Novel PEG-Modified Hybrid PLGA-Vegetable Oils Nanostructured Carriers for Improving Performances of Indomethacin Delivery
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Methods
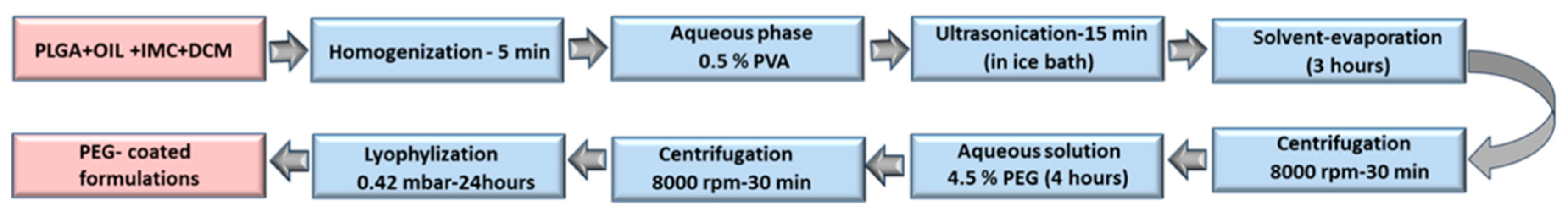
The Preparation of IMC-Loaded Hybrid Polymeric-Oil Nanoparticles and Standard Nanoparticles
2.3. Nanoparticles Surface Modification (Pegylation)
2.4. Characterization
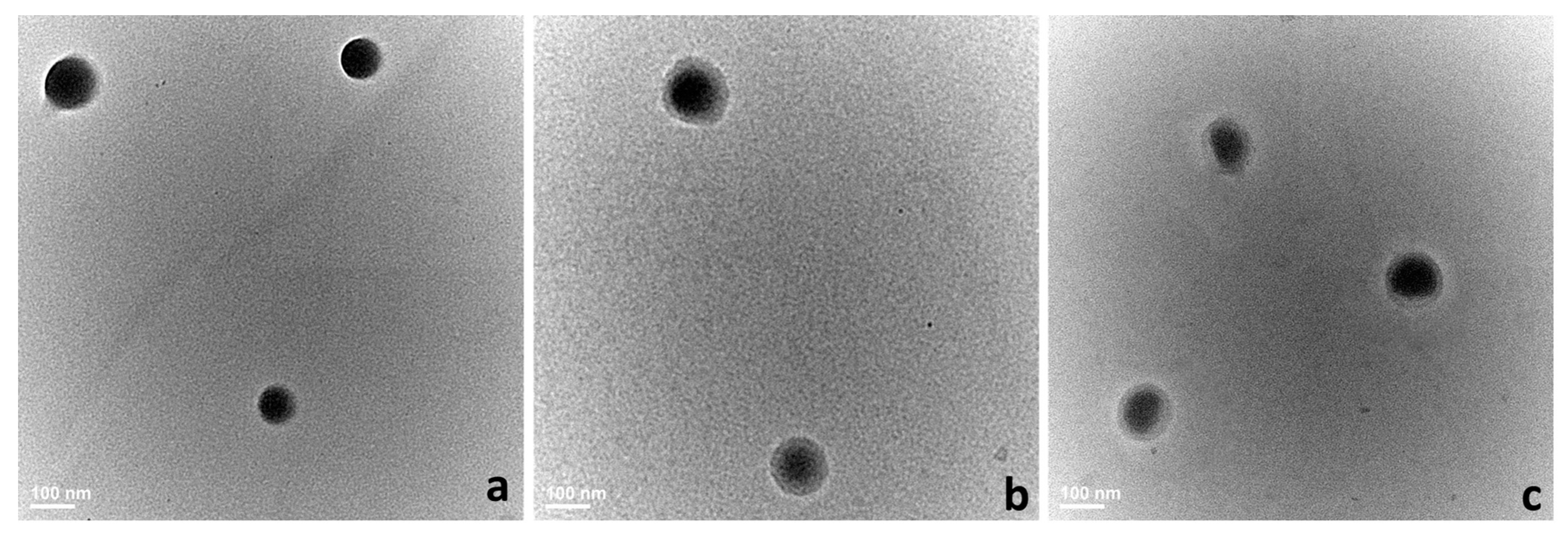
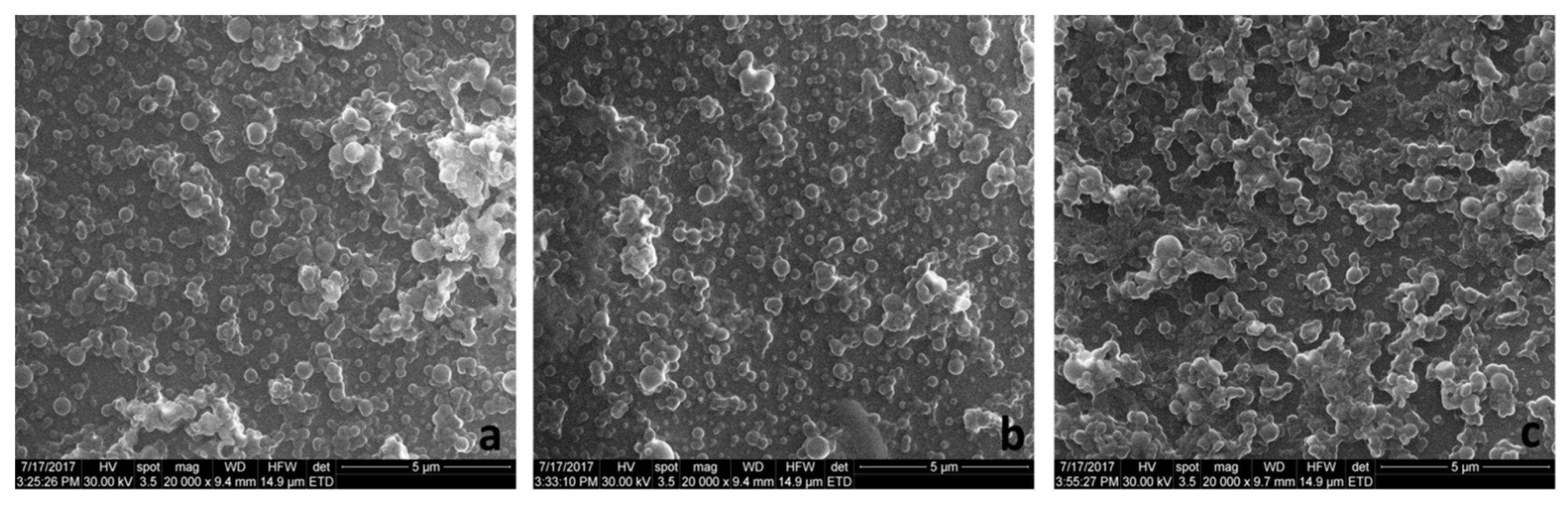
2.4.1. Size Distribution, Zeta Potential, and Morphology
2.4.2. Drug Loading (DL) and Encapsulation Efficiency (EE)
2.4.3. DSC Analyses
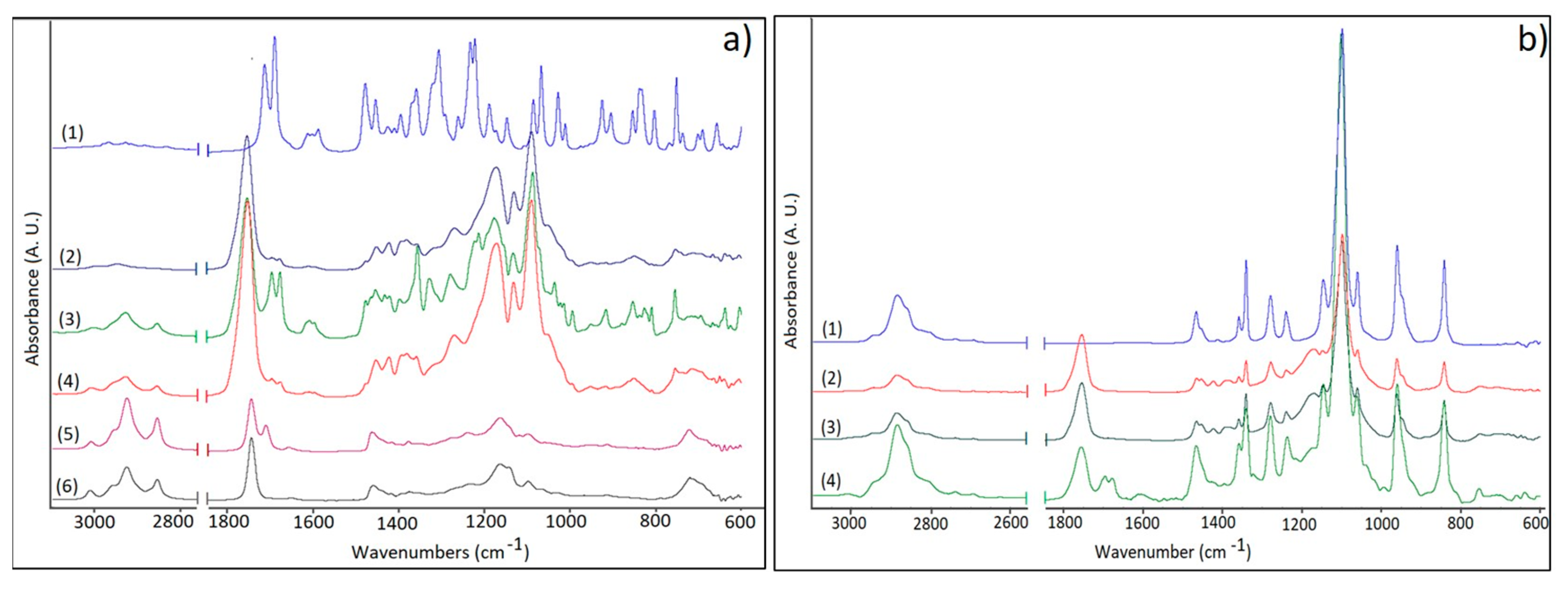
2.4.4. FTIR Measurements
2.4.5. Release Studies
2.4.6. In Vitro Drug Release Kinetics and Mechanism
2.4.7. Cytotoxicity Assay
2.4.8. Statistical Analyses
3. Results and Discussion
3.1. Morphology, Hydrodynamic Characteristics, DL, and EE
3.2. DSC Analyses
3.3. FTIR Studies
3.4. In Vitro Drug Release Studies
3.5. In Vitro Drug Release Kinetic Mechanisms
3.6. Cytotoxicity Studies
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pandita, D.; Kumar, S.; Lather, V. Hybrid poly(lactic-co-glycolic acid) nanoparticles: Design and delivery prospectives. Drug Discov. Today 2015, 20, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sangwana, P.; Lather, V.; Pandita, D. Biocompatible PLGA-oil hybrid nanoparticles for high loading and controlled delivery of resveratrol. J. Drug Deliv. Sci. Technol. 2015, 30, 54–62. [Google Scholar] [CrossRef]
- Narvekar, M.; Xue, H.Y.; Wong, H.L. A novel hybrid delivery system: Polymer-oil nanostructured carrier for controlled delivery of highly lipophilic drug all-trans-retinoic acid (ATRA). Int. J. Pharm. 2012, 436, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Narvekar, M.; Xue, H.Y.; Tran, N.T.; Mikhael, M.; Wong, H.L. A new nanostructured carrier design including oil to enhance the pharmaceutical properties of retinoid therapy and its therapeutic effects on chemo-resistant ovarian cancer. Eur. J. Pharm. Biopharm. 2014, 88, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Lacatusu, I.; Badea, N.; Badea, G.; Oprea, O.; Mihaila, M.A.; Kaya, M.A.; Stan, R.; Meghea, A. Lipid nanocarriers based on natural oils with high activity against oxygen free radicals and tumor cell proliferation. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Doolaanea, A.A.; Mansor, N.; Mohd Nor, N.H.; Farahidah, M. Co-encapsulation of Nigella sativa oil and plasmid DNA for enhanced gene therapy of Alzheimer’s disease. J. Microencapsul. 2016, 33, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Sah, H.; Thoma, L.A.; Desu, H.R.; Sah, E.; Wood, G.C. Concepts and practices used to develop functional PLGA-based nanoparticulate systems. Int. J. Nanomed. 2013, 8, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xua, Q.; Kima, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.; Vega, E.; Pérez, Y.; Gómara, M.J.; García, M.L.; Haro, I. Conjugation of cell-penetrating peptides with poly(lactic-co-glycolic acid)-polyethylene glycol nanoparticles improves ocular drug delivery. Int. J. Nanomed. 2015, 27, 609–631. [Google Scholar] [CrossRef]
- Xu, Q.; Ensign, L.M.; Boylan, N.J.; Schön, A.; Gong, X.; Yang, J.C.; Lamb, N.W.; Cai, S.; Yu, T.; Freire, E.; et al. Impact of Surface Polyethylene Glycol (PEG) Density on Biodegradable Nanoparticle Transport in Mucus Ex Vivo and Distribution In Vivo. ACS Nano 2015, 9, 9217–9227. [Google Scholar] [CrossRef] [PubMed]
- Duran-Lobato, M.; Martin-Banderas, L.; Goncalves, L.M.D.; Fernandez-Arevalo, M.; Almeida, A.J. Comparative study of chitosan- and PEG-coated lipid and PLGA nanoparticles as oral delivery systems for cannabinoids. J. Nanopart. Res. 2015, 17, 61. [Google Scholar] [CrossRef]
- Cheh, N.; Chipitsyna, G.; Gong, Q.; Yeo, C.J.; Arafa, H.A. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB 2009, 11, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, M.A.; Alghamdi, M.S. Anticancer activity of Nigella sativa (black seed)—A review. Am. J. Chin. Med. 2011, 39, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Botelho, P.B.; da Mariano, K.R.; Rogero, M.M.; de Castro, A.I. Effect of Echium oil compared with marine oils on lipid profile and inhibition of hepatic steatosis in LDLr knockout mice. Lipids Health Dis. 2013, 12, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.N.; Cho, J.Y.; Lee, M.C.; Kang, J.Y.; Park, N.G.; Fujii, H.; Hong, Y.K. Isolation of Two Anti-inflammatory and One Pro-inflammatory Polyunsaturated Fatty Acids from the Brown Seaweed Undaria pinnatifida. J. Agric. Food Chem. 2007, 55, 6984–6988. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Wua, J.; Yin, G.; Huang, Z.; Yao, Y.; Liao, X.; Chen, A.; Pu, X.; Liao, L. Preparation, characterization and in vitro cytotoxicity of indomethacin-loaded PLLA/PLGA microparticles using supercritical CO2 technique. Eur. J. Pharm. Biopharm. 2008, 70, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, C.; Amici, C.; Angelini, M.; Fracassi, C.; Belardo, G.; Santoro, M.G. The non-steroidal anti-inflammatory drug indomethacin activates the eIF2α kinase PKR, causing a translational block in human colorectal cancer cells. Biochem. J. 2012, 44, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.A.; Gardner, S.H.; Hawcroft, G. Activity of the non-steroidal anti-inflammatory drug indomethacin against colorectal cancer. Cancer Treat. Rev. 2003, 29, 309–320. [Google Scholar] [CrossRef]
- Castelli, F.; Puglia, C.; Sarpietro, M.G.; Rizza, L.; Bonina, F. Characterization of indomethacin-loaded lipid nanoparticles by differential scanning calorimetry. Int. J. Pharm. 2005, 304, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Dupeyrón, D.; Kawakami, M.; Ferreira, A.M.; Cáceres-Vélez, P.R.; Rieumont, J.; Azevedo, R.B.; Carvalho, J.C. Design of indomethacin-loaded nanoparticles: Effect of polymer matrix and surfactant. Int. J. Nanomed. 2013, 8, 3467–3477. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, K.; Yabuki, N.; Terada, H.; Makino, K. Surfactant free preparation of PLGA nanoparticles: The combination of antisolvent diffusion with preferential solvation. Colloids Surf. A 2014, 457, 88–93. [Google Scholar] [CrossRef]
- Ghitman, J.; Stan, R.; Iovu, H. Experimental contributions in the synthesis of PLGA nanoparticles with excellent properties for drug delivery: Investigation of key parameters. UPB Sci. Bull. Ser. B 2017, 79, 101–112. [Google Scholar]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Matthaiou, E.I.; Barar, J.; Sandaltzopoulos, R.; Li, C.; Coukos, G.; Omidi, Y. Shikonin-loaded antibody-armed nanoparticles for targeted therapy of ovarian cancer. Int. J. Nanomed. 2014, 15, 1855–1870. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Review: Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Seedat, N.; Kalhapure, R.S.; Mocktar, C.; Vepuri, S.; Jadhav, M.; Soliman, M.; Govender, T. Co-encapsulation of multi-lipids and polymers enhanges the performances of vancomycin in lipid-polymer hybrid nanoparticles: In vitro and in silico studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Pal, D. Formulation optimization and evaluation of jackfruit seed starch-alginate mucoadhesive beads of metformin HCl. Int. J. Biol. Macromol. 2013, 59, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.Y.; Su, C.S.; Wu, P.C.; Yeh, C.S. Multifunctional polymeric nanoparticles for combined chemotherapeutic and near-infrared photothermal cancer therapy in vitro and in vivo. Chem. Commun. 2010, 46, 3167–3169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Kong, L. Chitosan-Modified PLGA Nanoparticles with Versatile Surface for Improved Drug Delivery. APS Pharm. Sci. Tech. 2013, 14, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.N. Effect of a model lipophilic compound on the phase behavior of hydrophilic self-micro-emulsifying lipid formulations. J. Pharm. Res. 2016, 10, 647–654. [Google Scholar]
- Ueda, H.; Aikawa, S.; Kashima, Y.; Kikuchi, K.; Ida, Y.; Tanino, T.; Kadota, K.; Tozuka, Y. Anti-plasticizing Effect of Amorphous Indomethacin Induced by Specific Intermolecular Interactions with PVA Copolymer. J. Pharm. Sci. 2014, 103, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, K.; Terashima, H.; Suzuki, K.; Inagi, T.; Terada, H.; Makino, K. Enhanced transdermal delivery of indomethacin-loaded PLGA nanoparticles by iontophoresis. Colloids Surf. B Biointerfaces 2011, 88, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Gupta, V.; Ahsan, F. PEG-PLGA based large porous particles for pulmonary delivery of a highly soluble drug, low molecular weight heparin. J. Control. Release 2012, 162, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Lacatusu, I.; Badea, N.; Badea, G.; Brasoveanu, L.; Stan, R.; Ott, C.; Oprea, O.; Meghea, A. Ivy leaves extract based—Lipid nanocarriers and their bioefficacy on antioxidant and antitumor activities. RSC Adv. 2016, 6, 77243–77255. [Google Scholar] [CrossRef]
- Nartowski, K.P.; Tedder, J.; Braun, D.E.; Fábián, L.; Khimyak, Y.Z. Building solids inside nano-space: From confined amorphous through confined solvate to confined ‘metastable’ polymorph. Phys. Chem. Chem. Phys. 2015, 17, 24761–24773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graves, R.A.; Poole, D.; Moiseyev, R.; Bostanian, L.A.; Mandal, T.K. Encapsulation of indomethacin using coaxial ultrasonic atomization followed by solvent evaporation. Drug Dev. Ind. Pharm. 2008, 34, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.C.; Lin, H.L; Li, S.Y. Thermal analysis and FTIR spectral curve-fitting investigation of formation mechanism and stability of indomethacin-saccharin co crystals via solid-state grinding process. J. Pharm. Biomed. Anal. 2012, 66, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Cabo, N. Characterization of edible oils and lard by fourier transform infrared spectroscopy. Relationships between composition and frequency of concrete bands in the fingerprint region. J. Am. Oil Chem. Soc. 1997, 74, 1281–1286. [Google Scholar] [CrossRef]
- Petrova, S.; Miloshev, S.; Mateva, R.; Iliev, I. Synthesis of Amphiphilic PEG-PCL-PEG Triblock Copolymers. J. Univ. Chem. Technol. Metall. 2008, 43, 199–204. [Google Scholar]
- Kumar, R.; Nagarwal, R.C.; Dhanawat, M.; Pandit, J.K. In-Vitro and In-Vivo Study of Indomethacin Loaded Gelatin Nanoparticles. J. Biomed. Nanotechnol. 2011, 7, 1–9. [Google Scholar] [CrossRef]
- Durán-Lobato, M.; Muñoz-Rubio, I.; Holgado, M.A.; Álvarez-Fuentes, J.; Fernández-Arévalo, M.; Martín-Banderas, L. Enhanced Cellular Uptake and Biodistribution of a Synthetic Cannabinoid Loaded in Surface-Modified Poly(lactic-co-glycolic acid) Nanoparticles. J. Biomed. Nanotechnol. 2014, 10. [Google Scholar] [CrossRef]
- Yeo, Y.; Park, K. Control of Encapsulation Efficiency and Initial Burst in Polymeric Microparticle Systems. Arch. Pharm. Res. 2004, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vueba, M.L.; Batista de Carvalho, L.A.E.; Veiga, F.; Sousa, J.J.; Pina, M.E. Influence of cellulose ether polymers on ketoprofen release from hydrophilic matrix tablets. Eur. J. Pharm. Biopharm. 2004, 58, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, B.; Shou, D.; Xia, X.; Hu, Y. Preparation and Evaluationof Vancomycin-Loaded N-trimethyl Chitosan Nanoparticles. Polymers 2015, 7, 1850–1870. [Google Scholar] [CrossRef]
- Paul, D.R. Elaborations on the Higuchi model for drug delivery. Int. J. Pharm. 2011, 418, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Hines, D.J.; Kaplan, D.L. Poly(lactic-co-glycolic acid) controlled release systems: Experimental and modeling insights. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 257–276. [Google Scholar] [CrossRef]
- Shaikh, K.H.; Kshirsagar, R.V.; Patil, S.G. Mathematical Models for Drug Release Characterization: A Review. World J. Pharm. Pharm. Sci. 2015, 4, 324–338. [Google Scholar]
- Joy, M.; Iyengar, S.J.; Chakraborty, J.; Ghosh, S. Layered double hydroxide using hydrothermal treatment: Morphology evolution, intercalation and release kinetics of diclofenac sodium. Front. Mater. Sci. 2017, 11, 395–409. [Google Scholar] [CrossRef]
- Gbureck, U.; Vorndran, E.; Barralet, J.E. Modeling vancomycin release kinetics from microporous calcium phosphate ceramics comparing static and dynamic immersion conditions. Acta Biomater. 2008, 4, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lópeza, E.; Egea, M.A.; Cano, A.; Espina, M.; Calpena, A.C.; Ettcheto, M.; Camins, A.; Souto, E.B.; Silva, A.M.; García, M.L. PEGylated PLGA nanospheres optimized by design of experiments for ocular administration of dexibuprofen—In vitro, ex vivo and in vivo characterization. Colloids Surf. B 2016, 145, 241–250. [Google Scholar] [CrossRef] [PubMed]

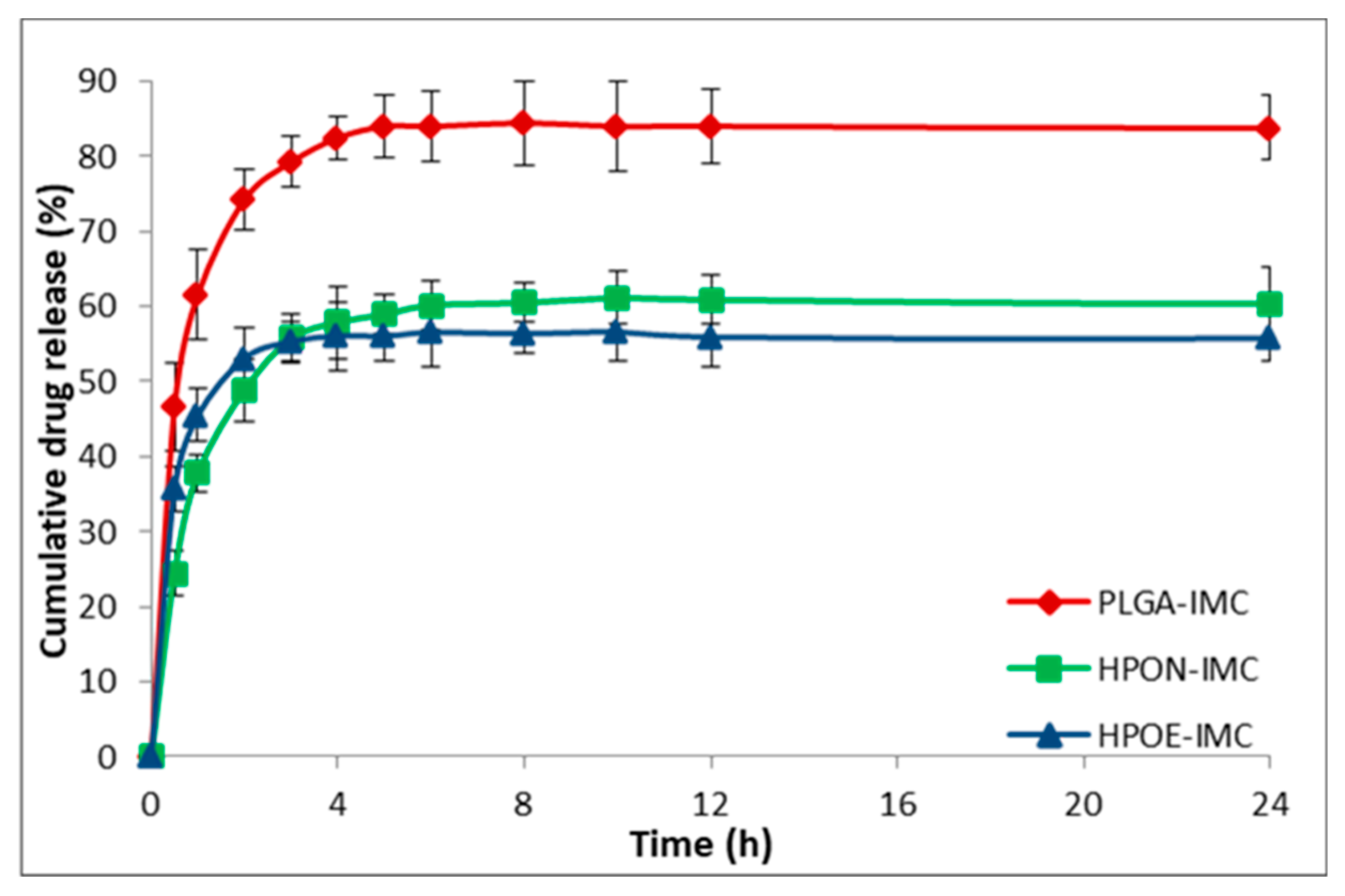
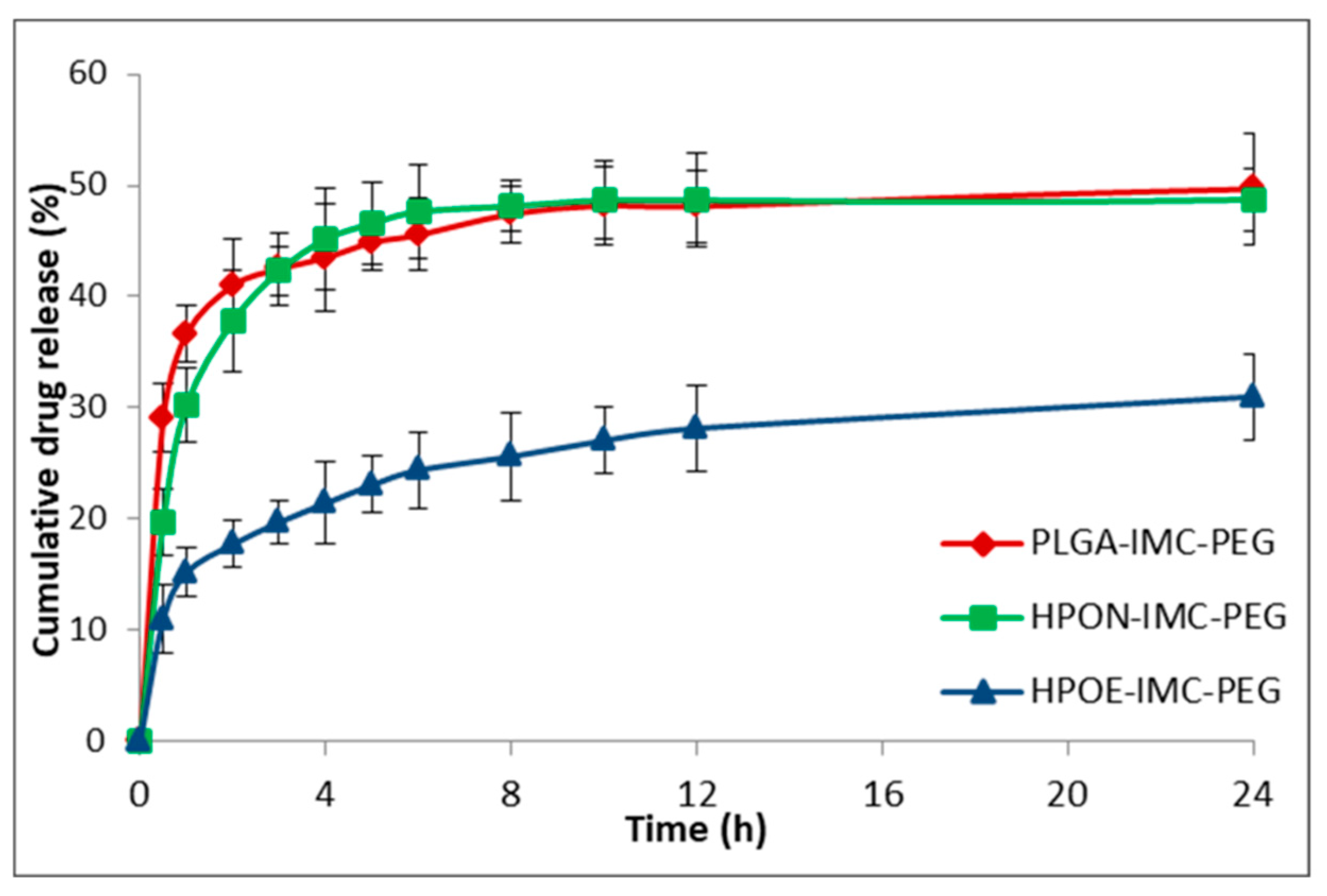
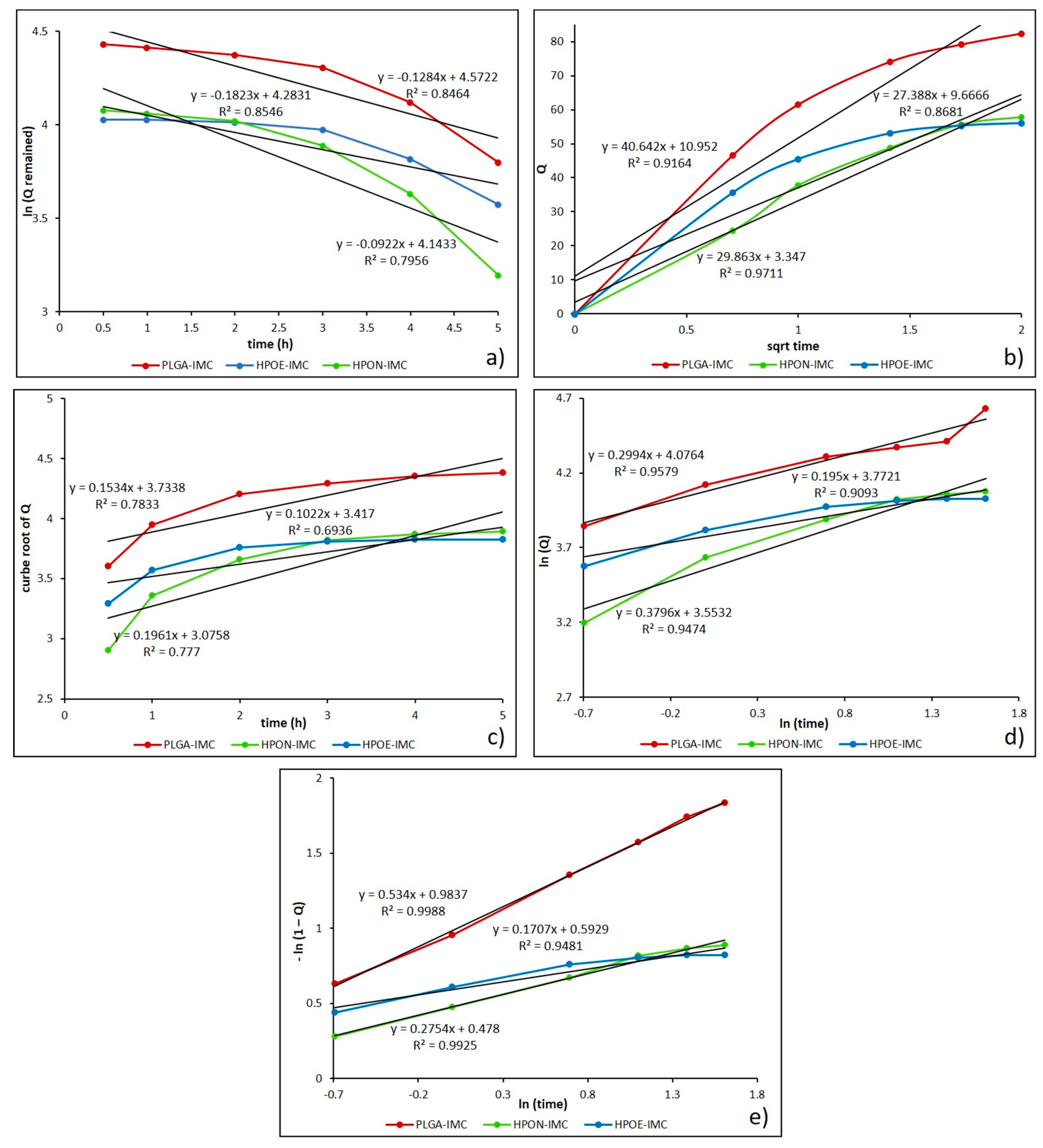
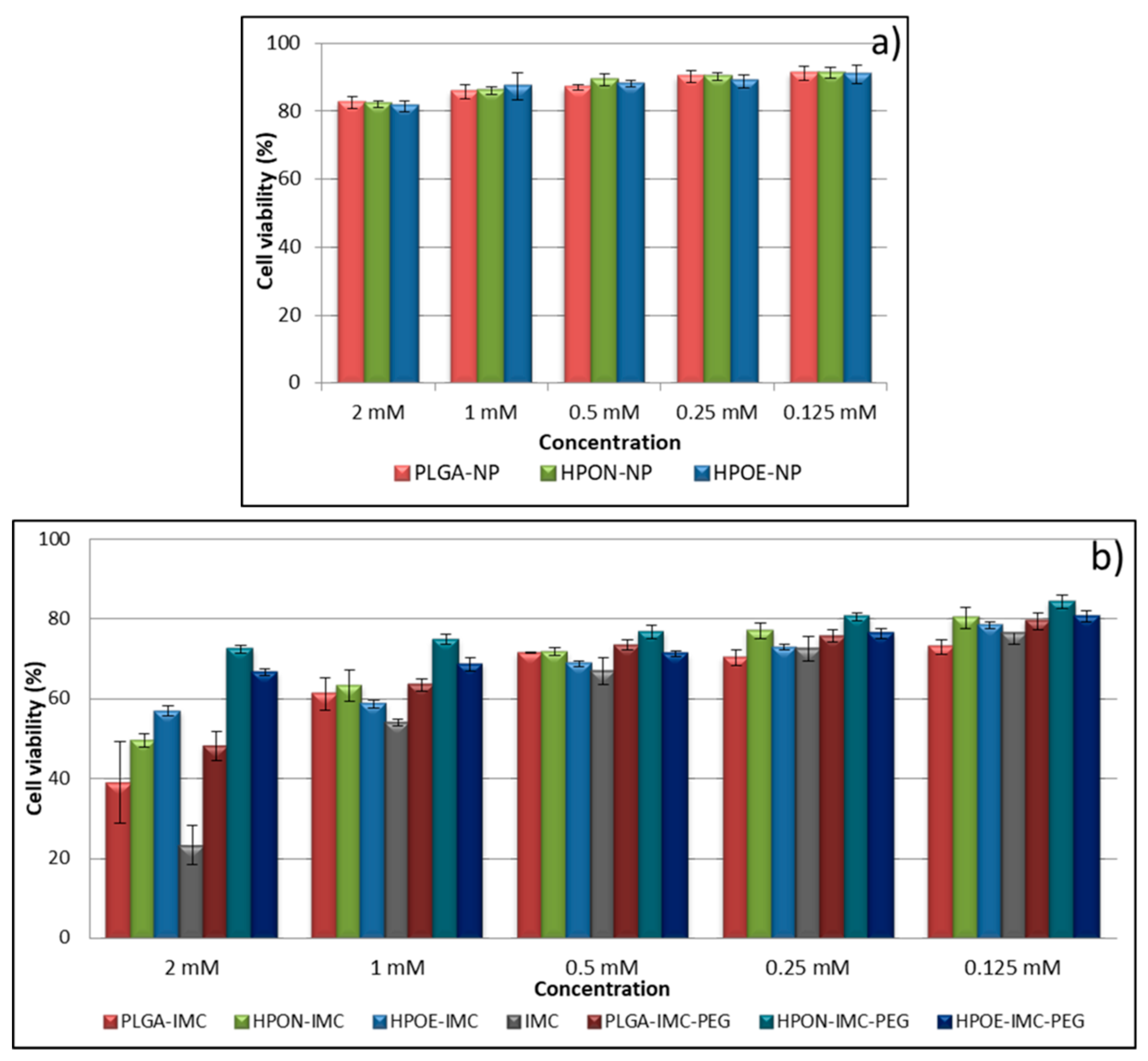
| Sample | Vegetable oils and its main components | Indomethacin IMC (wt %) | |
|---|---|---|---|
| PLGA-NP | - | - | - |
| PLGA-IMC | 10% | ||
| HPON-NP | Nigella sativa oil | 62.84% linoleic acid (C18:2) 20.80% oleic acid (C18:1) 11.42% palmitic acid (C16:0) 1.61% stearic acid (C18:0) 1.23%cis-11,14-eicosad acid (C20:2) 0.16% myristic acid (C14:0) 0.10% palmitoleic acid (C16:1) | - |
| HPON-IMC (PLGA/NSO ratio 1/1 wt) | 10% | ||
| HPOE-NP | Echium oil | 33.79% α-linolenic acid (C18:3) 16.10% oleic acid (C18:1) 14.83% linoleic acid (C18:2) 14.32% stearidonic acid (C18:4) 10.74% γ-linolenic acid (C18:3) 6.93% palmitic acid (C16:0) 3.26% stearic acid (C18:0) | - |
| HPOE-IMC (PLGA/EQ ratio 1/0.5 wt) | 10% | ||
| Release exponent (n) | Drug transport mechanism |
|---|---|
| n < 0.43 | Fickian release (diffusion-controlled release) |
| 0.43 < n < 0.85 | Non-Fickian release (anomalous transport, combination of diffusion and erosion drug release mechanism) |
| 0.85 < n < 1 | Case II transport (relaxation-controlled release) |
| n > 1 | Super case II transport (swelling and polymer chain relaxation controlled release) |
| Formulation | d (nm) | PdI | ZP (mV) |
|---|---|---|---|
| PLGA-NP | 106.0 ± 1.4 | 0.210 ± 0.016 | −12.2 ± 1.2 |
| HPON-NP | 144.5 ± 0.7 | 0.163 ± 0.031 | −22.0 ± 0.9 |
| HPOE-NP | 153.0 ± 2.5 | 0.164 ± 0.014 | −22.1 ± 1.3 |
| PLGA-IMC | 165.2 ± 0.7 | 0.134 ± 0.008 | −13.4 ± 0.1 |
| HPON-IMC | 160.9 ± 1.9 | 0.109 ± 0.020 | −15.2 ± 0.4 |
| HPOE-IMC | 167.1 ± 1.8 | 0.107 ± 0.025 | −14.4 ± 0.5 |
| PLGA-IMC-PEG | 275.8 ± 2.7 | 0.230 ± 0.003 | −10.1 ± 0.2 |
| HPON-IMC-PEG | 179.0 ± 2.5 | 0.179 ± 0.004 | −14.0 ± 0.8 |
| HPOE-IMC-PEG | 200.9 ± 2.2 | 0.108 ± 0.015 | −13.6 ± 1.2 |
| Formulation | DL (%) | EE (%) |
|---|---|---|
| PLGA-IMC | 4.6 ± 0.2 | 28.7 ± 2.4 |
| HPON-IMC | 16.4 ± 0.4 | 61.4 ± 2.6 |
| HPOE-IMC | 8.5 ± 0.1 | 37.7 ± 1.9 |
| Formulation | First order | Higuchi | Hixson-crowell | Korsmeyer-peppas | Weibull | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | k | R2 | k | R2 | k | R2 | n | k | R2 | a | b | |
| PLGA-IMC | 0.846 | 0.147 | 0.916 | 47.490 | 0.783 | 0.153 | 0.957 | 0.213 | 62.452 | 0.998 | 0.983 | 0.534 |
| HPON-IMC | 0.749 | 0.136 | 0.971 | 30.212 | 0.777 | 0.196 | 0.943 | 0.380 | 34.925 | 0.992 | 1.613 | 0.275 |
| HPOE-IMC | 0.795 | 0.036 | 0.868 | 33.697 | 0.693 | 0.138 | 0.909 | 0.218 | 43.489 | 0.948 | 1.809 | 0.170 |
| PLGA-IMC-PEG | 0.854 | 0.066 | 0.766 | 26.266 | 0.733 | 0.073 | 0.979 | 0.178 | 34.713 | 0.971 | 1.548 | 0.101 |
| HPON-IMC-PEG | 0.854 | 0.129 | 0.950 | 24.690 | 0.780 | 0.169 | 0.976 | 0.259 | 30.898 | 0.994 | 1.419 | 0.170 |
| HPOE-IMC-PEG | 0.931 | 0.125 | 0.911 | 11.393 | 0.880 | 0.083 | 0.981 | 0.305 | 14.216 | 0.990 | 1.172 | 0.060 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghitman, J.; Stan, R.; Ghebaur, A.; Cecoltan, S.; Vasile, E.; Iovu, H. Novel PEG-Modified Hybrid PLGA-Vegetable Oils Nanostructured Carriers for Improving Performances of Indomethacin Delivery. Polymers 2018, 10, 579. https://doi.org/10.3390/polym10060579
Ghitman J, Stan R, Ghebaur A, Cecoltan S, Vasile E, Iovu H. Novel PEG-Modified Hybrid PLGA-Vegetable Oils Nanostructured Carriers for Improving Performances of Indomethacin Delivery. Polymers. 2018; 10(6):579. https://doi.org/10.3390/polym10060579
Chicago/Turabian StyleGhitman, Jana, Raluca Stan, Adi Ghebaur, Sergiu Cecoltan, Eugeniu Vasile, and Horia Iovu. 2018. "Novel PEG-Modified Hybrid PLGA-Vegetable Oils Nanostructured Carriers for Improving Performances of Indomethacin Delivery" Polymers 10, no. 6: 579. https://doi.org/10.3390/polym10060579





