Processing and Thermal Response of Temperature-Sensitive-Gel(TSG)/Polymer Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TSG Particles
2.3. Preparation of TSG Films
2.4. Preparation of TSG/LLDPE/CaCO3 Composites and Films
2.5. Characterization
2.5.1. Differential Scanning Calorimetry (DSC)
2.5.2. Wide-Angle X-Ray Scattering (WAXS)
2.5.3. Thermal Gravimetric Analysis (TGA)
2.5.4. Thermal Mechanical Analysis (TMA)
2.5.5. Pressure-Volume-Temperature (PVT) Test
2.5.6. Tensile Testing
2.5.7. Scanning Electron Microscope (SEM)
2.5.8. Air Permeability Measurement
2.5.9. Temperature Control Experiment
3. Results and Discussion
3.1. Size, Morphology, and Thermal Properties of TSG Particles
3.2. Linear and Volumue Thermal Expansion of TSG
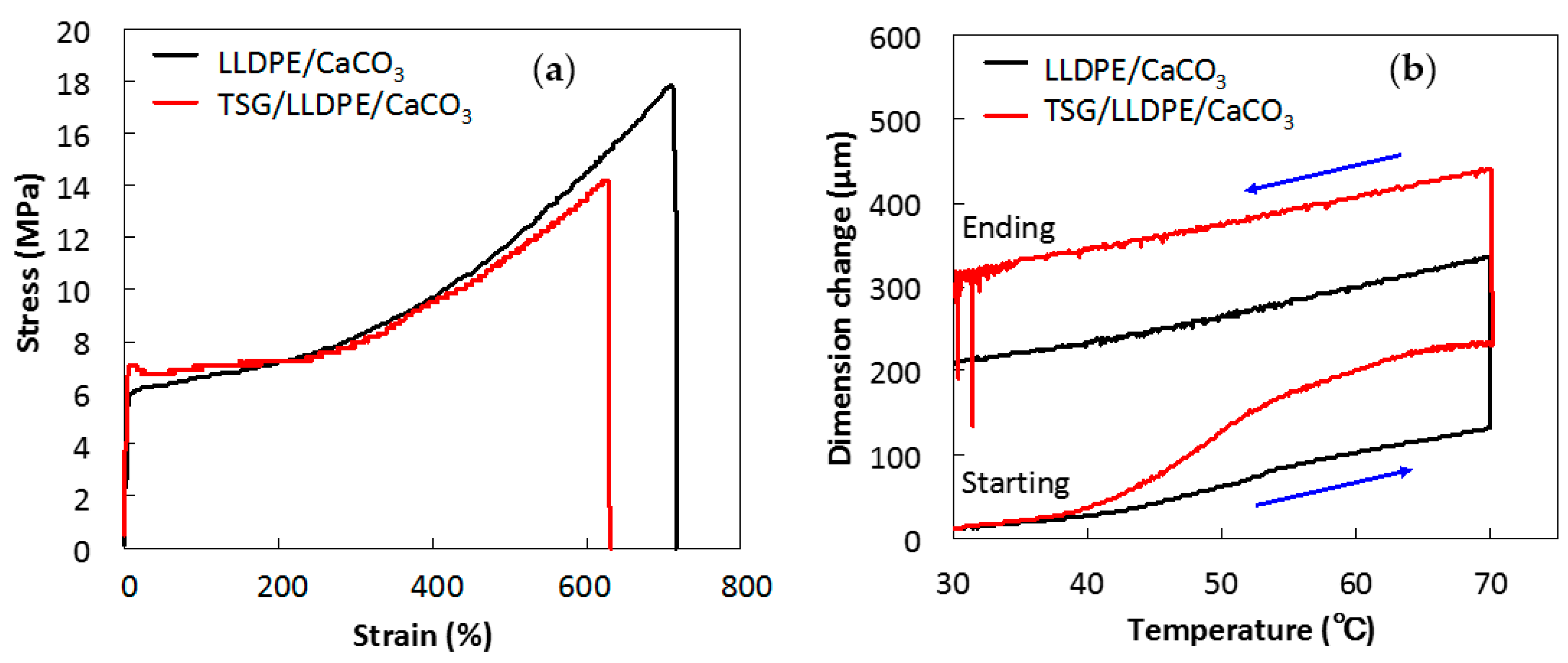
3.3. Effect of TSG on Mechanical Properties and Thermal Expansion
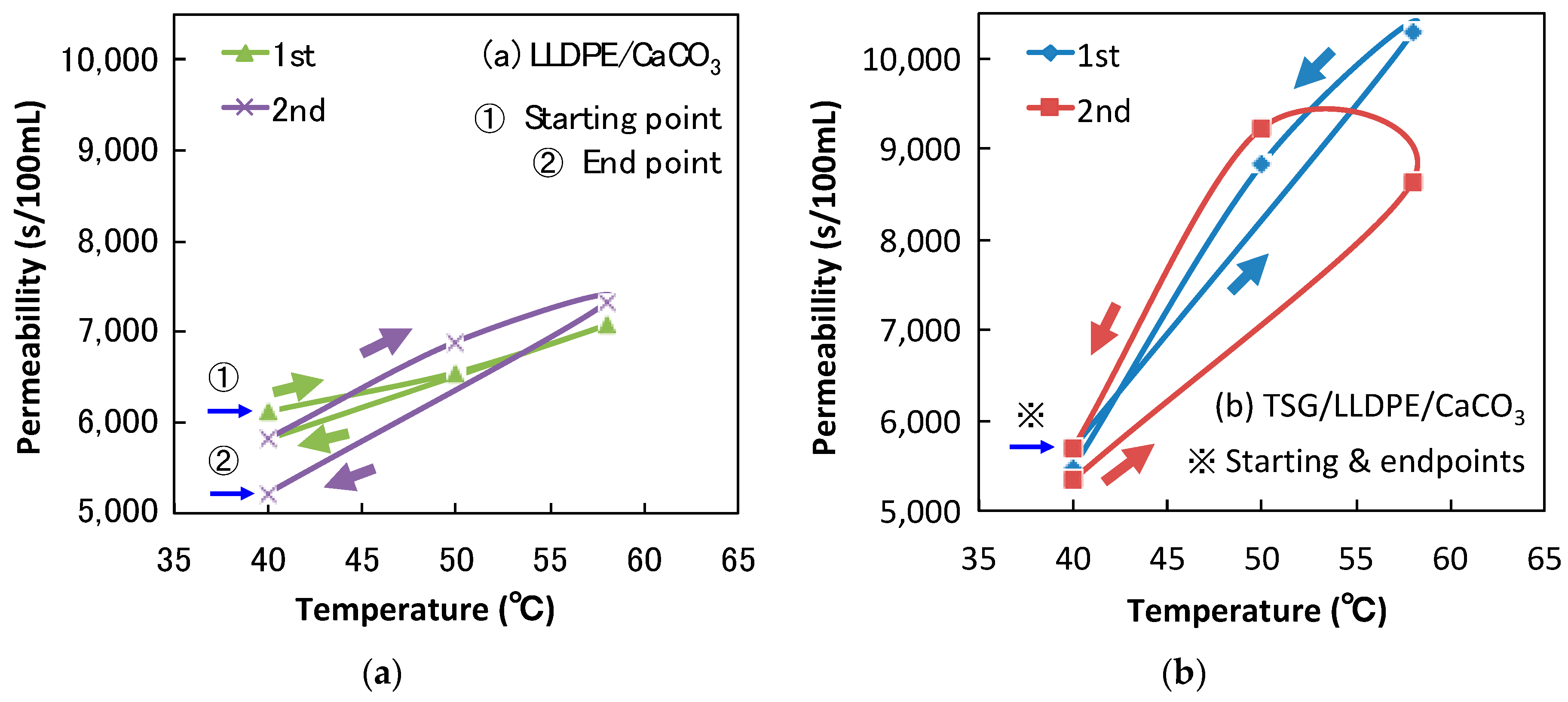
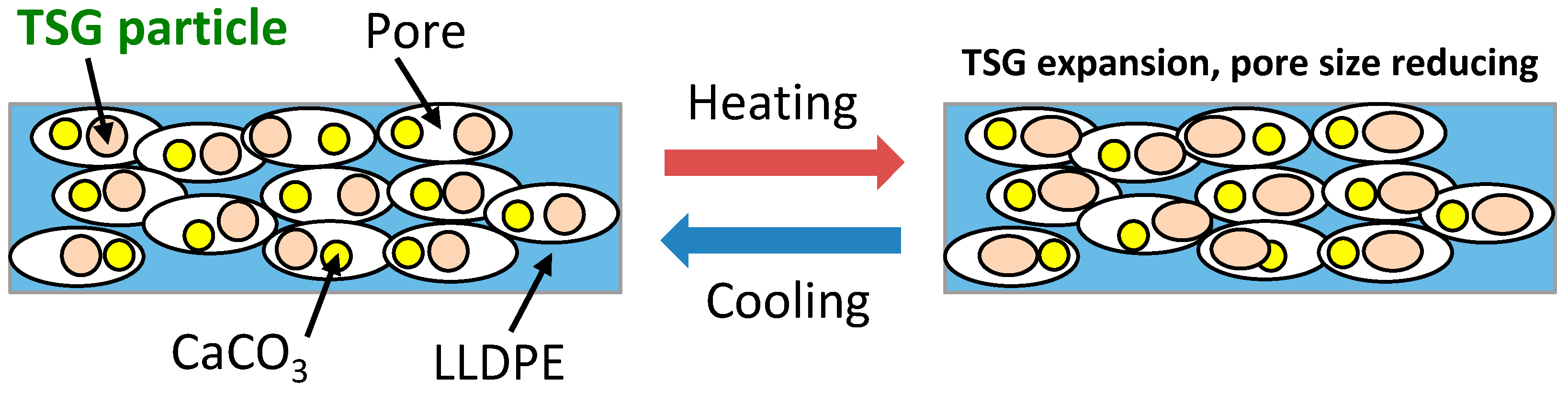
3.4. Effect of TSG on Air Permeability
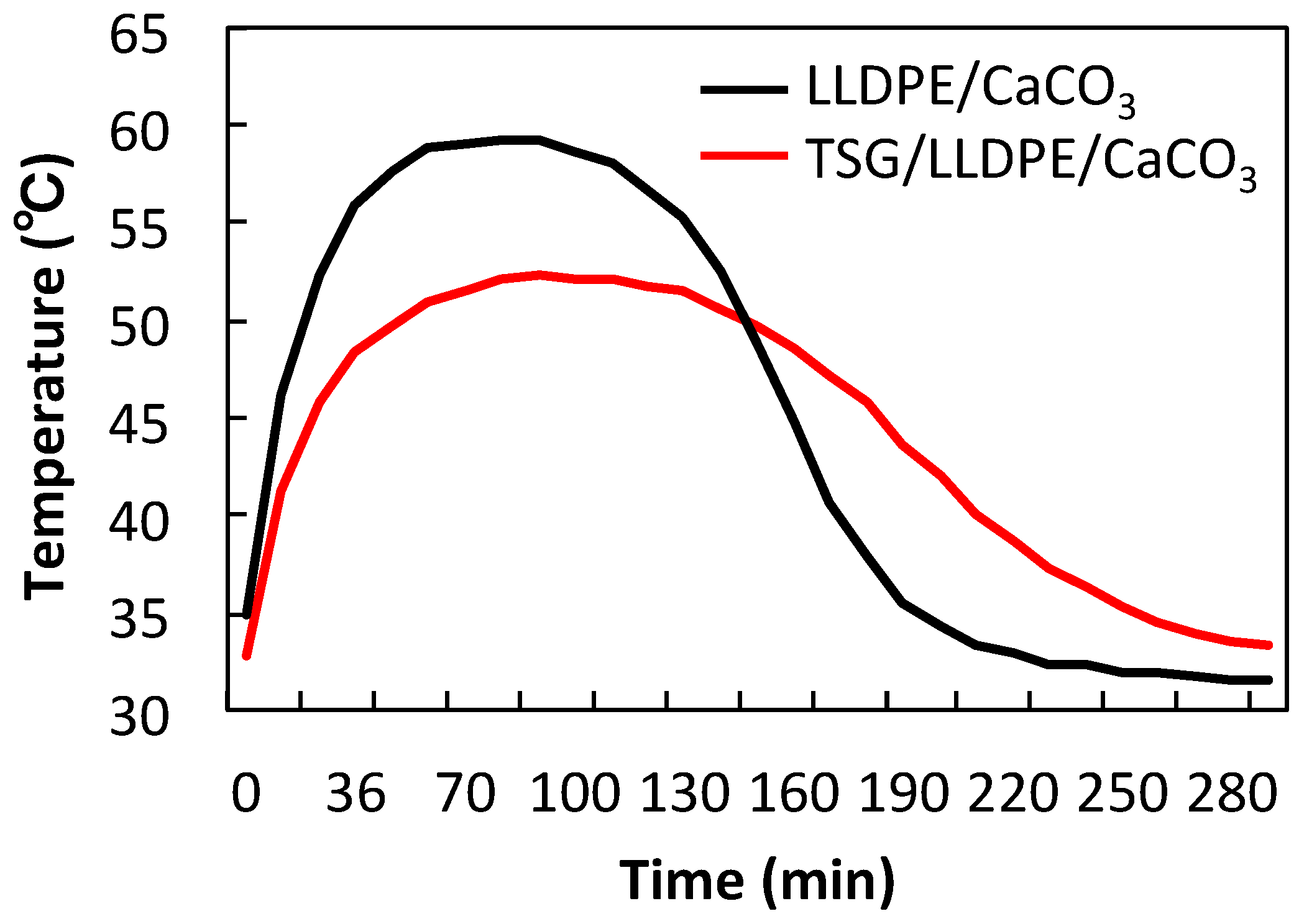
3.5. Effect of TSG on Temperature Control
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Gong, J.; Shitara, M.; Serizawa, R.; Makino, M.; Kabir, M.H.; Furukawa, H. 3D printing of meso-decorated gels and foods. Mater. Sci. Forum 2014, 783–786, 1250–1254. [Google Scholar] [CrossRef]
- Gong, J.; Igarashi, S.; Sawamura, K.; Furukawa, H. Gel engineering materials meso-decorated with polymorphic crystals. Adv. Mater. Res. 2013, 746, 325–329. [Google Scholar] [CrossRef]
- Gong, J.; Watanabe, Y.; Watanabe, Y.; Hidema, R.; Kabir, M.H.; Furukawa, H. Development of a novel standard type of gel engineering materials via simple bulk polymerization. J. Solid Mech. Mater. Eng. 2013, 7, 455–462. [Google Scholar] [CrossRef]
- Kabir, M.H.; Gong, J.; Watanabe, Y.; Makino, M.; Furukawa, H. Hard-to-soft transition of transparent shape memory gels and the first observation of their critical temperature studied with scanning microscopic light scattering. Mater. Lett. 2013, 108, 239–242. [Google Scholar] [CrossRef]
- Gong, J.; Furukawa, H. Smart optical device of varifocal lens developed with high transparent shape memory gels. Expect. Mater. Future 2013, 13, 5–8. [Google Scholar]
- Amano, Y.; Hidema, R.; Gong, J.; Furukawa, H. Creation of shape memory gels with inter-crosslinking network structure. Chem. Lett. 2012, 41, 1029–1031. [Google Scholar] [CrossRef]
- Harada, S.; Hidema, R.; Gong, J.; Furukawa, H. Intelligent button developed with smart soft and wet materials. Chem. Lett. 2012, 41, 1047–1049. [Google Scholar] [CrossRef]
- Kwon, Y.M.; Kim, S.W. Thermosensitive biodegradable hydrogels for the delivery of therapeutic agents. In Drugs and the Pharmaceutical Sciences, Polymer Drug Delivery System, 1st ed.; Kwon, G.S., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; Volume 148, pp. 251–274. ISBN 0-8247-2532-8. [Google Scholar]
- Chandra, A.; Meyer, W.H.; Best, A.; Hanewald, A.; Wegner, G. Modifying thermal expansion of polymer composites by blending with a negative thermal expansion material. Macromol. Mater. Eng. 2007, 292, 295–301. [Google Scholar] [CrossRef]
- Takenaka, K.; Ichigo, M. Thermal expansion adjustable polymer matrix composites with giant negative thermal expansion filler. Compos. Sci. Technol. 2014, 104, 47–51. [Google Scholar] [CrossRef]
- Poveda, R.L.; Gupta, N. Thermal expansion of CNF/polymer composites. In Carbon Nanofiber Reinforced Polymer Composites; Springer eBook; Springer: Berlin, Germany, 2016; pp. 53–62. ISBN 978-3-319-23787-9. [Google Scholar]
- Kiba, S.; Suzuki, N.; Okawauchi, Y.; Yamauchi, Y. Prototype of low thermal expansion materials: Fabrication of mesoporous silica/polymer composites with densely filled polymer inside mesopore space. Chem. Asian J. 2010, 5, 2100–2105. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, N.; Isobe, T.; Ando, S. Low Thermal Expansion Composites Prepared from Polyimide and ZrW2O8 Particles with Negative Thermal Expansion. J. Photopolym. Sci. Technol. 2012, 25, 385–388. [Google Scholar] [CrossRef]
- Wei, C.; Srivastava, D.; Cho, K. Thermal Expansion and Diffusion Coefficients of Carbon Nanotube-Polymer Composites. Nano Lett. 2002, 2, 647–650. [Google Scholar] [CrossRef]
- Nishino, T.; Kotera, M.; Sugiura, Y. Residual stress of particulate polymer composites with reduced thermal expansion. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2009; Volume 184. [Google Scholar]
- Sideridou, I.; Achilias, D.S.; Kyrikou, E. Thermal expansion characteristics of light-cured dental resins and resin composites. Biomaterials 2004, 25, 3087–3097. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yang, W. Thermo-sensitive switching membranes regulated by pore-covering polymer brushes. J. Membr. Sci. 2003, 218, 247–255. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.; Tsuru, T. Gas transport properties of interfacially polymerized polyamide composite membranes under different pre-treatments and temperatures. J. Membr. Sci. 2014, 449, 109–118. [Google Scholar] [CrossRef]
- Pilar Castejón, P.; Habibi, K.; Saffar, A.; Ajji, A.; Martínez, A.B.; Arencón, D. Polypropylene-Based Porous Membranes: Influence of Polymer Composition, Extrusion Draw Ratio and Uniaxial Strain. Polymers 2018, 33, 1–21. [Google Scholar] [CrossRef]
- Park, Y.; Gutierrez, M.P.; Lee, L.P. Reversible self-actuated thermo-responsive pore membrane. Sci. Rep. 2016, 6, 2045–2322. [Google Scholar] [CrossRef] [PubMed]
- Sershen, S.R.; Westcott, S.L.; Halas, N.J.; West, J.L. Temperature-sensitive polymer-nanoshell composites for photothermally modulated drug delivery. J. Biomed. Mater. Res. Part A 2000, 51, 293–298. [Google Scholar] [CrossRef]
- He, Y.; Moreira, E.; Overson, A.; Nakamura, S.H.; Bider, C.; Briscoe, J.F. Thermal characterization of an epoxy-based underfill material for flip chip packaging. Thermochim. Acta 2000, 357–358, 1–8. [Google Scholar] [CrossRef]
- Choi, Y.J.; Yamaguchi, T.; Nakao, S.I. A novel separation system using porous thermosensitive membranes. Ind. Eng. Chem. Res. 2000, 39, 2491–2495. [Google Scholar] [CrossRef]
- Park, Y.S.; Ito, Y.; Imanishi, Y. Permeation control through porous membranes immobilized with thermosensitive polymer. Langmuir 1998, 14, 910–914. [Google Scholar] [CrossRef]
- Shtanko, N.I.; Kabano, V.Y.; Apel, P.Y.; Yoshida, M. The use of radiation-induced graft polymerization for modification of polymer track membranes. Nucl. Instrum. Methods Phys. Res. Sect. B 1999, 151, 416–422. [Google Scholar] [CrossRef]
- Chung, D.J.; Ito, Y.; Imanishi, Y. Preparation of porous membranes grafted with poly (spiropyran-containing methacrylate) and photocontrol of permeability. J. Appl. Polymer Sci. 1994, 51, 2027–2033. [Google Scholar] [CrossRef]
- Iwata, H.; Oodate, M.; Uyama, Y.; Amemiya, H.; Ikada, Y. Preparation of temperature-sensitive membranes by graft polymerization onto a porous membrane. J. Membr. Sci. 1991, 55, 119–130. [Google Scholar] [CrossRef]
- Hotpress Gilfillan, W.N.; Moghaddam, L.; Bartley, J.; Doherty, W.O.S. Thermal extrusion of starch film with alcohol. J. Food Eng. 2015, 170, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Ruland, W. X-ray determination of crystalinity and diffuse disorder scattering. Acta Crystallogr. 1961, 14, 1180–1185. [Google Scholar] [CrossRef]
- Roger, B. Handbook of Polymer Testing—Physical Methods, 1st ed.; Marcel Dekker, Inc.: New York, NY, USA, 1999; pp. 341–342. ISBN 0-8247-0171-2. [Google Scholar]
- Landsberg, M.I.; Winston, G. Relationship between measurements of air Permeability by two machines. Text. Res. J. 1947, 17, 214–221. [Google Scholar] [CrossRef]
- Chakravorty, S. PVT testing of polymers under industrial processing conditions. Polym. Test. 2002, 21, 313–317. [Google Scholar] [CrossRef]
- Huang, D.H.; Tran, T.N.; Yang, B. Investigation on the reaction of iron powder mixture as a portable heat source for thermoelectric power generators. J. Therm. Anal. Calorim. 2014, 116, 1047–1053. [Google Scholar] [CrossRef]
- Mo, Z.; Zhang, H. The degree of crystallinity in polymers by wide-angle x-ray diffraction (WAXD). J. Macromol. Sci. Part C Polym. Rev. 1995, 35, 55–580. [Google Scholar] [CrossRef]
- Hosaka, E.; Gong, J.; Ito, H.; Shibata, Y.; Nakanishi, D.; Ishihara, S. Processing and Thermal Response Properties of Temperature Sensitive Gel/Polymer composites. Design, Manufacturing and Applications of Composites. In Proceedings of the Eleventh Joint Canada-Japan Workshop on Composites: First Joint Canada-Japan-Vietnam Workshop on Composites, Ho Chi Minh, Vietnam, 8–10 August 2016; Available online: dpi-proceedings.com/index.php/dmac/article/view/5246/0 (accessed on 15 May 2016).
- Technavio. Top 5 Vendors in the Breathable Films Market from 2017 to 2021; Technavio: London, UK, August 2017; pp. 1–71. Available online: technavio.com/report/global-breathable-films-market (accessed on 14 September 2017).
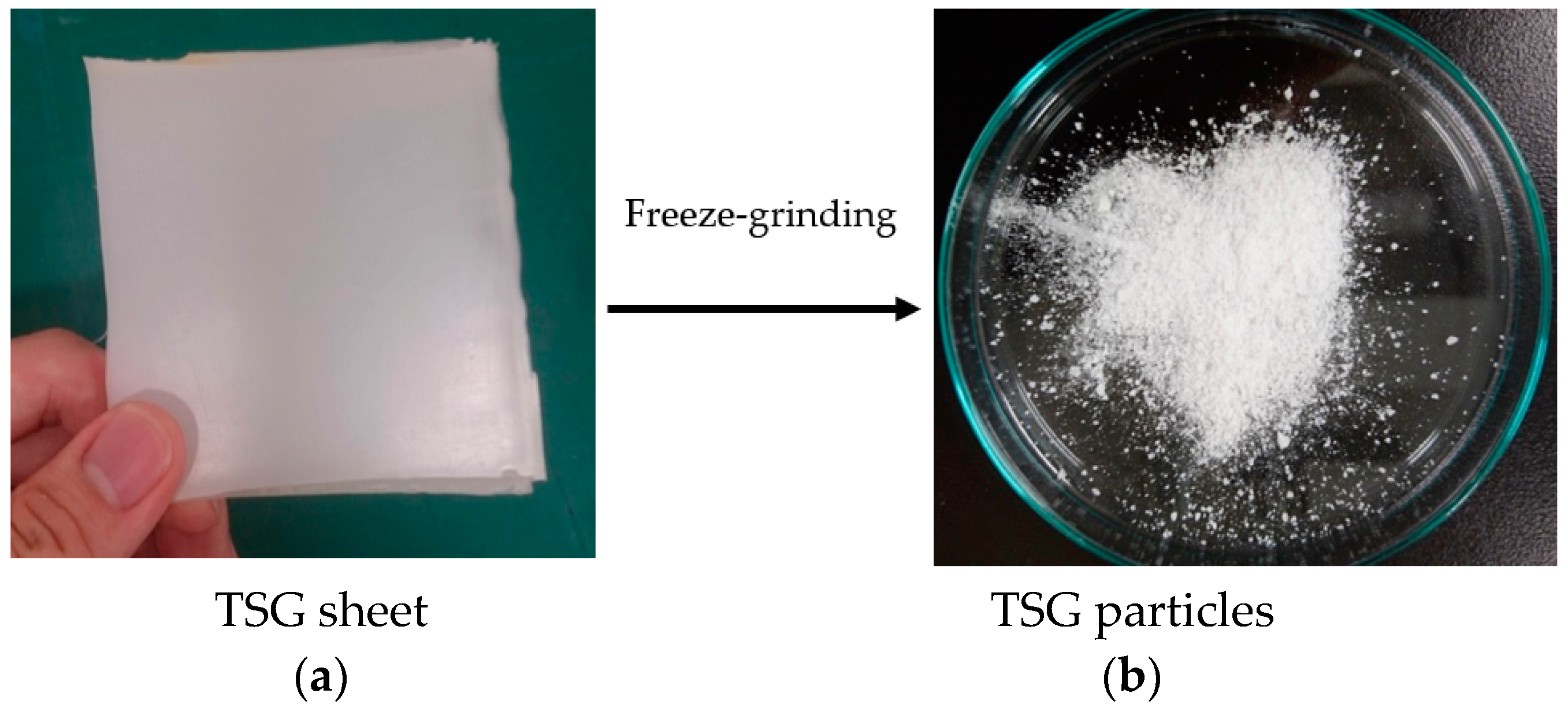

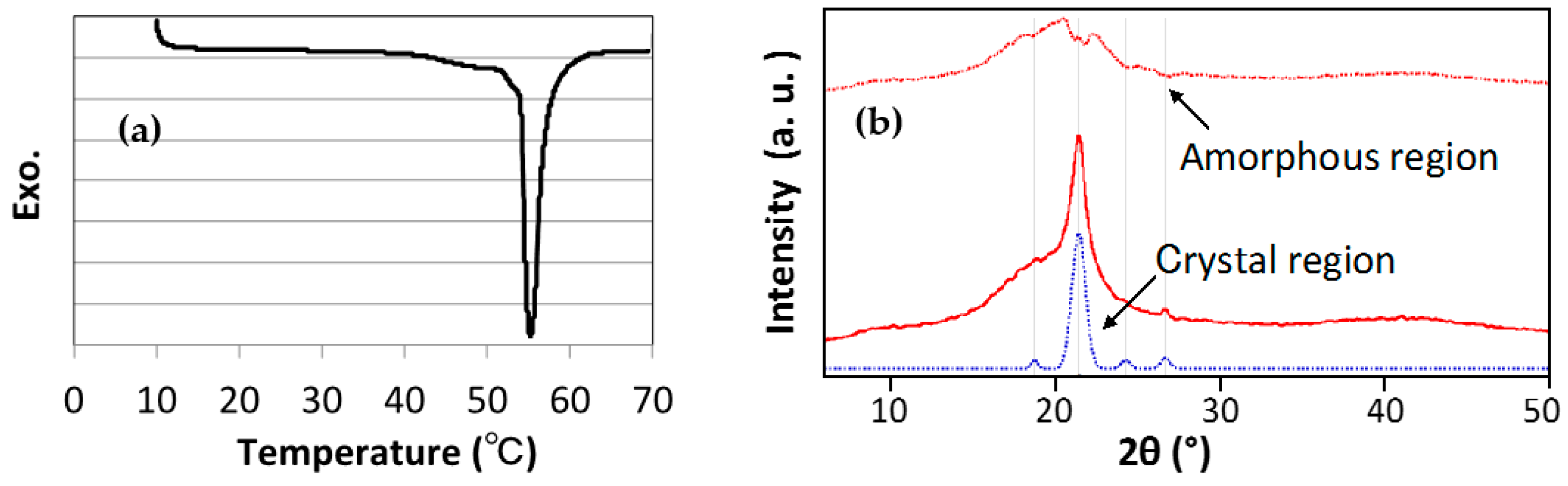
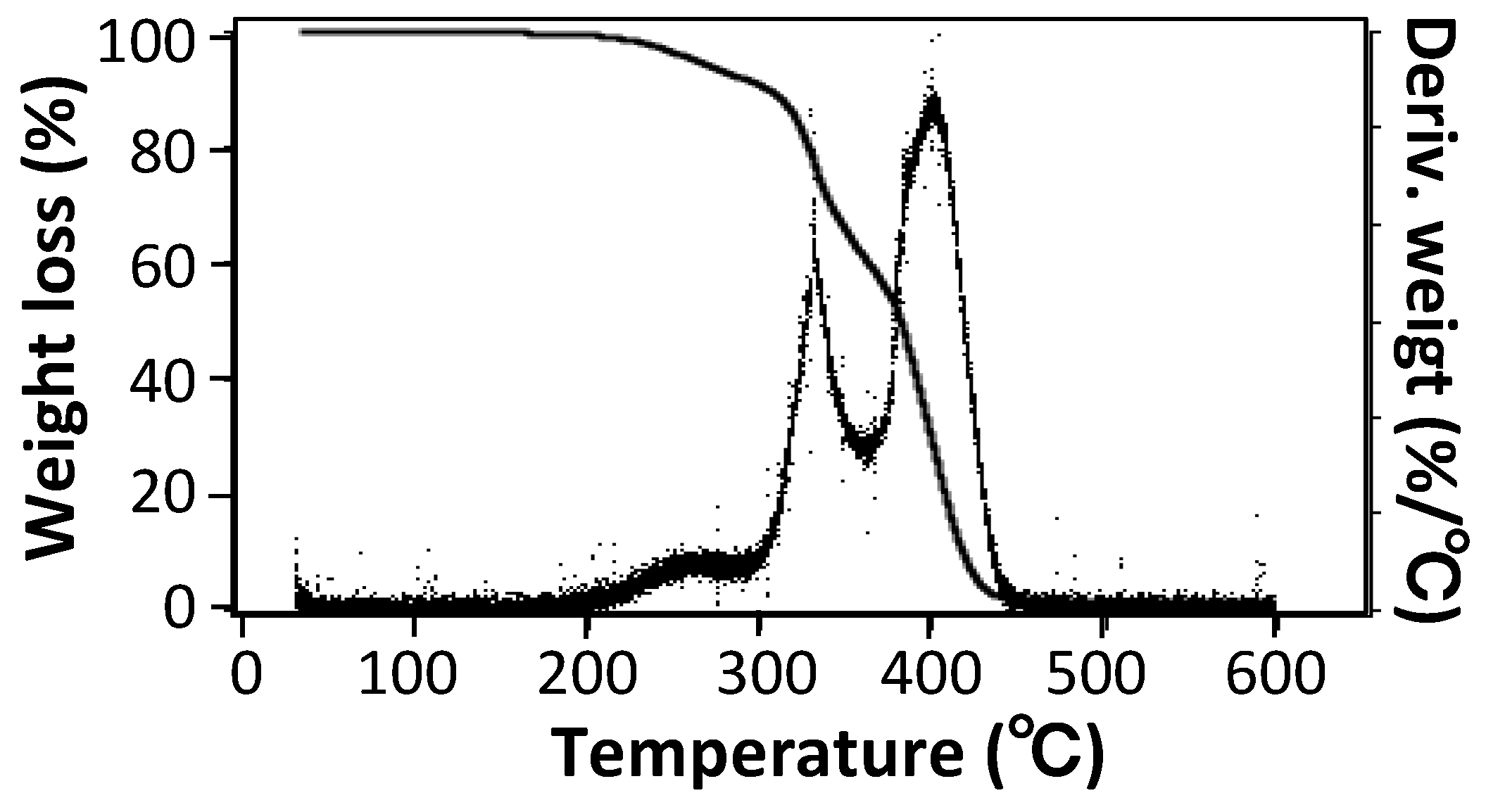
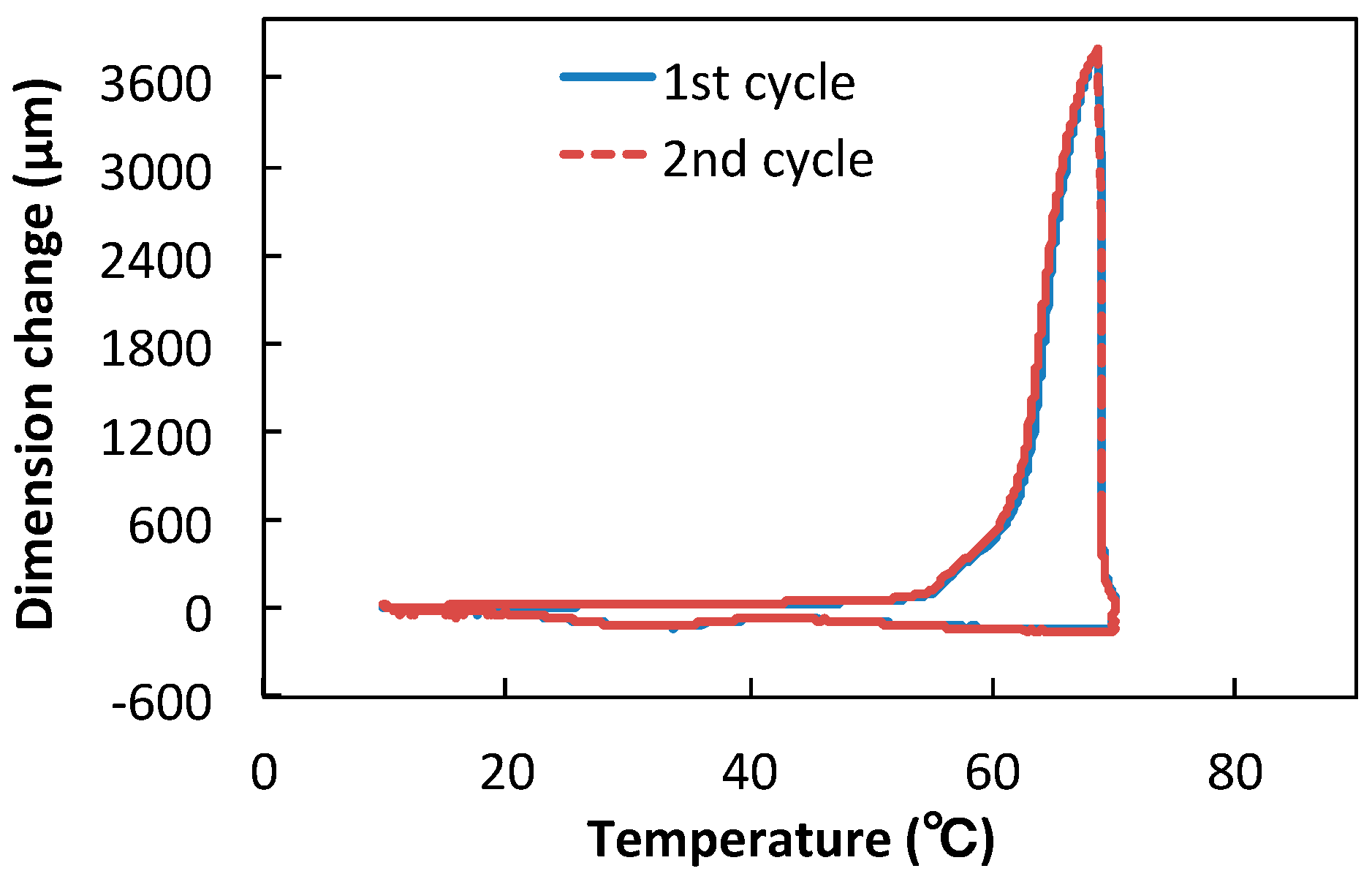


| Sample | Coefficient of Linear Thermal Expansion, α (10−5/°C) | ||
|---|---|---|---|
| 10–50 °C | 55–65 °C | 65–70 °C | |
| TSG | 4.5 | 322.2 | 2817.0 |
| Samples | Volume (cm3) | Change Rate of Volume (%) | |
|---|---|---|---|
| 30 °C | 60 °C | 30–60 °C | |
| TSG | 0.443 | 0.459 | 3.530 |
| LLDPE/CaCO3 | 0.391 | 0.395 | 1.111 |
| Samples | Tensile Strength at Break (MPa) | Young’s Modulus (MPa) | Elongation (%) |
|---|---|---|---|
| LLDPE/CaCO3 | 39 ± 2 | 147.7 ± 0.3 | 571 ± 5 |
| TSG /LLDPE/CaCO3 | 28.32 ± 2 | 145.5 ± 0.5 | 699 ± 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.; Hosaka, E.; Sakai, K.; Ito, H.; Shibata, Y.; Sato, K.; Nakanishi, D.; Ishihara, S.; Hamada, K. Processing and Thermal Response of Temperature-Sensitive-Gel(TSG)/Polymer Composites. Polymers 2018, 10, 486. https://doi.org/10.3390/polym10050486
Gong J, Hosaka E, Sakai K, Ito H, Shibata Y, Sato K, Nakanishi D, Ishihara S, Hamada K. Processing and Thermal Response of Temperature-Sensitive-Gel(TSG)/Polymer Composites. Polymers. 2018; 10(5):486. https://doi.org/10.3390/polym10050486
Chicago/Turabian StyleGong, Jin, Eiichi Hosaka, Kohei Sakai, Hiroshi Ito, Yoshikazu Shibata, Kosei Sato, Dai Nakanishi, Shinichiro Ishihara, and Kazuhiro Hamada. 2018. "Processing and Thermal Response of Temperature-Sensitive-Gel(TSG)/Polymer Composites" Polymers 10, no. 5: 486. https://doi.org/10.3390/polym10050486
APA StyleGong, J., Hosaka, E., Sakai, K., Ito, H., Shibata, Y., Sato, K., Nakanishi, D., Ishihara, S., & Hamada, K. (2018). Processing and Thermal Response of Temperature-Sensitive-Gel(TSG)/Polymer Composites. Polymers, 10(5), 486. https://doi.org/10.3390/polym10050486






