A Temperature-Tunable Thiophene Polymer Laser
Abstract
:1. Introduction
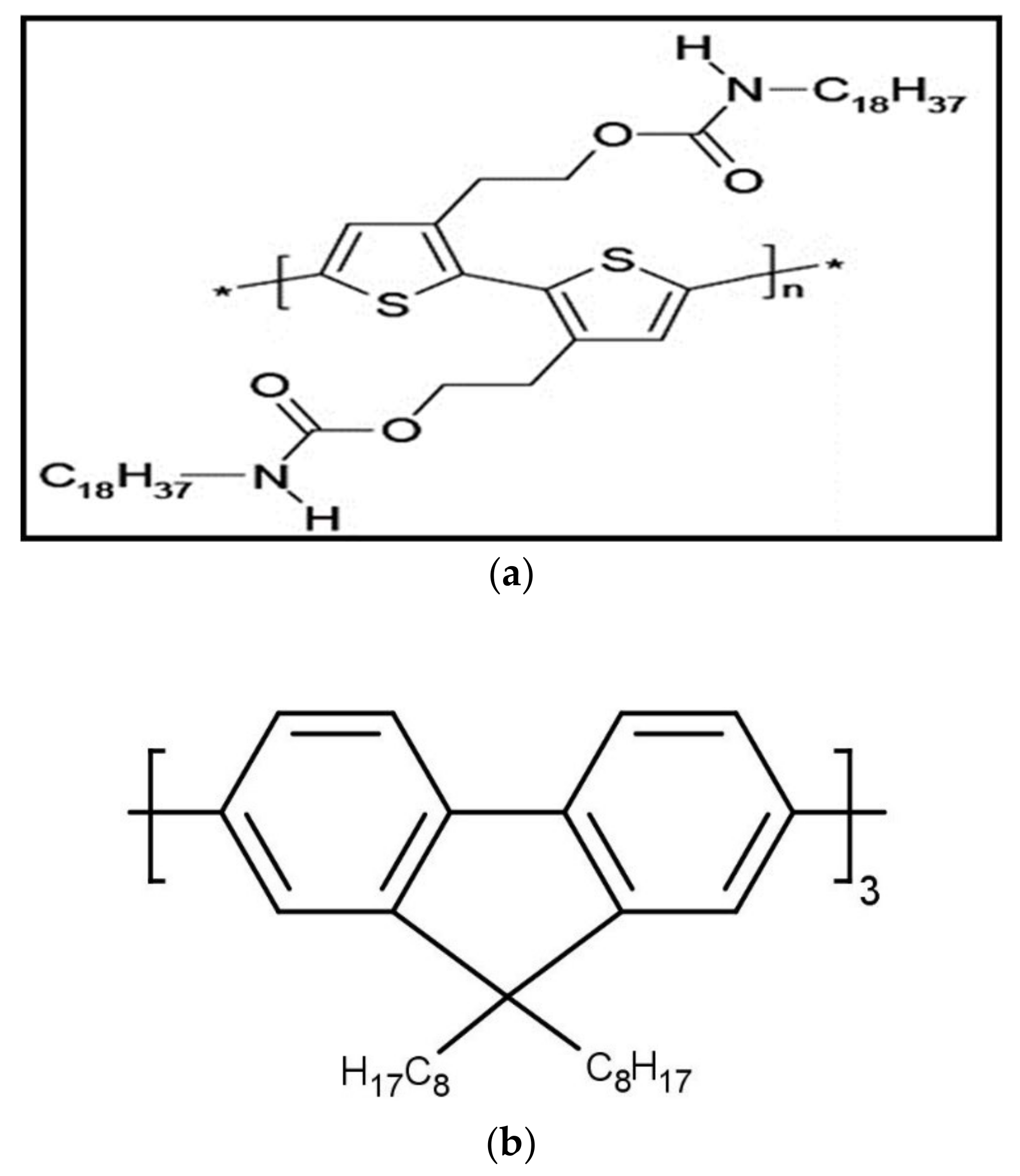
2. Materials and Methods
3. Results and Discussion
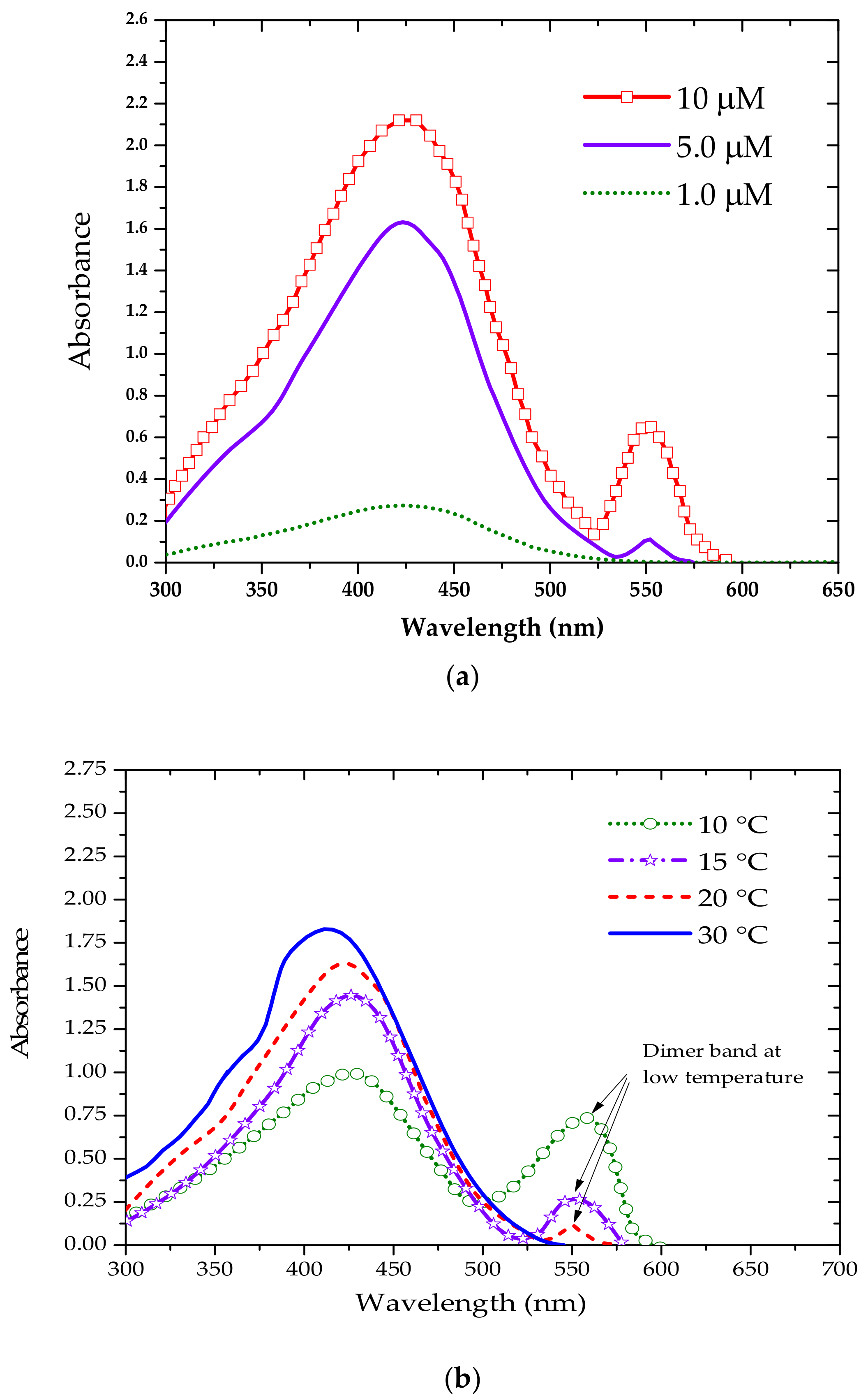
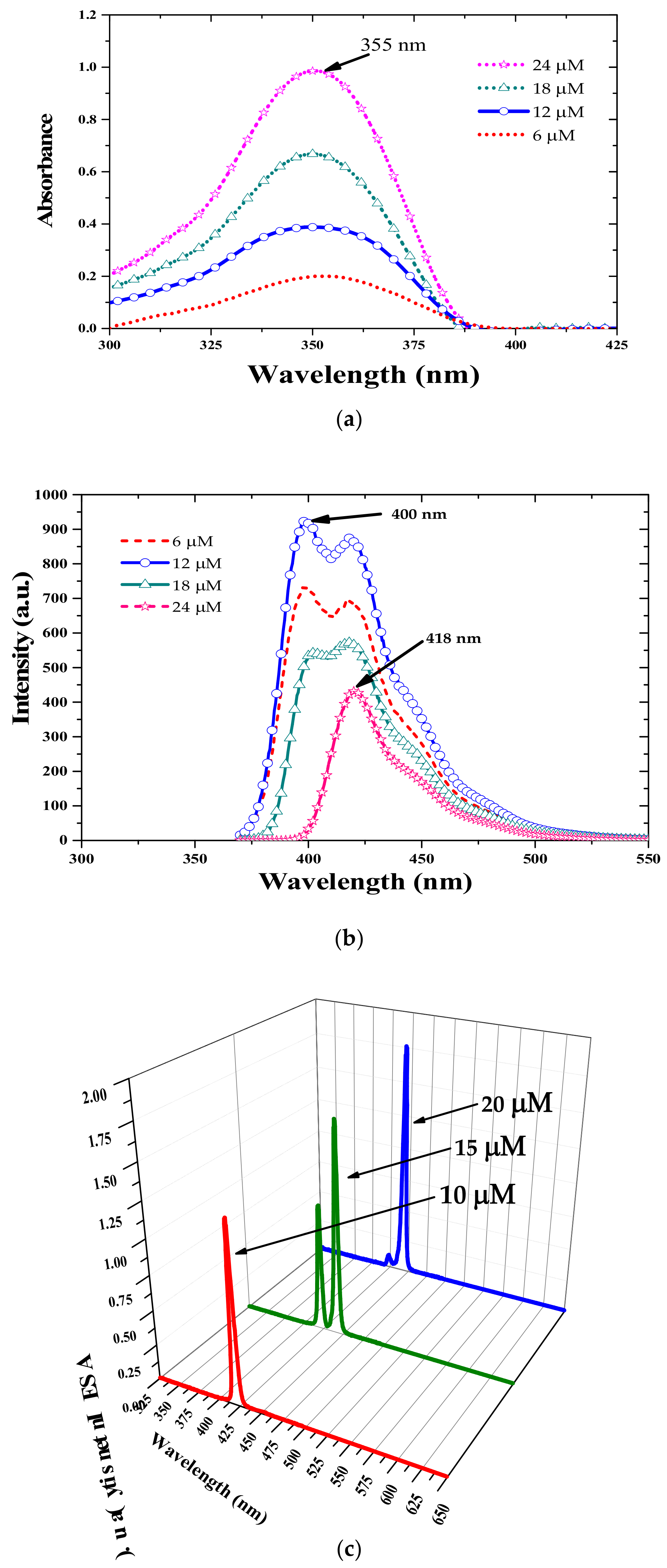
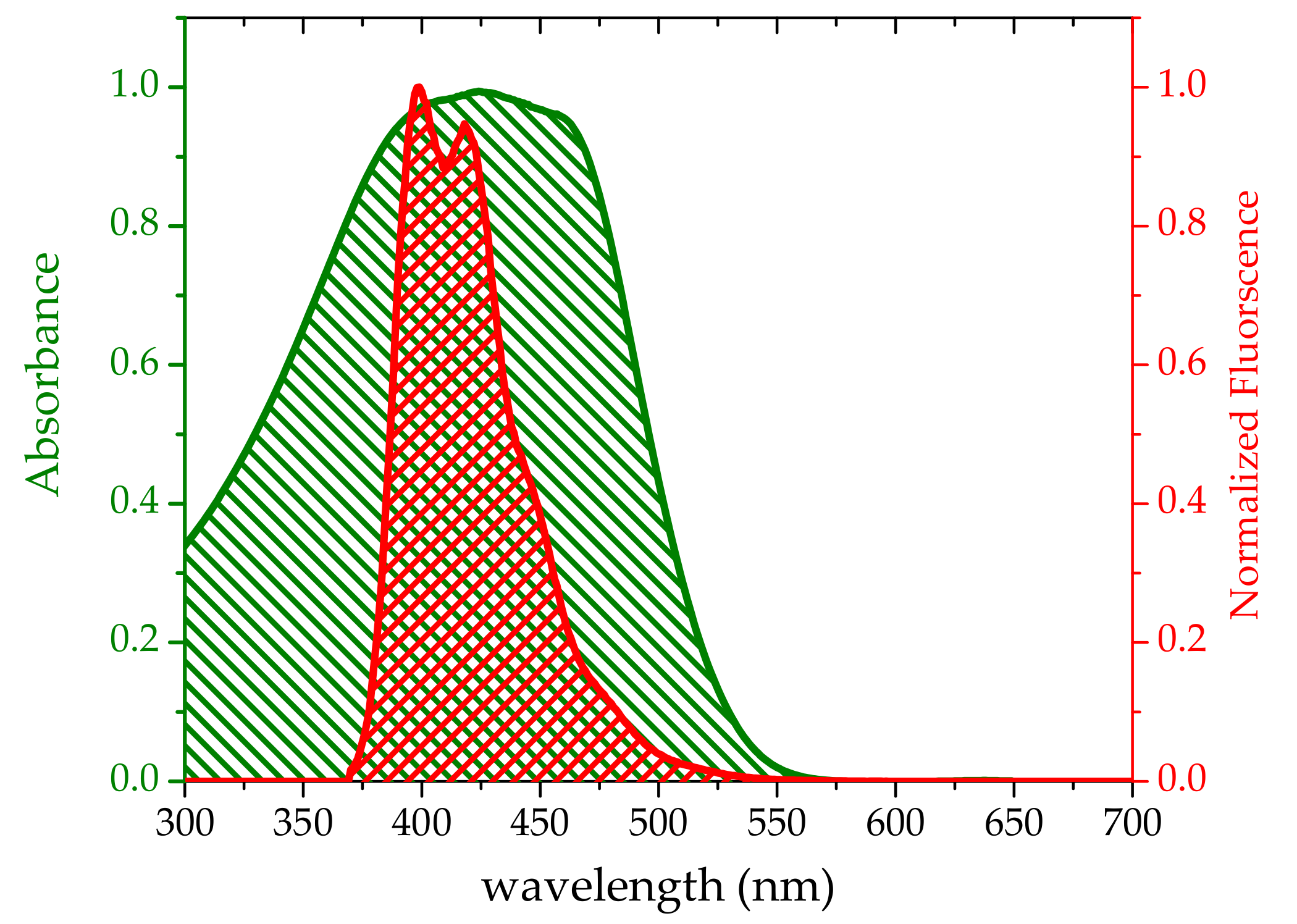
3.1. Absorption and Fluorescence Properties
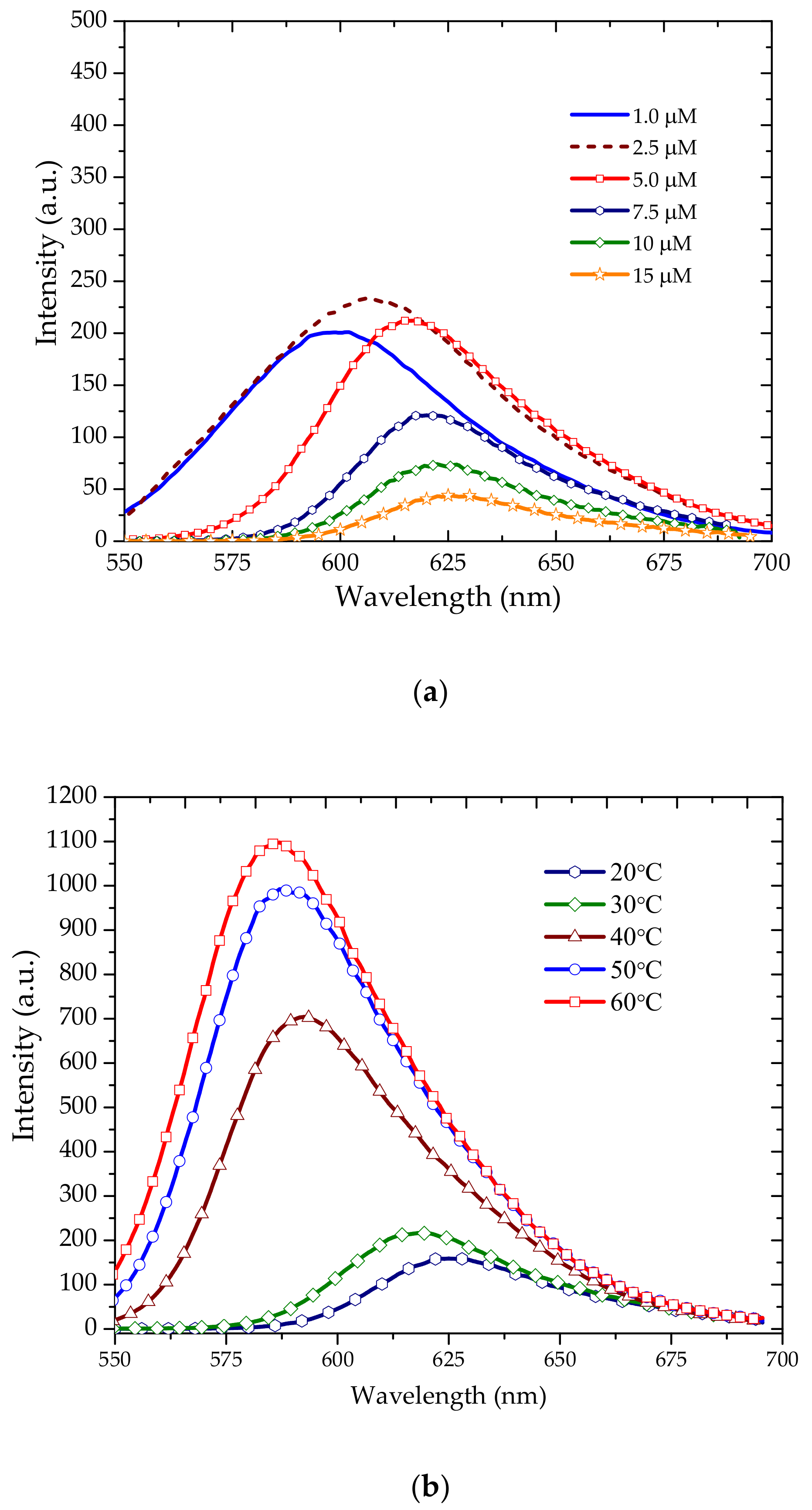
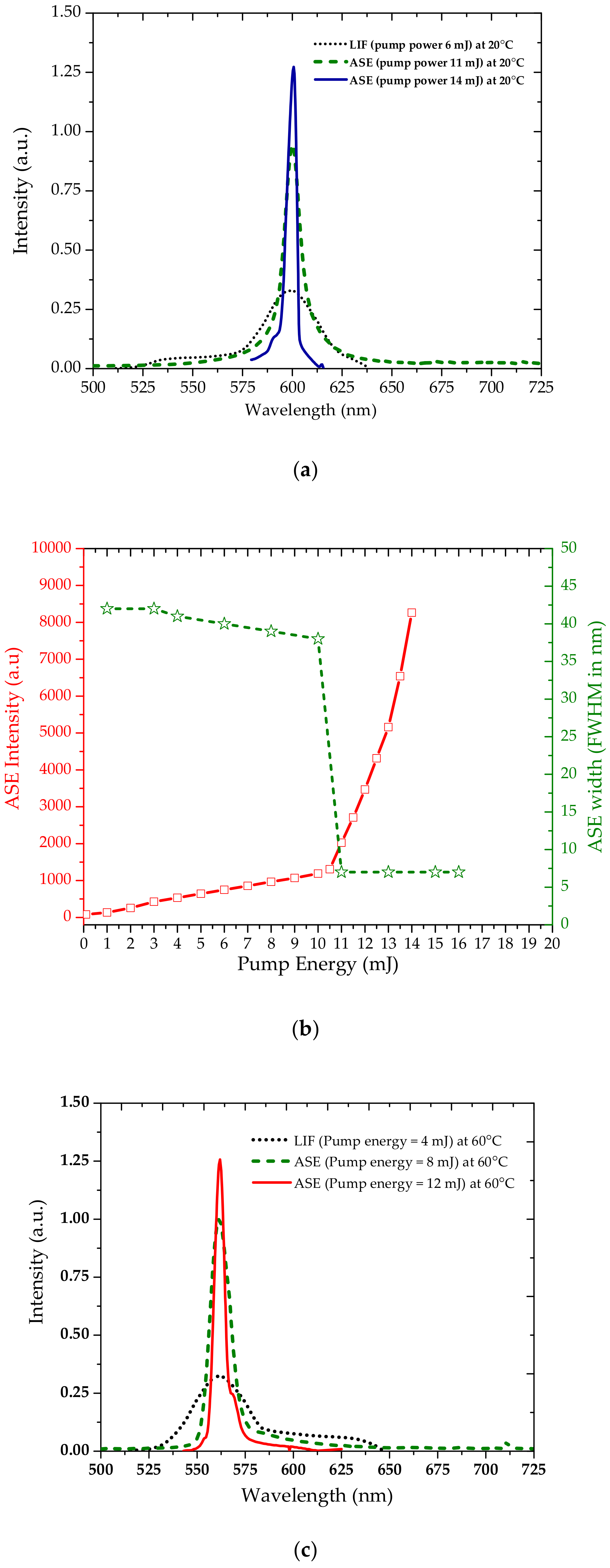
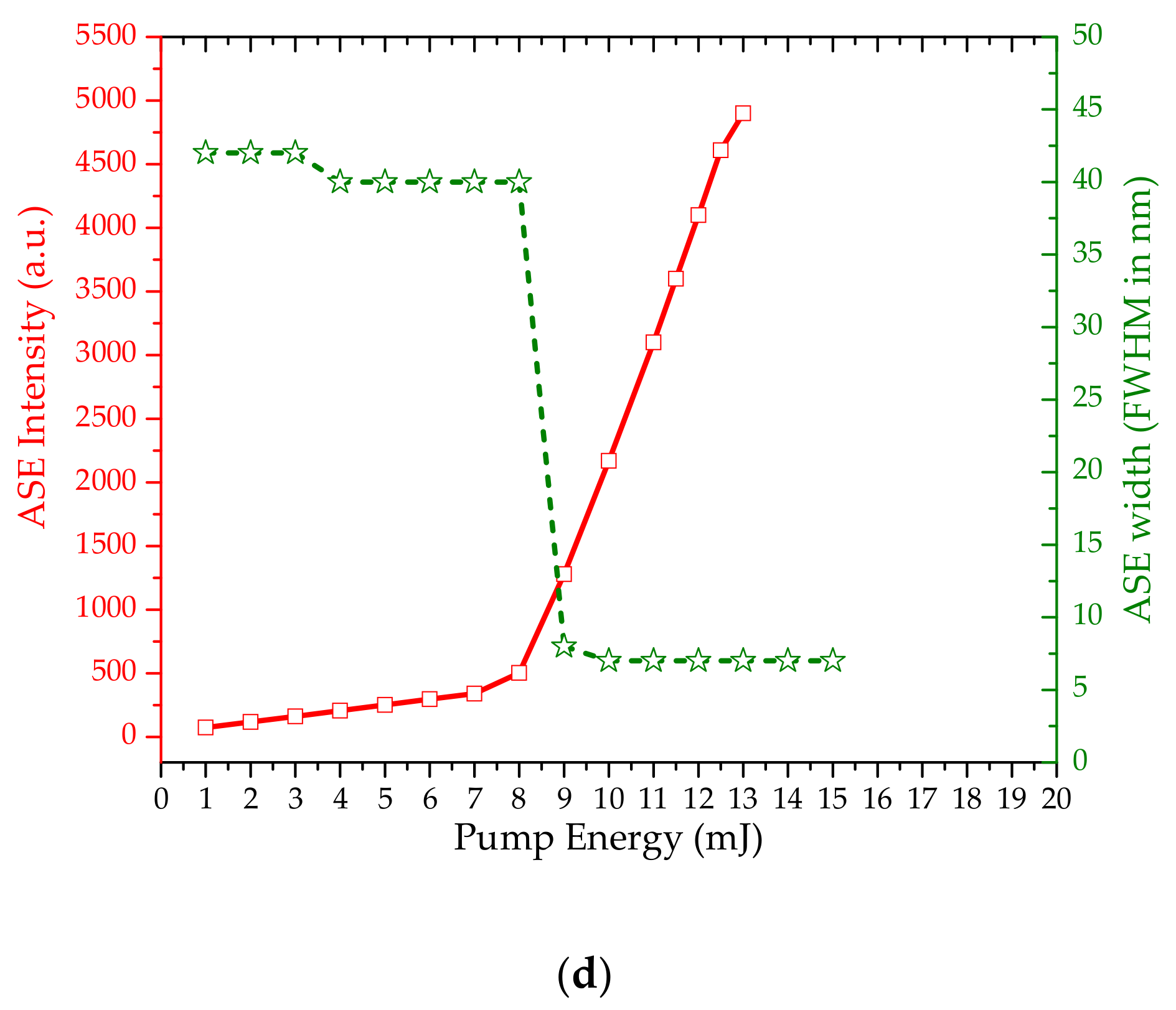
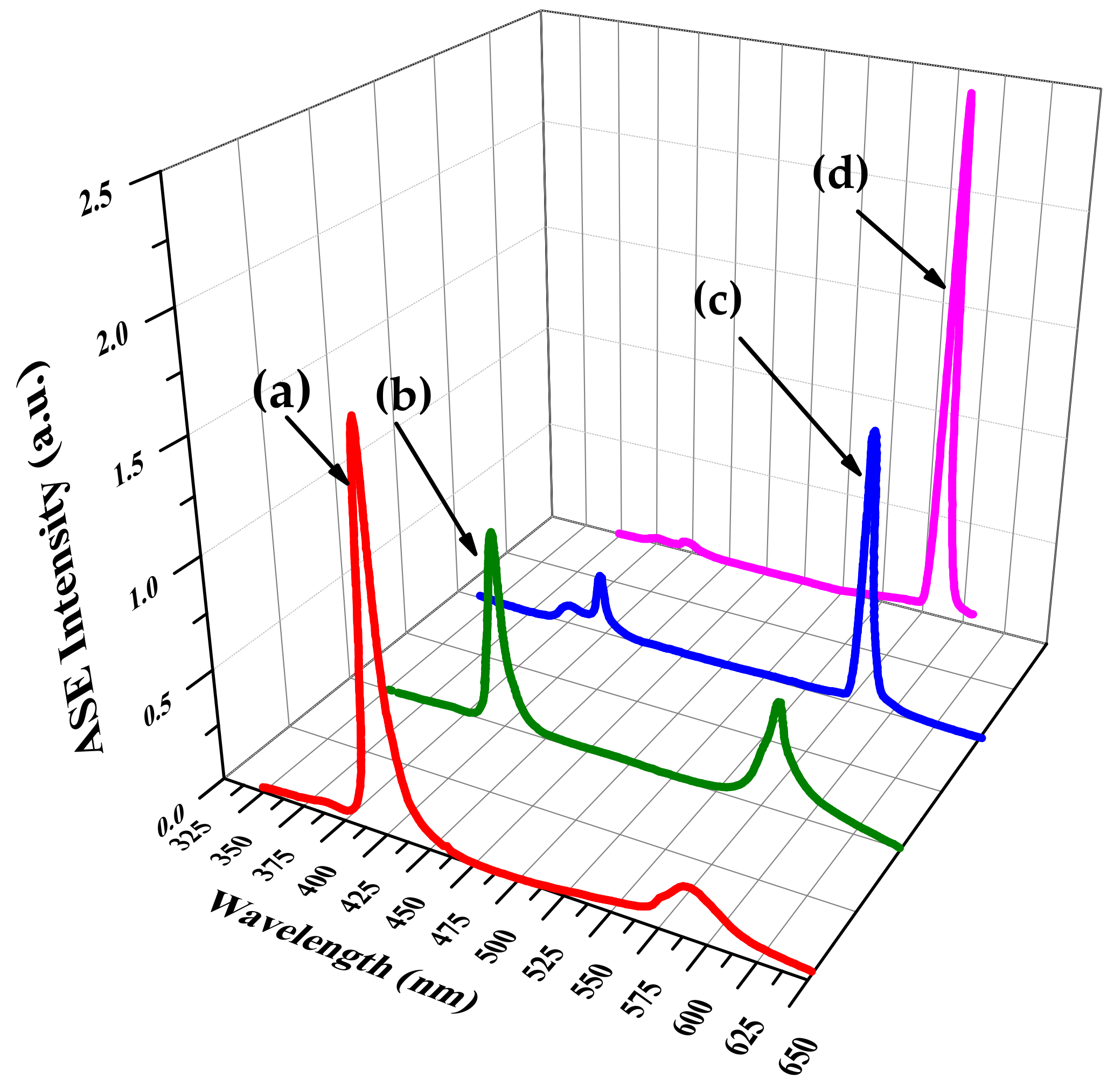
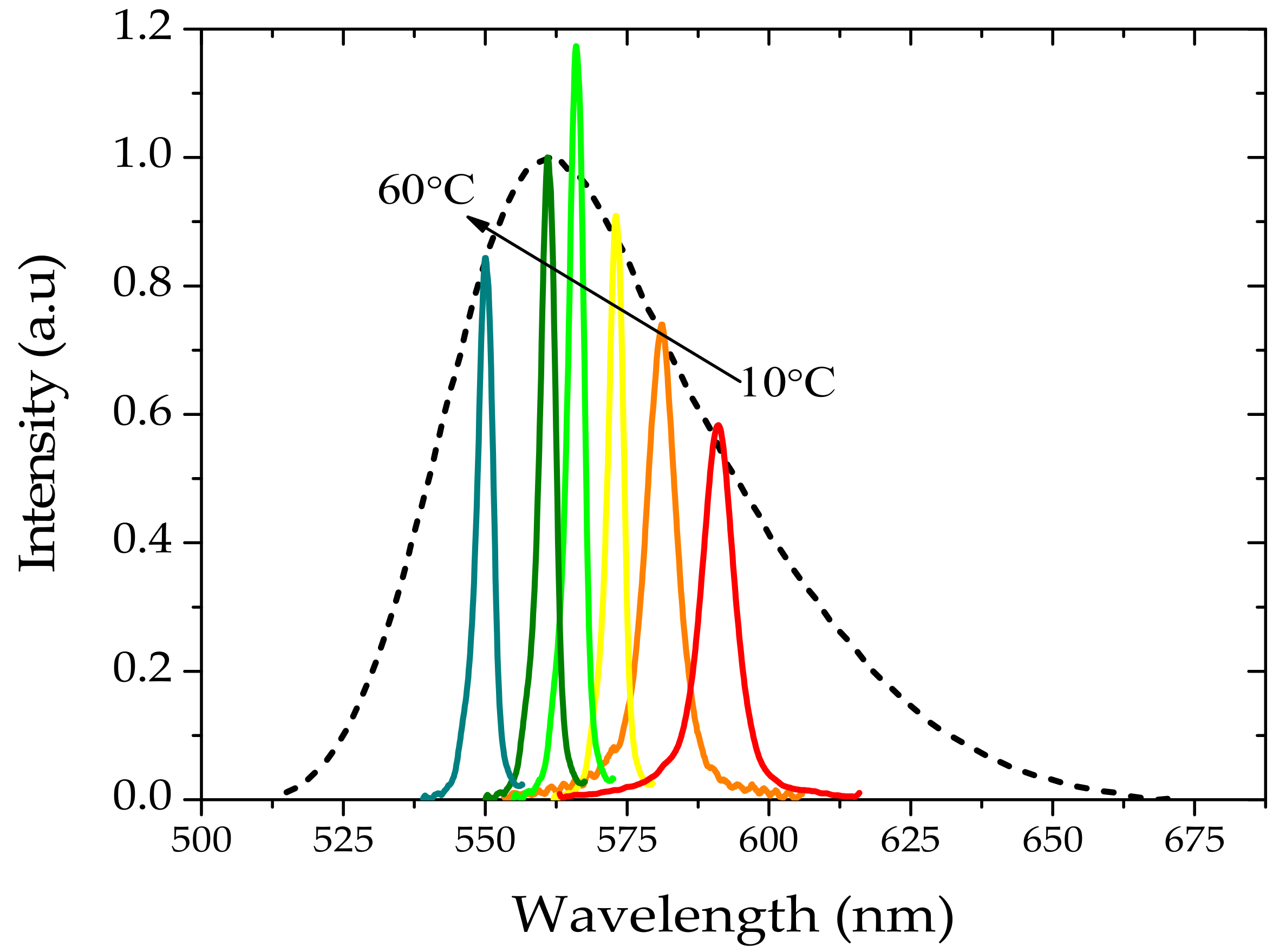
3.2. Amplified Spontaneous Emission
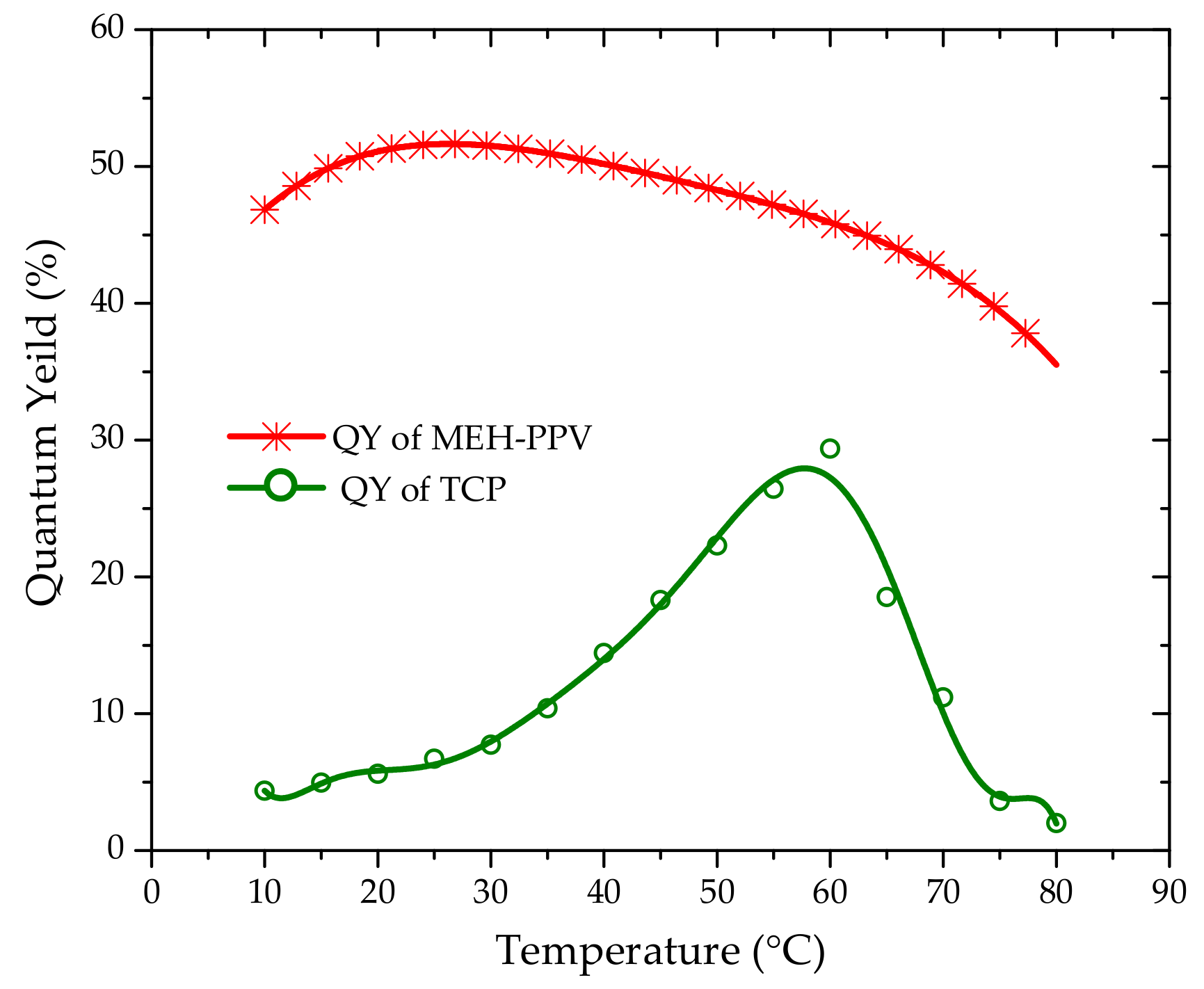
3.3. Energy Transfer between Oligomer and TCP
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Blinova, N.V.; Stejskal, J.; Trchová, M.; Prokeš, J.; Omastová, M. Polyaniline and polypyrrole: A comparative study of the preparation. Eur. Polym. J. 2007, 43, 2331–2341. [Google Scholar] [CrossRef]
- Kim, Y.; Cook, S.; Tuladhar, S.M.; Choulis, S.A.; Nelson, J.; Durrant, J.R.; Bradley, D.D.; Giles, M.; McCulloch, I.; Ha, C.-S. A strong regioregularity effect in self-organizing conjugated polymer films and high-efficiency polythiophene: Fullerene solar cells. Nat. Mater. 2006, 5, 197–203. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Ohshita, J.; Tada, Y.; Kunai, A.; Harima, Y.; Kunugi, Y. Hole-injection properties of annealed polythiophene films to replace PEDOT–PSS in multilayered OLED systems. Synth. Met. 2009, 159, 214–217. [Google Scholar] [CrossRef]
- Gadisa, A.; Svensson, M.; Andersson, M.R.; Inganäs, O. Correlation between oxidation potential and open-circuit voltage of composite solar cells based on blends of polythiophenes/fullerene derivative. Appl. Phys. Lett. 2004, 84, 1609–1611. [Google Scholar] [CrossRef]
- Beek, W.J.; Wienk, M.M.; Janssen, R.A. Hybrid solar cells from regioregular polythiophene and zno nanoparticles. Adv. Funct. Mater. 2006, 16, 1112–1116. [Google Scholar] [CrossRef]
- Briseno, A.L.; Holcombe, T.W.; Boukai, A.I.; Garnett, E.C.; Shelton, S.W.; Fréchet, J.J.; Yang, P. Oligo-and polythiophene/ZnO hybrid nanowire solar cells. Nano Lett. 2009, 10, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Plante, M.-P.; Bérubé, E.V.; Bissonnette, L.; Bergeron, M.G.; Leclerc, M. Polythiophene biosensor for rapid detection of microbial particles in water. ACS Appl. Mater. Interfaces 2013, 5, 4544–4548. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Nakanotani, H.; Yasuda, T.; Adachi, C. Light-emitting organic field-effect transistors based on highly luminescent single crystals of thiophene/phenylene co-oligomers. J. Mater. Chem. C 2014, 2, 4918–4921. [Google Scholar] [CrossRef]
- Pisignano, D.; Anni, M.; Gigli, G.; Cingolani, R.; Zavelani-Rossi, M.; Lanzani, G.; Barbarella, G.; Favaretto, L. Amplified spontaneous emission and efficient tunable laser emission from a substituted thiophene-based oligomer. Appl. Phys. Lett. 2002, 81, 3534–3536. [Google Scholar] [CrossRef]
- Anni, M.; Gigli, G.; Cingolani, R.; Zavelani-Rossi, M.; Gadermaier, C.; Lanzani, G.; Barbarella, G.; Favaretto, L. Amplified spontaneous emission from a soluble thiophene-based oligomer. Appl. Phys. Lett. 2001, 78, 2679–2681. [Google Scholar] [CrossRef]
- Mizuno, H.; Ohnishi, I.; Yanagi, H.; Sasaki, F.; Hotta, S. Lasing from epitaxially oriented needle crystals of a thiophene/phenylene co-oligomer. Adv. Mater. 2012, 24, 2404–2408. [Google Scholar] [CrossRef] [PubMed]
- Bando, K.; Nakamura, T.; Masumoto, Y.; Sasaki, F.; Kobayashi, S.; Hotta, S. Origin of the amplified spontaneous emission from thiophene/phenylene co-oligomer single crystals: Towards co-oligomer lasers. J. Appl. Phys. 2006, 99, 013518. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Hoshino, D.; Hotta, S. Unusually narrowed emission lines from a single crystal of thiophene/phenylene co-oligomer. Appl. Phys. Lett. 2003, 83, 4494–4496. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, H.; Xiao, Y. Replacing phenyl ring with thiophene: An approach to longer wavelength Aza-dipyrromethene boron difluoride (Aza-BODIPY) dyes. J. Ganic Chem. 2011, 77, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Granlund, T.; Theander, M.; Berggren, M.; Andersson, M.; Ruzeckas, A.; Sundström, V.; Björk, G.; Granström, M.; Inganäs, O. A polythiophene microcavity laser. Chem. Phys. Lett. 1998, 288, 879–884. [Google Scholar] [CrossRef]
- Rajendra, S.P.; Alsalhi, M.S.; Masilamani, V. Temperature Tuned Conjugated Polymer Laser. U.S. Patent US9698561B1, 4 July 2017. [Google Scholar]
- Prasad, S.; AlHesseny, H.S.; AlSalhi, M.S.; Devaraj, D.; Masilamai, V. A high power, frequency tunable colloidal quantum dot (CdSe/ZnS) laser. Nanomaterials 2017, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Ibnaouf, K.; AlSalhi, M.; Devaraj, D.; Masilamani, V. High power amplified spontaneous emission from an oligomer in solution. J. Lumin. 2015, 168, 109–113. [Google Scholar] [CrossRef]
- Masilamani, V.; Ibnaouf, K.; Alsalhi, M.; Yassin, O. Laser properties of a conjugate polymer (MEH-PPV) in the liquid-excimeric state. Laser Phys. 2007, 17, 1367–1373. [Google Scholar] [CrossRef]
- Moses, D. High quantum efficiency luminescence from a conducting polymer in solution: A novel polymer laser dye. Appl. Phys. Lett. 1992, 60, 3215–3216. [Google Scholar] [CrossRef]
- Prasad, S.; Ibnaouf, K.; AlSalhi, M.; Masilamani, V. Laser from the dimer state of a conjugated polymer (PFO) in solution. Polymer 2014, 55, 727–732. [Google Scholar] [CrossRef]
- Al-Asbahi, B.; Alsalhi, M.; Al-Dwayyan, A.; Jumali, M.H. Förster-type energy transfer mechanism in PF2/6 to MEH-PPV conjugated polymers. J. Lumin. 2012, 132, 386–390. [Google Scholar] [CrossRef]
- Gilbert, A.; Baggott, J. Essentials of Molecular Photochemistry; Blackwell Science: Oxford, UK, 1991. [Google Scholar]
| Temperature | R0 (nm) | |
|---|---|---|
| 10 | 0.64 | 3.61 |
| 20 | 0.62 | 3.63 |
| 30 | 0.55 | 3.55 |
| 40 | 0.50 | 3.52 |
| 50 | 0.48 | 3.31 |
| 60 | 0.44 | 3.18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlSalhi, M.S.; Almotiri, A.R.; Prasad, S.; Aljaafreh, M.J.; Othman, A.H.S.; Masilamai, V. A Temperature-Tunable Thiophene Polymer Laser. Polymers 2018, 10, 470. https://doi.org/10.3390/polym10050470
AlSalhi MS, Almotiri AR, Prasad S, Aljaafreh MJ, Othman AHS, Masilamai V. A Temperature-Tunable Thiophene Polymer Laser. Polymers. 2018; 10(5):470. https://doi.org/10.3390/polym10050470
Chicago/Turabian StyleAlSalhi, Mohamad S., Ahlam Rashed Almotiri, Saradh Prasad, Mamduh J. Aljaafreh, Ahmad H. S. Othman, and Vadivel Masilamai. 2018. "A Temperature-Tunable Thiophene Polymer Laser" Polymers 10, no. 5: 470. https://doi.org/10.3390/polym10050470
APA StyleAlSalhi, M. S., Almotiri, A. R., Prasad, S., Aljaafreh, M. J., Othman, A. H. S., & Masilamai, V. (2018). A Temperature-Tunable Thiophene Polymer Laser. Polymers, 10(5), 470. https://doi.org/10.3390/polym10050470







