Structure-Function Relationships in PMA and PMAT Series Copolymers for Polymer Solar Cells
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
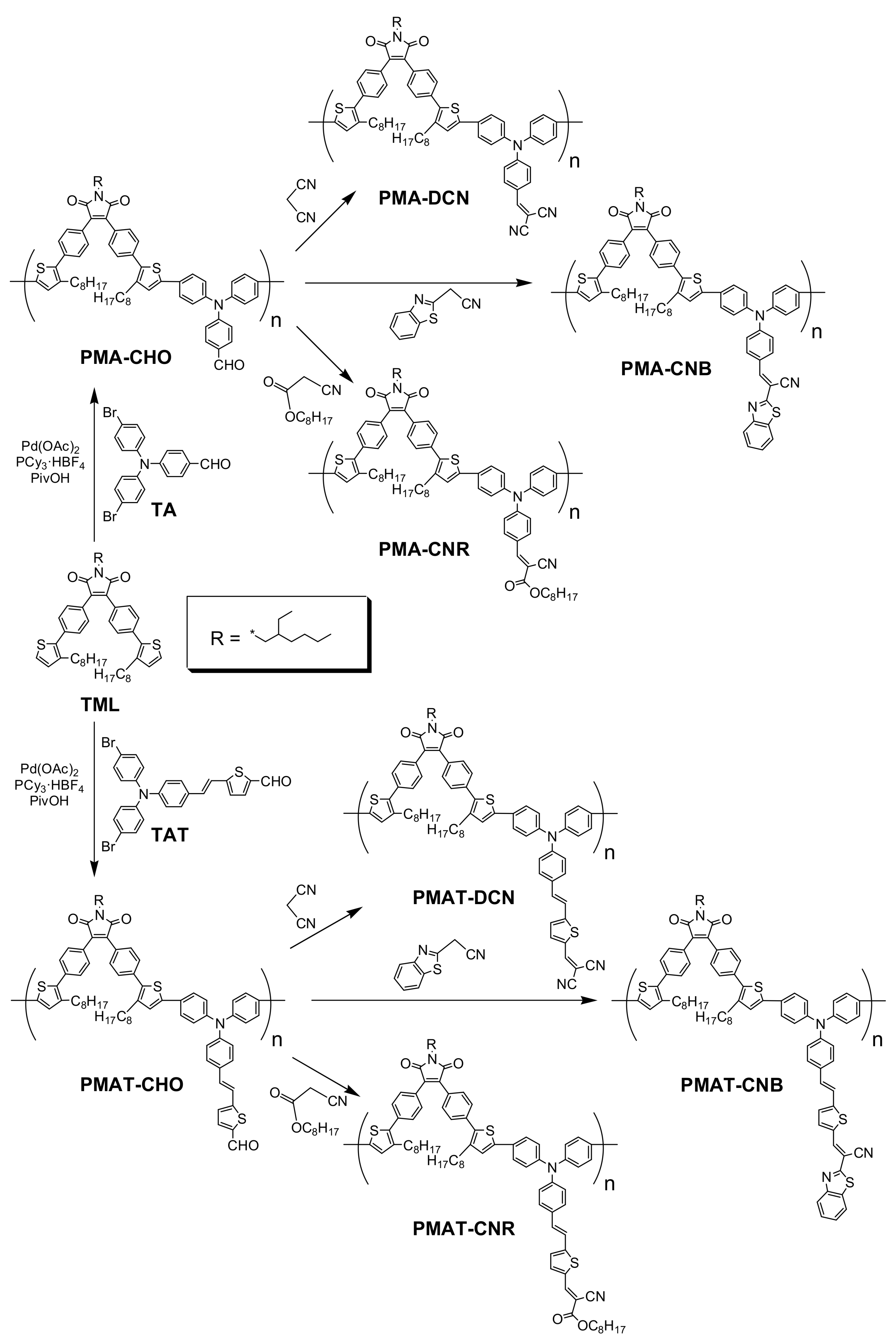
2.1.1. Synthesis of PMA-CHO
2.1.2. Synthesis of PMAT-CHO
2.1.3. Synthesis of PMA-DCN
2.1.4. Synthesis of PMA-CNR
2.1.5. Synthesis of PMA-CNB
2.1.6. Synthesis of PMAT-DCN
2.1.7. Synthesis of PMAT-CNR
2.1.8. Synthesis of PMAT-CNB
2.2. Characterization of Copolymers
2.3. Fabrication and Characterization of PSCs
3. Results and Discussion
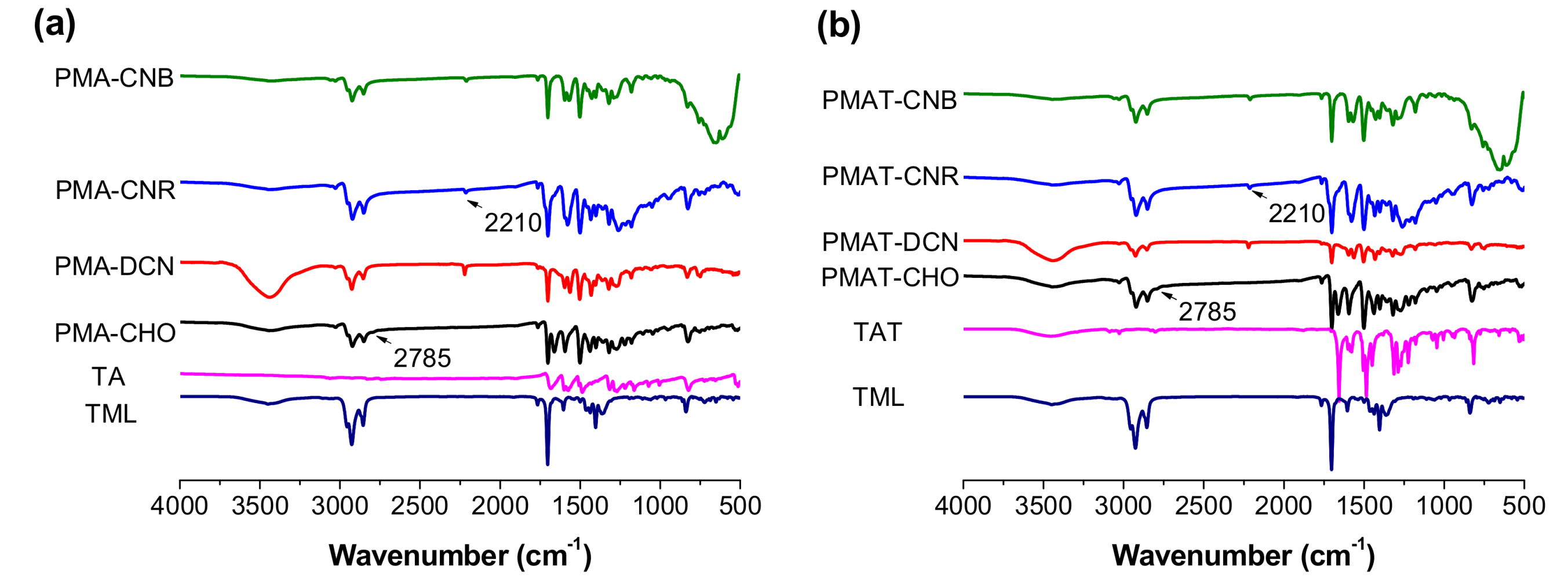
3.1. Characterization of Copolymers
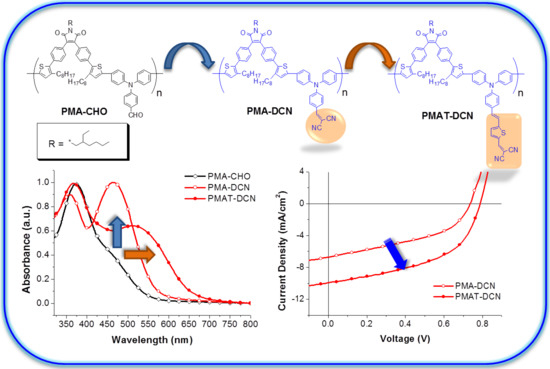
3.2. Optical Properties
3.3. Electrochemical Properties
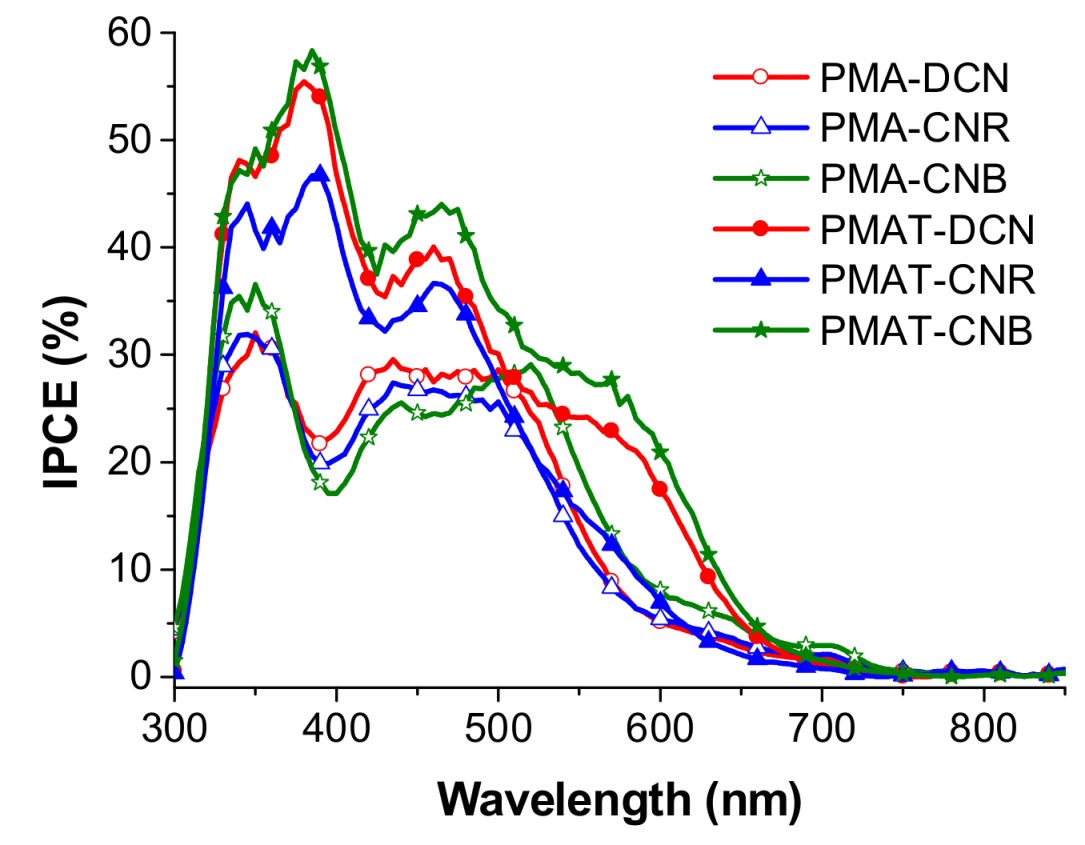
3.4. PV Properties of PSCs
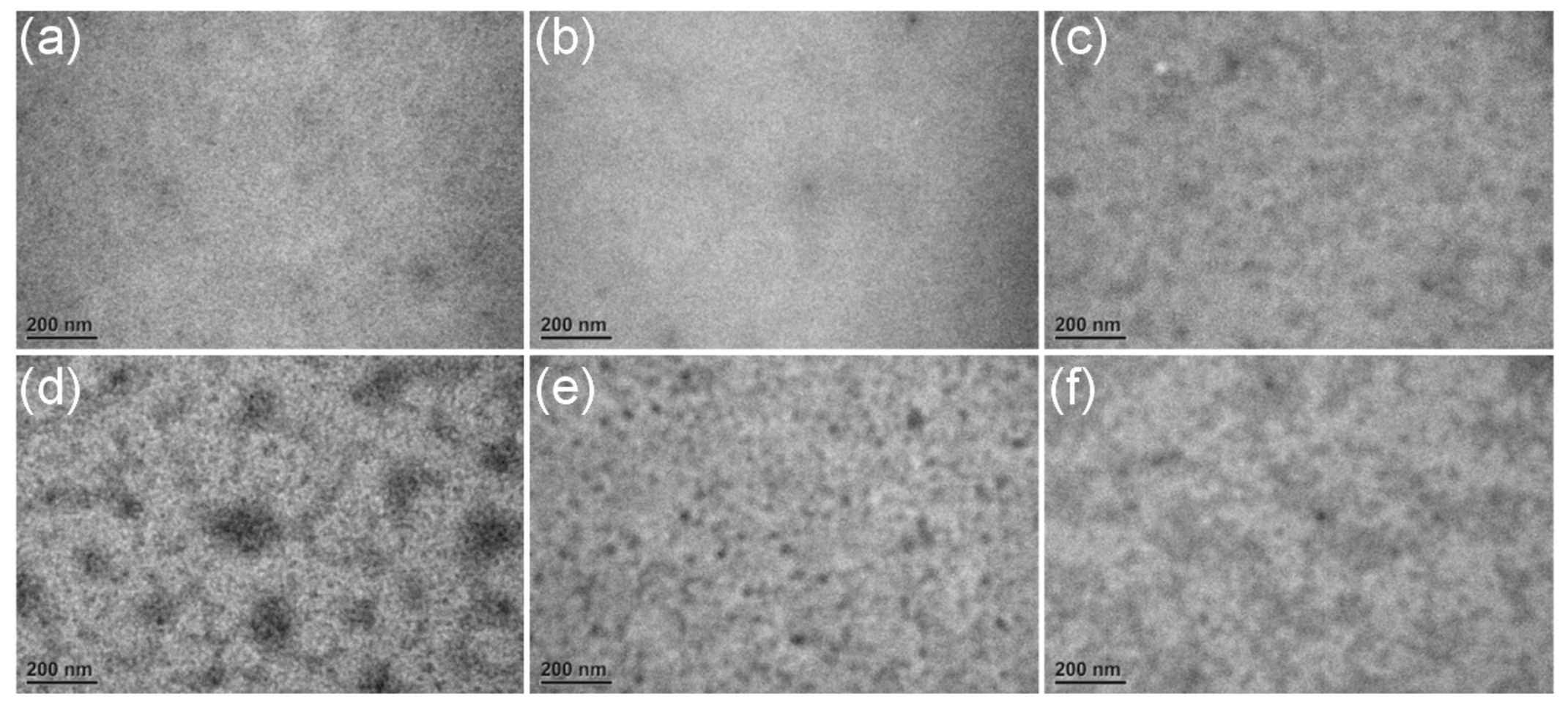
3.5. Transmission Electron Microscopy Investigation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153–161. [Google Scholar]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent advances in bulk heterojunction polymer solar cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 2016, 1, 15027. [Google Scholar] [CrossRef]
- Kawashima, K.; Fukuhara, T.; Suda, Y.; Suzuki, Y.; Koganezawa, T.; Yoshida, H.; Ohkita, H.; Osaka, I.; Takimiya, K. Implication of fluorine atom on electronic properties, ordering structures, and photovoltaic performance in naphthobisthiadiazole-based semiconducting polymers. J. Am. Chem. Soc. 2016, 138, 10265–10275. [Google Scholar] [PubMed]
- Zhou, H.Q.; Zhang, Y.; Mai, C.-K.; Collins, S.D.; Bazan, G.C.; Nguyen, T.-Q.; Heeger, A.J. Polymer homo-tandem solar cells with best efficiency of 11.3%. Adv. Mater. 2015, 27, 1767–1773. [Google Scholar] [PubMed]
- Zheng, Z.; Zhang, S.; Zhang, J.; Qin, Y.; Li, W.; Yu, R.; Wei, Z.; Hou, J. Over 11% Efficiency in tandem polymer solar cells featured by a low-band-gap polymer with fine-tuned properties. Adv. Mater. 2016, 28, 5133–5138. [Google Scholar] [CrossRef] [PubMed]
- Price, S.C.; Stuart, A.C.; Yang, L.; Zhou, H.; You, W. Fluorine substituted conjugated polymer of medium band gap yields 7% efficiency in polymer–fullerene solar cells. J. Am. Chem. Soc. 2011, 133, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, H.A.; Lu, L.; He, F.; Bullock, J.E.; Wang, W.; Carsten, B.; Yu, L. Polyselenopheno[3,4-b]selenophene for highly efficient bulk heterojunction solar cells. ACS Macro Lett. 2012, 1, 361–365. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, M.; Ma, W.; Ye, L.; Zhang, S.; Liu, S.; Ade, H.; Huang, F.; Hou, J. Enhanced photovoltaic performance by modulating surface composition in bulk heterojunction polymer solar cells based on PBDTTT-C-T/PC71BM. Adv. Mater. 2014, 26, 4043–4049. [Google Scholar] [PubMed]
- Wang, D.H.; Moon, J.S.; Seifter, J.; Jo, J.; Park, J.H.; Park, O.O.; Heeger, A.J. Sequential processing: Control of nanomorphology in bulk heterojunction solar cells. Nano Lett. 2011, 11, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wu, H.; Liu, S.; Luo, G.; Wang, W.; Guo, Z.; Wei, W.; Gao, C.; An, Z. Efficient alternating polymer based on benzodithiophene and di-fluorinated quinoxaline derivatives for bulk heterojunction photovoltaic cells. Polymer 2017, 116, 35–42. [Google Scholar] [CrossRef]
- Li, Y. Molecular Design of photovoltaic materials for polymer solar cells: Toward suitable electronic energy levels and broad absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Roelofs, W.S.C.; Wienk, M.M.; Janssen, R.A.J. Enhancing the photocurrent in diketopyrrolopyrrole-based polymer solar cells via energy level control. J. Am. Chem. Soc. 2012, 134, 13787–13795. [Google Scholar] [CrossRef] [PubMed]
- Cong, Z.; Zhao, B.; Wu, H.; Guo, Z.; Wang, W.; Luo, G.; Xu, J.; Xia, Y.; Gao, C.; An, Z. Synthesis of copolymers based on benzo[1,2-b:4,5-b′]difuran and fluorinated quinoxaline derivatives and their photovoltaic properties. Polymer 2015, 67, 55–62. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, L.; Zhao, W.; Yang, B.; Wang, Q.; Hou, J. Realizing over 10% efficiency in polymer solar cell by device optimization. Sci. China Chem. 2015, 58, 248–256. [Google Scholar] [CrossRef]
- Hou, J.; Tan, Z.; Yan, Y.; He, Y.; Yang, C.; Li, Y. Synthesis and photovoltaic properties of two-dimensional conjugated polythiophenes with bi(thienylenevinylene) side chains. J. Am. Chem. Soc. 2006, 128, 4911–4916. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Cai, W.; Huang, F.; Zhang, J.; Wang, M.; Yang, T.; Zhong, C.; Gong, X.; Cao, Y. Novel Silafluorene-Based Conjugated Polymers with Pendant Acceptor Groups for High Performance Solar Cells. Macromolecules 2010, 43, 5262–5268. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, L.; Zhao, W.; Liu, D.; Yao, H.; Hou, J. Side chain selection for designing highly efficient photovoltaic polymers with 2D-conjugated structure. Macromolecules 2014, 47, 4653–4659. [Google Scholar] [CrossRef]
- Shen, P.; Bin, H.; Xiao, L.; Li, Y. Enhancing photovoltaic performance of copolymers containing thiophene unit with D–A conjugated side chain by rational molecular design. Macromolecules 2013, 47, 9575–9586. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.-H.; Moon, B.; Kim, H.G.; Kim, M.; Shin, J.; Hwang, H.; Cho, K. Two-dimensionally extended π-conjugation of donor–acceptor copolymers via oligothienyl side chains for efficient polymer solar cells. Macromolecules 2017, 50, 7984–7992. [Google Scholar] [CrossRef]
- Katz, H.E.; Lovinger, A.J.; Johnson, J.; Kloc, C.; Siegrist, T.; Li, W.; Lin, Y.-Y.; Dodabalapur, A. A soluble and air-stable organic semiconductor with high electron mobility. Nature 2000, 404, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Malenfant, P.R.L.; Dimitrakopoulos, C.D.; Gelorme, J.D.; Kosbar, L.L.; Graham, T.O. N-type organic thin-film transistor with high field-effect mobility based on a N,N′-dialkyl-3,4,9,10-perylene tetracarboxylic diimide derivative. Appl. Phys. Lett. 2002, 80, 2517–2519. [Google Scholar] [CrossRef]
- Unni, K.N.N.; Pandey, A.K.; Nunzi, J.-M. N-channel organic field-effect transistors using N,N′-ditridecylperylene-3, 4, 9, 10-tetracarboxylic diimide and a polymeric dielectric. Chem. Phys. Lett. 2005, 407, 95–99. [Google Scholar] [CrossRef]
- Chen, H.-Z.; Shi, M.-M.; Aernouts, T.; Wang, M.; Borghs, G.; Heremans, P. A novel organic n-type material: Fluorinated perylene diimide. Sol. Energy Mater. Sol. Cells 2005, 87, 521–527. [Google Scholar] [CrossRef]
- Wu, W.-C.; Yeh, H.-C.; Chan, L.-H.; Chen, C.-T. Red organic light-emitting diodes with a non-doping amorphous red emitter. Adv. Mater. 2002, 14, 1072–1075. [Google Scholar] [CrossRef]
- Chan, L.-H.; Juang, S.-Y.; Chen, M.-C.; Lin, Y.-J. A new series of random conjugated copolymers containing 3,4-diphenyl-maleimide and thiophene units for organic photovoltaic cell applications. Polymer 2012, 53, 2334–2346. [Google Scholar] [CrossRef]
- Wang, J.; Liu, K.; Ma, L.; Zhan, X. Triarylamine: Versatile platform for organic, dye-sensitized, and perovskite solar cells. Chem. Rev. 2016, 116, 14675–14725. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.-H.; Lee, Y.-D.; Chen, C.-T. Achieving saturated red photoluminescence and electroluminescence with readily synthesized maleimide-arylamine copolymers. Tetrahedron 2006, 62, 9541–9547. [Google Scholar] [CrossRef]
- Chan, L.-H.; Lee, Y.-D.; Chen, C.-T. Synthesis and characterization of 3,4-diphenylmaleimide copolymers that exhibit orange to red photoluminescence and electroluminescence. Macromolecules 2006, 39, 3262–3269. [Google Scholar] [CrossRef]
- Chan, L.-H.; Lee, Y.-D.; Chen, C.-T. 3,4-Diphenylmaleimide-thiophene-fluorene copolymers for polymeric orange–red light-emitting diodes. Org. Electron. 2006, 7, 55–59. [Google Scholar] [CrossRef]
- Wood, C.J.; Summers, G.H.; Gibson, E.A. Increased photocurrent in a tandem dye-sensitized solar cell by modifications in push–pull dye-design. Chem. Commun. 2015, 51, 3915–3918. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chen, K.-S.; Yip, H.-L.; Hau, S.K.; Acton, O.; Zhang, Y.; Luo, J.; Jen, A.K.-Y. Development of new conjugated polymers with donor–π-bridge–acceptor side chains for high performance solar cells. J. Am. Chem. Soc. 2009, 131, 13886–13887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-G.; Liu, Y.-L.; Yang, Y.; Hou, K.; Peng, B.; Zhao, G.; Zhang, M.; Guo, X.; Kang, E.-T.; Li, Y. Alternating copolymers of carbazole and triphenylamine with conjugated side chain attaching acceptor groups: Synthesis and photovoltaic application. Macromolecules 2010, 43, 9376–9383. [Google Scholar] [CrossRef]
- Guo, Q.; Dong, J.; Wan, D.; Wu, D.; You, J. Modular establishment of a diketopyrrolopyrrole-based polymer library via Pd-catalyzed direct C–H (Hetero)arylation: A highly efficient approach to discover low-bandgap polymers. Macromol. Rapid Commun. 2013, 34, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-M.; Lin, H.-K.; Lin, Y.-C.; Chen, H.-C.; Lan, S.-C.; Chang, C.-K.; Wei, K.-H. Side chain structure affects the photovoltaic performance of two-dimensional conjugated polymers. Macromolecules 2014, 47, 70–78. [Google Scholar] [CrossRef]
- Wang, X.; Tong, J.; Guo, P.; Li, Y.; Li, H.; Xia, Y.; Wang, F. Low-bandgap conjugated polymers based on alkylthiothienyl-substituted benzodithiophene for efficient bulk heterojunction polymer solar cells. Polymer 2017, 122, 96–104. [Google Scholar] [CrossRef]
- Qi, B.; Wang, J. Open-circuit voltage in organic solar cells. J. Mater. Chem. 2012, 22, 24315–24325. [Google Scholar] [CrossRef]
- Jackson, N.E.; Savoie, B.M.; Marks, T.J.; Chen, L.X.; Ratner, M.A. The next breakthrough for organic photovoltaics? J. Phys. Chem. Lett. 2015, 6, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-J.; Li, H.; Courtright, B.A.E.; Subramaniyan, S.; Jenekhe, S.A. Nonfullerene polymer solar cells with 8.5% efficiency enabled by a new highly twisted electron acceptor dimer. Adv. Mater. 2016, 28, 124–131. [Google Scholar] [CrossRef] [PubMed]



| Copolymers | Mn a (g/mol) | Mw a (g/mol) | PDI (Mw/Mn) | Td b (°C) |
|---|---|---|---|---|
| PMA-CHO | 7620 | 9220 | 1.21 | 418 |
| PMA-DCN | 8830 | 11,480 | 1.30 | 385 |
| PMA-CNR | 7830 | 11,350 | 1.45 | 325 |
| PMA-CNB | 7390 | 10,200 | 1.38 | 419 |
| PMAT-CHO | 7880 | 9300 | 1.18 | 388 |
| PMAT-DCN | 8200 | 11,890 | 1.45 | 421 |
| PMAT-CNR | 9600 | 13,350 | 1.39 | 346 |
| PMAT-CNB | 8020 | 12,110 | 1.51 | 410 |
| Copolymers | In Solution a (nm) | In Film b (nm) | HOMO/LUMO c (eV) | d (V) |
|---|---|---|---|---|
| PMA-CHO | 374 | 372 | −5.44/−3.43 | 2.01 |
| PMA-DCN | 355, 455 | 358, 465 | −5.46/−3.65 | 1.81 |
| PMA-CNR | 363, 448 | 363, 452 | −5.45/−3.70 | 1.75 |
| PMA-CNB | 360, 470 | 365, 483 | −5.42/−3.62 | 1.80 |
| PMAT-CHO | 358, 442 | 362, 444 | −5.30/−3.45 | 1.86 |
| PMAT-DCN | 363, 501 | 367, 513 | −5.29/−3.80 | 1.49 |
| PMAT-CNR | 367, 472 | 371, 486 | −5.30/−3.76 | 1.54 |
| PMAT-CNB | 367, 484 | 371, 493 | −5.31/−3.78 | 1.53 |
| Active Layer | Voc (mV) | Jsc [Jsc] a (mA/cm2) | FF (%) | PCE (%) | Thickness (nm) |
|---|---|---|---|---|---|
| PMA-DCN | 742 | 6.70 [6.41] | 43.02 | 2.14 | 95 |
| PMA-CNR | 766 | 6.72 [6.59] | 39.86 | 2.05 | 110 |
| PMA-CNB | 742 | 6.95 [7.09] | 41.93 | 2.16 | 102 |
| PMAT-DCN | 782 | 9.89 [9.85] | 51.85 | 4.01 | 113 |
| PMAT-CNR | 762 | 8.84 [8.72] | 46.55 | 3.14 | 90 |
| PMAT-CNB | 769 | 10.42 [10.36] | 47.73 | 3.82 | 121 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-H.; Liu, C.-K.; Chang, W.-C.; Sah, P.-T.; Chan, L.-H. Structure-Function Relationships in PMA and PMAT Series Copolymers for Polymer Solar Cells. Polymers 2018, 10, 384. https://doi.org/10.3390/polym10040384
Chen J-H, Liu C-K, Chang W-C, Sah P-T, Chan L-H. Structure-Function Relationships in PMA and PMAT Series Copolymers for Polymer Solar Cells. Polymers. 2018; 10(4):384. https://doi.org/10.3390/polym10040384
Chicago/Turabian StyleChen, Jhe-Han, Chi-Kan Liu, Wei-Che Chang, Pai-Tao Sah, and Li-Hsin Chan. 2018. "Structure-Function Relationships in PMA and PMAT Series Copolymers for Polymer Solar Cells" Polymers 10, no. 4: 384. https://doi.org/10.3390/polym10040384
APA StyleChen, J.-H., Liu, C.-K., Chang, W.-C., Sah, P.-T., & Chan, L.-H. (2018). Structure-Function Relationships in PMA and PMAT Series Copolymers for Polymer Solar Cells. Polymers, 10(4), 384. https://doi.org/10.3390/polym10040384