vitro three-dimensional macroporous scaffolds from bacterial cellulose for culture of breast cancer cells. J. Biomater. Nanobiotechnol. 2013, 4, 316–326. [Google Scholar] [CrossRef]
- Xiong, G.Y.; Luo, H.L.; Zhu, Y. Creation of macropores in three-dimensional bacterial cellulose scaffold for potential cancer cell culture. Carbohyd. Polym. 2014, 114, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, Q.; Nakamoto, T.; Kawazoe, N.; Chen, G. Gelatin Scaffolds with Controlled Pore Structure and Mechanical Property for Cartilage Tissue Engineering. Tissue Eng. Part. C Methods 2016, 22, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Vlierberghe, S.V. Crosslinking strategies for porous gelatin scaffolds. J. Mater. Sci. 2016, 51, 4349–4357. [Google Scholar] [CrossRef]
- Wang, J.; Luo, H.L.; Gao, C.; Huang, Y. Immobilization of gelatin on bacterial cellulose nanofibers surface via crosslinking technique. Mater. Sci. Eng. C 2012, 32, 536–541. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Hossana, M.J.; Gafurb, M.A.; Kadirb, R.; Karima, M.M. Preparation and characterization of gelatin-hydroxyapatite composite for bone tissue engineering. J. Int. Eng. Technol. 2012, 14, 24–33. [Google Scholar]
- Saska, S.; Teixeira, L.N.; de Oliveira, P.T.; Gaspar, A.M.M.; Ribeiro, S.J.L.; Messaddeq, Y.; Marchetto, R. Bacterial cellulose-collagen nanocomposite for bone tissue engineering. J. Mater. Chem. 2012, 22, 22102–22112. [Google Scholar] [CrossRef]
- Liao, G.Y.; Zhou, X.P.; Xie, X.L.; Mai, Y.W. Electrospun polymer scaffolds: Their biomedical and mechanical properties. In Biomaterials for Implants and Scaffolds; Springer: Berlin/Heidelberg, Germany, 2017; pp. 237–270. [Google Scholar]
- DeBaradinis, R.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Nio, K.; Yamashita, T.; Kaneko, S. The evolving concept of liver cancer stem cells. Mol. Cancer 2017, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Kievit, F.M.; Florczyk, S.J.; Leung, M.C.; Veiseh, O.; Park, J.O.; Disis, M.L.; Zhang, M. Chitosan-alginate 3d scaffolds as a mimic of the glioma tumor microenvi-ronment. Biomaterials 2010, 31, 5903–5910. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.; Ma, J.M.; Bryan, L.; Jemal, A. Breast Cancer Statistics, 2013. CA Cancer J. Clin. 2014, 64, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.M.; Suresh, P.K.; Pai, R.R. Clinicopathological features of triple negative breast carcinoma. J. Clin. Diagn. Res. 2017, 11, EC05–EC08. [Google Scholar] [CrossRef] [PubMed]
- Aljarroudi, O.; Abda, N.; Brahmi, S.A.; Afqir, S. Triple Negative Breast Cancer at the University Hospital Mohammed VI—Oujda. APJCP 2017, 18, 195–200. [Google Scholar]
- Thike, A.A.; Cheok, P.Y.; Jara-Lazaroar, A.R.; Tan, B.; Tan, P.; Tan, PH. Triple-negative breast cancer: Clinicopathological characteristics and relationship with basal-like breast cancer. Mod. Pathol. 2010, 23, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Yuka, S.; Hitoshi, T. Clinicopathological characteristics of triple-negative breast Cancers. Breast Cancer 2009, 16, 254–259. [Google Scholar]
- Goldhirsch, A.; Wood, W.; Celber, R.; Coats, A.; Thurlimann, B.; Senn, H.J. Progress and promise: Highlights of the international expert consensus on the primary therapy of early breast cancer. Ann. Oncol. 2007, 18, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Cronan, M.R.; Nakamura, K.; Johnson, N.L.; Granger, D.A.; Cuevas, B.D.; Wang, J.G.; Mackman, N.; Scott, J.E.; Dohlman, H.G.; Johnson, G.L. Defining MAP3Kinases required for MDA-MB-231 cell tumor growth and metastasis. Oncogene 2012, 31, 3889–3900. [Google Scholar] [CrossRef] [PubMed]
- Khadidiatou, G.; Shyam, A.P.; Steven, J.G.; Pranela, R.; Treena, L.A. Investigating breast cancer cell behavior using tissue engineering scaffolds. PLoS ONE 2015, 4, e0118724. [Google Scholar]
- Kim, H.J.; Cho, S.D.; Kim, J.; Kim, S.J.; Choi, C.S.; Kin, J.S.; Nam, J.-S.; Kwon, K.H.; Kang, K.-S.; Jung, J.-Y. Apoptotic effect of tolfenamic acid on MDAMB231 breast cancer cells and xenograft tumors. J. Clin. Biochem. Nutr. 2013, 53, 21–26. [Google Scholar] [CrossRef] [PubMed]



| Samples | Porosity (%) | Surface Area (m2/g) |
|---|---|---|
| BC | 92.1 | 91.5 |
| BC/gelatin | 86.1 | 84.3 |
| Samples | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) | p |
|---|---|---|---|---|
| BC | 0.600 ± 0.002 | 11.8 ± 0.6 | 6.3 ± 0.3 | — |
| BC/gelatin | 0.543 ± 0.003 | 10.4 ± 0.2 | 5.6 ± 0.3 | 0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhao, L.; Zhang, A.; Huang, Y.; Tavakoli, J.; Tang, Y. Novel Bacterial Cellulose/Gelatin Hydrogels as 3D Scaffolds for Tumor Cell Culture. Polymers 2018, 10, 581. https://doi.org/10.3390/polym10060581
Wang J, Zhao L, Zhang A, Huang Y, Tavakoli J, Tang Y. Novel Bacterial Cellulose/Gelatin Hydrogels as 3D Scaffolds for Tumor Cell Culture. Polymers. 2018; 10(6):581. https://doi.org/10.3390/polym10060581
Chicago/Turabian StyleWang, Jing, Li Zhao, Aixia Zhang, Yuan Huang, Javad Tavakoli, and Youhong Tang. 2018. "Novel Bacterial Cellulose/Gelatin Hydrogels as 3D Scaffolds for Tumor Cell Culture" Polymers 10, no. 6: 581. https://doi.org/10.3390/polym10060581
APA StyleWang, J., Zhao, L., Zhang, A., Huang, Y., Tavakoli, J., & Tang, Y. (2018). Novel Bacterial Cellulose/Gelatin Hydrogels as 3D Scaffolds for Tumor Cell Culture. Polymers, 10(6), 581. https://doi.org/10.3390/polym10060581







