Investigation on the Nanomechanics of Liposome Adsorption on Titanium Alloys: Temperature and Loading Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Liposomes and Adsorbed Surfaces
2.3. Atomic Force Microscopy
3. Results and Discussion
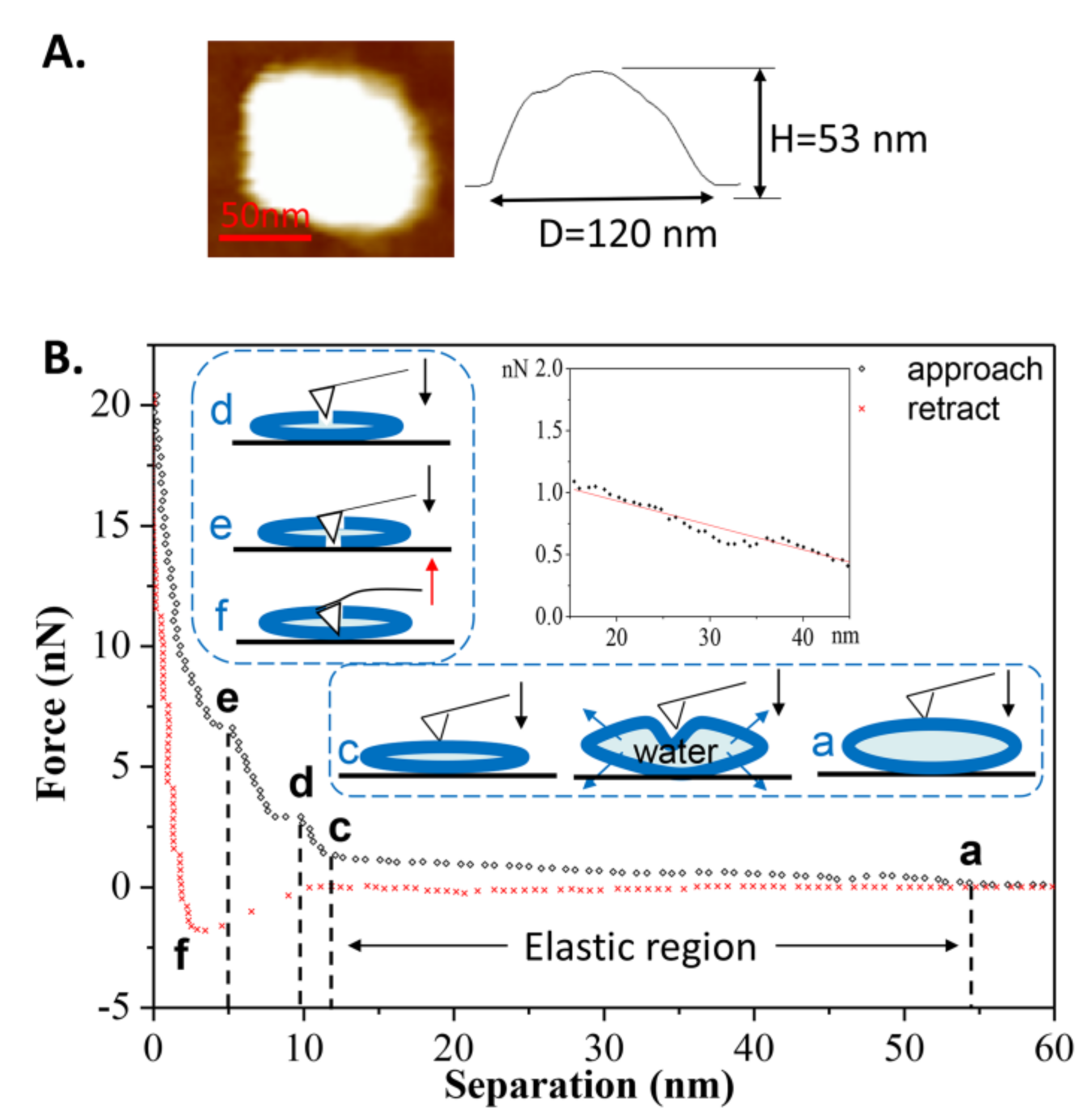
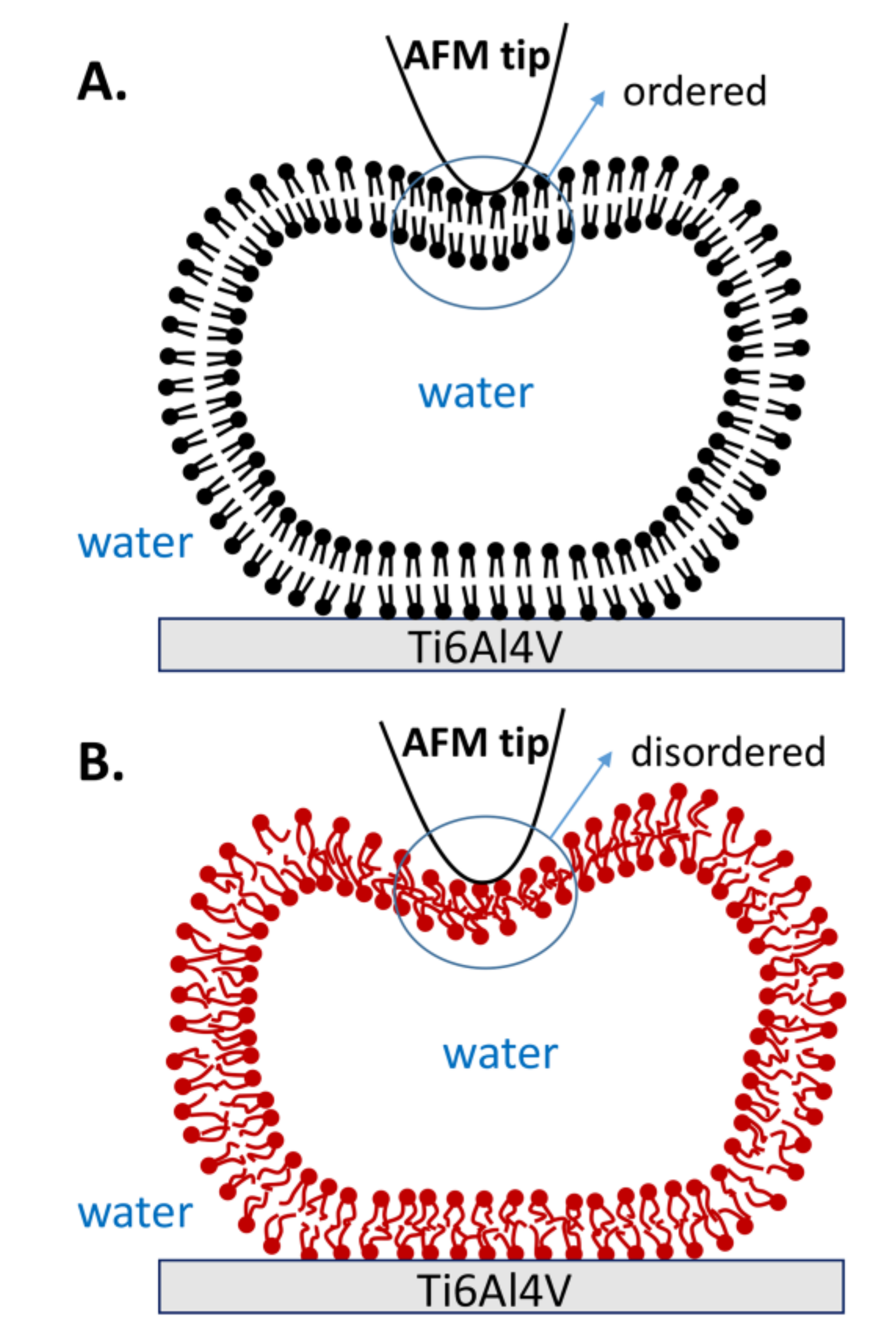
3.1. Characterization of Liposomes Adsorbing on Surfaces
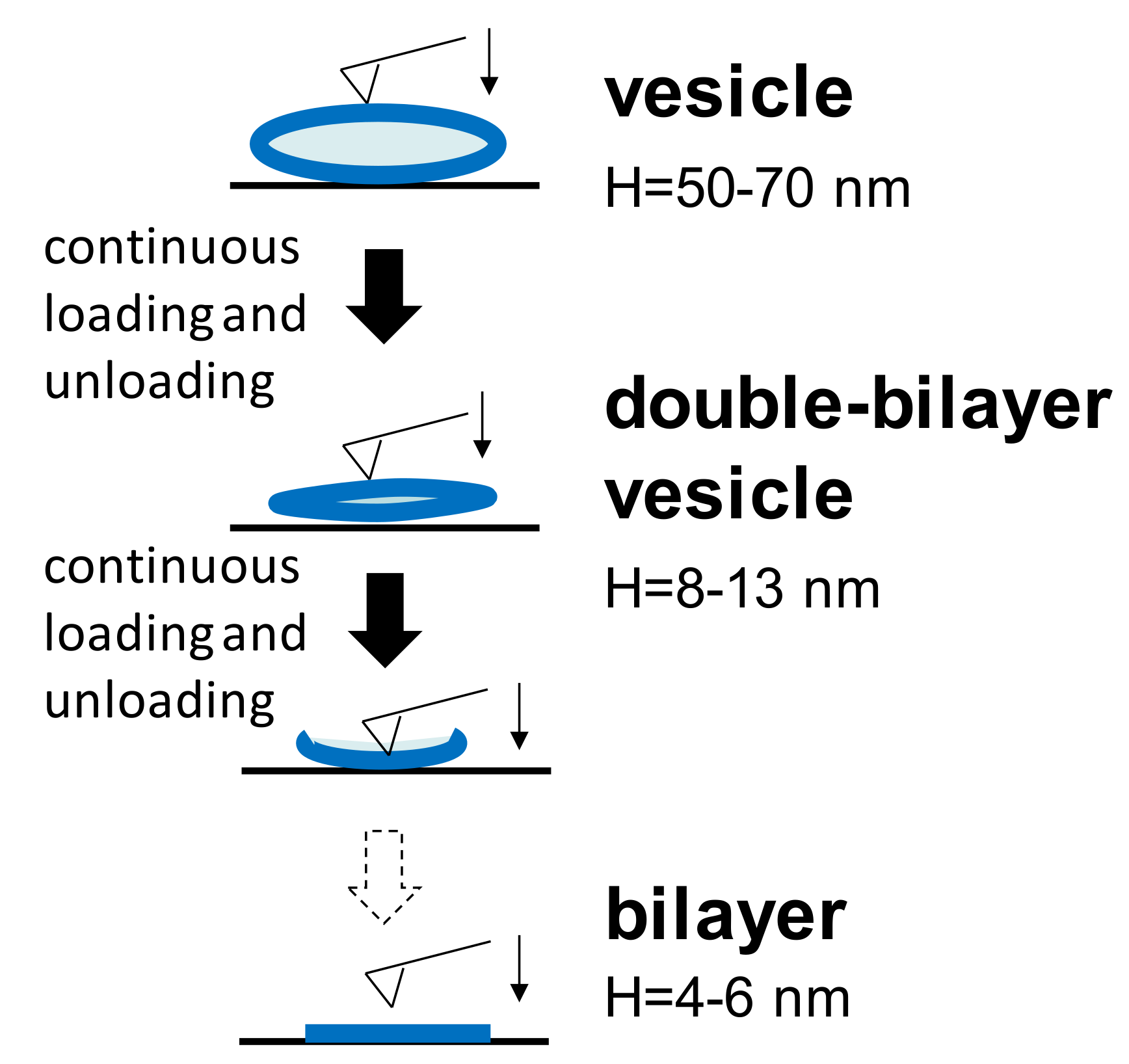
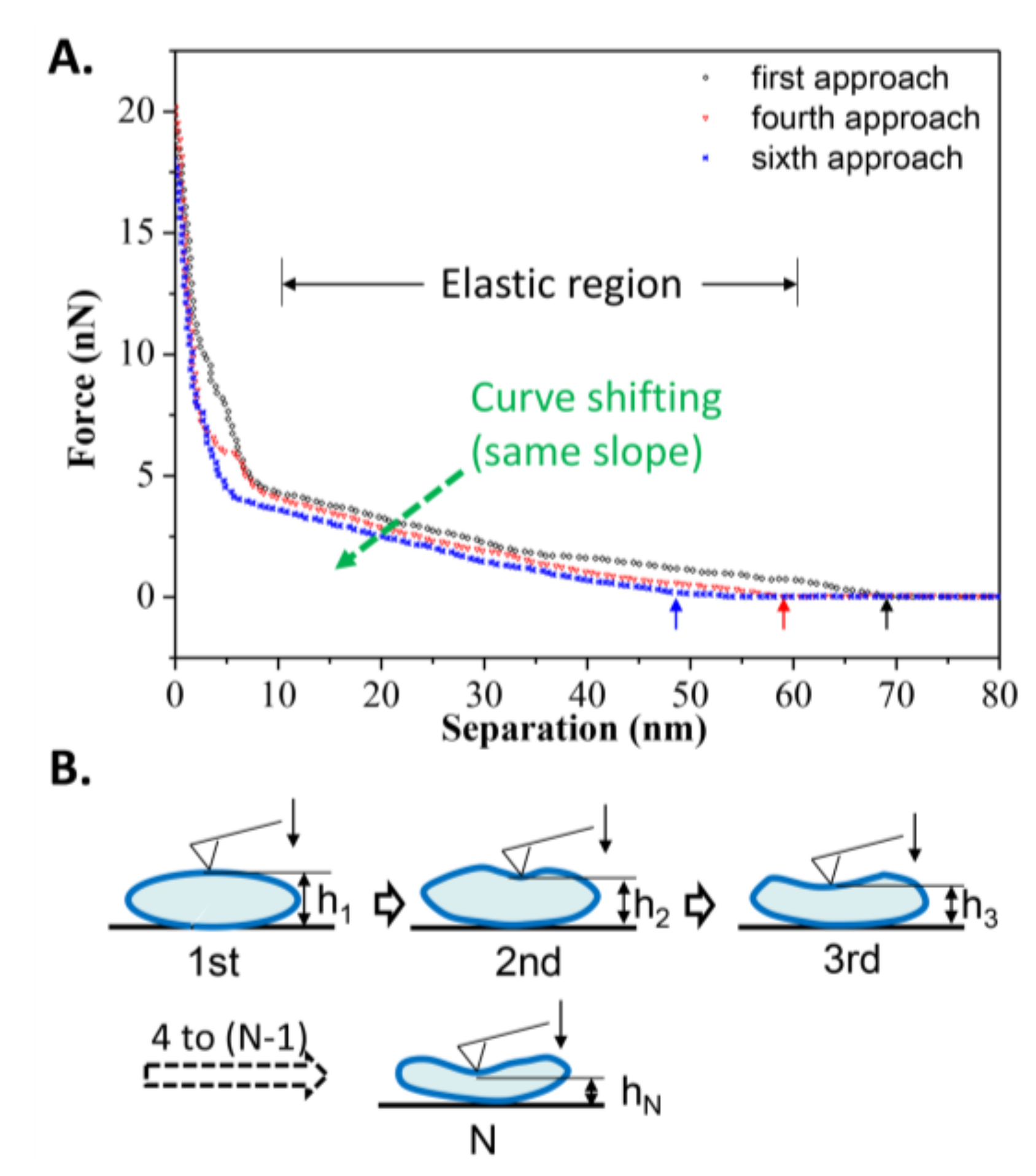
3.2. Three Different Structures of DPPC Vesicle Observed in a Continuous Loading and Unloading Process
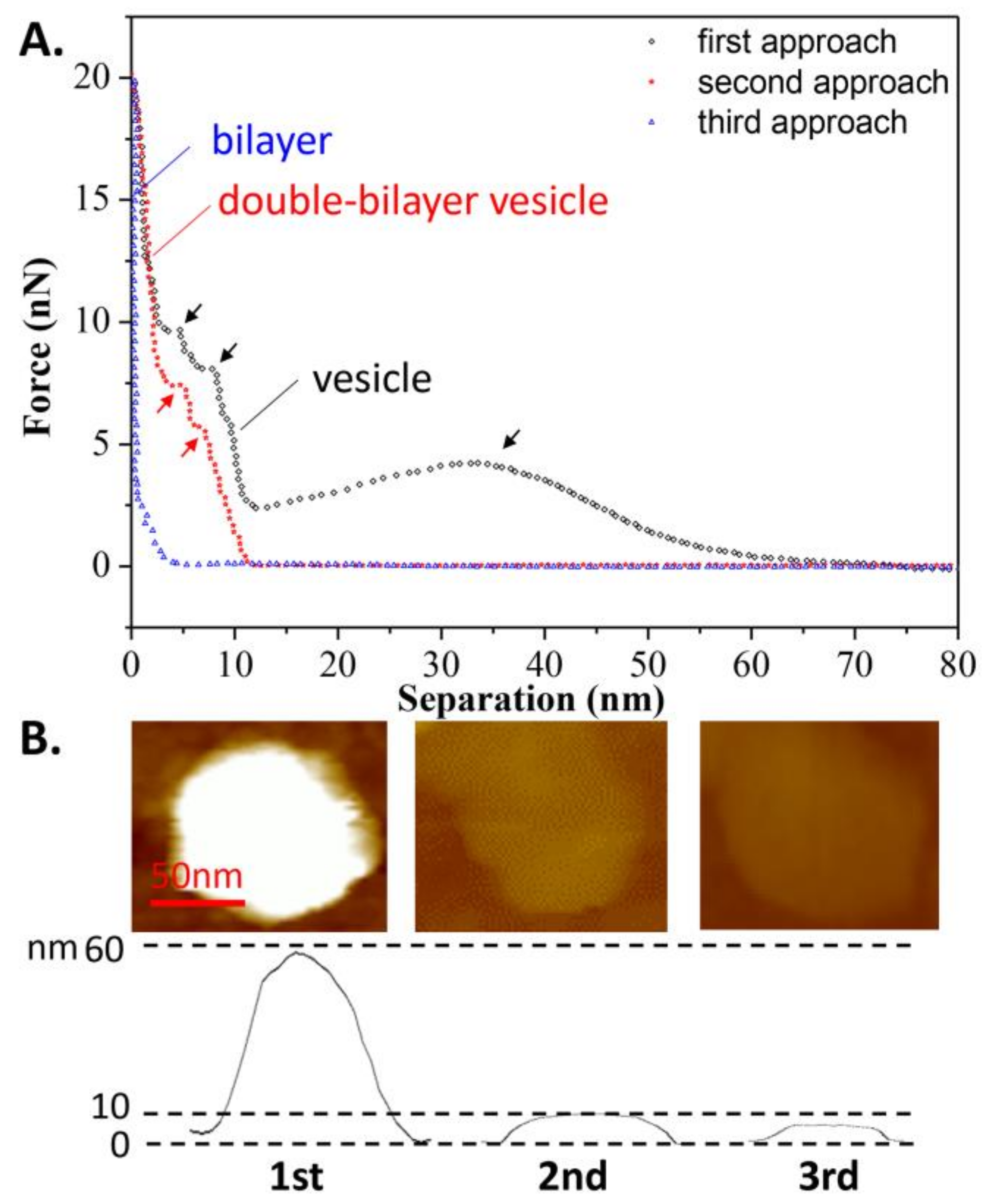
3.3. Force Measurements of DPPC Vesicle Obtained at T1 = 60 °C
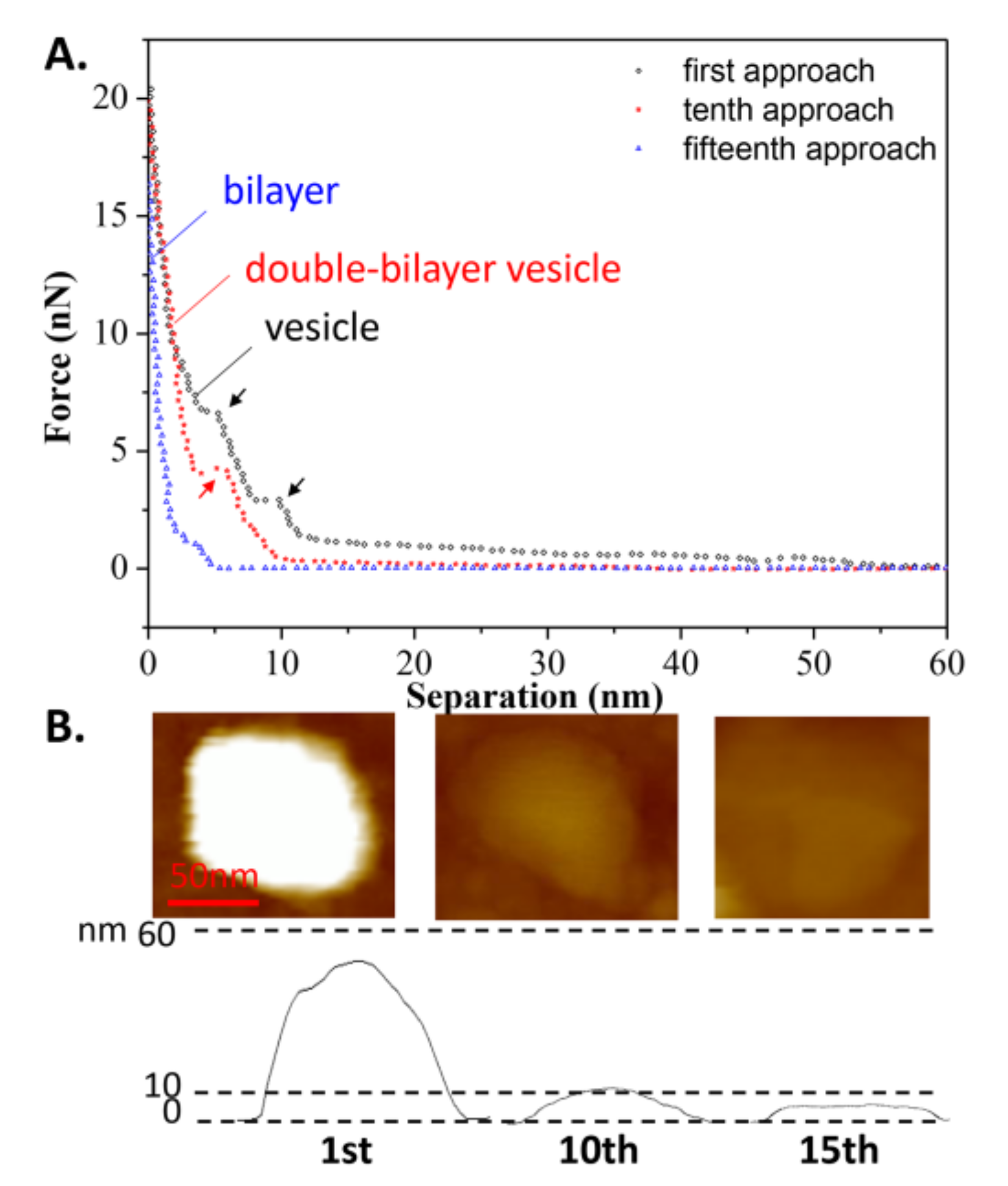
3.4. Force Measurements of DPPC Vesicles Obtained at T2 = 25 °C
3.5. Statistical Research on Comprehensive Bearing Capacity of DPPC Vesicles
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jesorka, A.; Orwar, O. Liposomes: Technologies and Analytical Applications. Ann. Rev. Anal. Chem. 2008, 1, 801–832. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of Liposomes in Medicine and Drug Delivery. Artif. Cell Nanomed. B 2016, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Velez, M.A.; Perotti, M.C.; Zanel, P.; Hynes, E.R.; Gennaro, A.M. Soy PC Liposomes as CLA Carriers for Food Applications: Preparation and Physicochemical Characterization. J. Food Eng. 2017, 212, 174–180. [Google Scholar] [CrossRef]
- Fakhravar, Z.; Ebrahimnejad, P.; Daraee, H.; Akbarzadeh, A. Nanoliposomes: Synthesis Methods and Applications in Cosmetics. J. Cosmet. Laser Ther. 2016, 18, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Pinnapireddy, S.R.; Duse, L.; Strehlow, B.; Schafer, J.; Bakowsky, U. Composite liposome-PEI/nucleic Acid Lipopolyplexes for Safe and Efficient Gene Delivery and Gene Knockdown. Colloids Surf. B Biointerfaces 2017, 158, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.; Schroeder, A.; Silbert, G.; Turjeman, K.; Barenholz, Y.; Klein, J. Boundary Lubricants with Exceptionally Low Friction Coefficients Based on 2D Close-Packed Phosphatidylcholine Liposomes. Adv. Mater. 2011, 23, 3517–3521. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, R.; Kampf, N.; Dror, Y.; Shimoni, E.; Klein, J. Origins of extreme boundary lubrication by phosphatidylcholine liposomes. Biomaterials 2013, 34, 5465–5475. [Google Scholar] [CrossRef] [PubMed]
- Gaisinskaya-Kipnis, A.; Klein, J. Normal and Frictional Interactions between Liposome-Bearing Biomacromolecular Bilayers. Biomacromolecules 2016, 17, 2591–2602. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, R.; Kampf, N.; Zhu, L.; Klein, J. Hydration lubrication and shear-induced self-healing of lipid bilayer boundary lubricants in phosphatidylcholine dispersions. Soft Matter 2016, 12, 2773–2784. [Google Scholar] [CrossRef] [PubMed]
- Sotres, J.; Arnebrant, T. Experimental Investigations of Biological Lubrication at the Nanoscale: The Cases of Synovial Joints and the Oral Cavity. Lubricants 2013, 1, 102–131. [Google Scholar] [CrossRef]
- Gardner, D.L.; O‘Connor, P.; Middleton, J.F.; Oates, K.; Orford, C.R. An investigation by transmission electron microscopy of freeze replicas of dog articular cartilage surfaces: The fibre-rich surface structure. J. Anat. 1983, 137, 573–582. [Google Scholar] [PubMed]
- Watanabe, M.; Leng, C.; Toriumi, H.; Hamada, Y.; Akamatsu, N.; Ohno, S. Ultrastructural study of upper surface layer in rat articular cartilage by “in vivo cryotechnique” combined with various treatments. Med. Electron. Microsc. 2000, 33, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Klein, J. Hydration lubrication. Friction 2013, 1, 1–23. [Google Scholar] [CrossRef]
- Pabst, G.; Rappolt, M.; Amenitsch, H.; Laggner, P. Structural information from multilamellar liposomes at full hydration: Full q-range fitting with high quality X-ray data. Phys. Rev. E 2000, 62, 4000–4009. [Google Scholar] [CrossRef]
- Seror, J.; Zhu, L.; Goldberg, R.; Day, A.J.; Klein, J. Supramolecular synergy in the boundary lubrication of synovial joints. Nat. Commun. 2015, 6, 6497. [Google Scholar] [CrossRef] [PubMed]
- Sivan, S.; Schroeder, A.; Verberne, G.; Merkher, Y.; Diminsky, D.; Priev, A.; Maroudas, A.; Halperin, G.; Nitzan, D.; Etsion, I.; et al. Liposomes Act as Effective Biolubricants for Friction Reduction in Human Synovial Joints. Langmuir 2010, 26, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Liu, Y.; Zhang, C.; Chen, Z.; Wen, S. Insight into the Tribological Behavior of Liposomes in Artificial Joints. Langmuir 2016, 32, 10957–10966. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, M.P.; Poehlmann, M.; Fery, A. Microcapsule mechanics: From stability to function. Adv. Colloid Interface Sci. 2014, 207, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Lipowsky, R. Remodeling of membrane compartments: Some consequences of membrane fluidity. Biol. Chem. 2014, 395, 253. [Google Scholar] [CrossRef] [PubMed]
- Duangjit, S.; Opanasopit, P.; Rojanarata, T.; Obata, Y.; Takayama, K.; Ngawhirunpat, T.; Pamornpathomkul, B. Role of the charge, carbon chain length, and content of surfactant on the skin penetration of meloxicam-loaded liposomes. Int. J. Nanomed. 2014, 9, 2005–2017. [Google Scholar] [CrossRef] [PubMed]
- Trunfio-Sfarghiu, A.; Berthier, Y.; Meurisse, M.; Rieu, J. Role of Nanomechanical Properties in the Tribological Performance of Phospholipid Biomimetic Surfaces. Langmuir 2008, 24, 8765–8771. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, R.; Dror, Y.; Kampf, N.; Klein, J. Mechanical Stability and Lubrication by Phosphatidylcholine Boundary Layers in the Vesicular and in the Extended Lamellar Phases. Langmuir 2014, 30, 5005–5014. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Manzanares, G.; González-Bermúdez, B.; Cruces, J.; De La Fuente, M.; Li, Q.; Guinea, G.V.; Pérez-Rigueiro, J.; Elices, M.; Plaza, G.R. Improved Measurement of Elastic Properties of Cells by Micropipette Aspiration and Its Application to Lymphocytes. Ann. Biomed. Eng. 2017, 45, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Dimova, R. Recent developments in the field of bending rigidity measurements on membranes. Adv. Colloid Interface Sci. 2014, 208, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Marie, H.; Plassat, V.; Lesieur, S. Magnetic-fluid-loaded liposomes for MR imaging and therapy of cancer. J. Drug Deliv. Sci. Technol. 2013, 23, 25–37. [Google Scholar] [CrossRef]
- Schaefer, E.; Vache, M.; Kliesch, T.; Janshoff, A. Mechanical response of adherent giant liposomes to indentation with a conical AFM-tip. Soft Matter 2015, 11, 4487–4495. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.S.; Rangamani, P.; Liedberg, B.; Parikh, A.N. Mixing Water, Transducing Energy, and Shaping Membranes: Autonomously Self-Regulating Giant Vesicles. Langmuir 2016, 32, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Spyratou, E.; Cunaj, E.; Tsigaridas, G.; Mourelatou, E.A.; Demetzos, C.; Serafetinides, A.A.; Makropoulou, M. Measurements of liposome biomechanical properties by combining line optical tweezers and dielectrophoresis. J. Liposome Res. 2015, 25, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Mao, G.; Simon Ng, K.Y. Probing small unilamellar EggPC vesicles on mica surface by atomic force microscopy. Colloids Surf. B Biointerfaces 2004, 34, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Mao, G.; Ng, K.Y.S. Mechanical properties and stability measurement of cholesterol-containing liposome on mica by atomic force microscopy. J. Colloid Interface Sci. 2004, 278, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Et-Thakafy, O.; Delorme, N.; Gaillard, C.; Meriadec, C.; Artzner, F.; Lopez, C.; Guyomarc, H.F. Mechanical Properties of Membranes Composed of Gel-Phase or Fluid-Phase Phospholipids Probed on Liposomes by Atomic Force Spectroscopy. Langmuir 2017, 33, 5117–5126. [Google Scholar] [CrossRef] [PubMed]
- Attwood, S.; Choi, Y.; Leonenko, Z. Preparation of DOPC and DPPC Supported Planar Lipid Bilayers for Atomic Force Microscopy and Atomic Force Spectroscopy. Int. J. Mol. Sci. 2013, 14, 3514–3539. [Google Scholar] [CrossRef] [PubMed]
- Picas, L.; Milhiet, P.; Hernández-Borrell, J. Atomic force microscopy: A versatile tool to probe the physical and chemical properties of supported membranes at the nanoscale. Chem. Phys. Lipids 2012, 165, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Brochu, H.; Vermette, P. Young’s Moduli of Surface-Bound Liposomes by Atomic Force Microscopy Force Measurements. Langmuir 2008, 24, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Delorme, N.; Fery, A. Direct method to study membrane rigidity of small vesicles based on atomic force microscope force spectroscopy. Phys. Rev. E Stat. Nonliner Soft Matter Phys. 2006, 74, 030901. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manyes, S.; Sanz, F. Nanomechanics of lipid bilayers by force spectroscopy with AFM: A perspective. Biochim. Biophys. Acta Biomembr. 2010, 1798, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Laney, D.E.; Garcia, R.A.; Parsons, S.M.; Hansma, H.G. Changes in the elastic properties of cholinergic synaptic vesicles as measured by atomic force microscopy. Biophys. J. 1997, 72, 806–813. [Google Scholar] [CrossRef]
- Chapman, D. Phase transitions and fluidity characteristics of lipids and cell membranes. Q. Rev. Biophys. 1975, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, Y.; Wen, S.; Wang, S. Poly(vinylphosphonic acid) (PVPA) on Titanium Alloy Acting as Effective Cartilage-like Superlubricity Coatings. ACS Appl. Mater. Interfaces 2014, 6, 17571–17578. [Google Scholar] [CrossRef] [PubMed]
- Arifin, A.; Sulong, A.B.; Muhamad, N.; Syarif, J.; Ramli, M.I. Material processing of hydroxyapatite and titanium alloy (HA/Ti) composite as implant materials using powder metallurgy: A review. Mater. Des. 2014, 55, 165–175. [Google Scholar] [CrossRef]
- Florin, E.L.; Rief, M.; Lehmann, H.; Ludwig, M.; Dornmair, C.; Moy, V.T.; Gaub, H.E. sensing specific molecular-interactions with the atomic-force microscope. Biosens. Bioelectron. 1995, 10, 895–901. [Google Scholar] [CrossRef]
- Ngwa, W.; Chen, K.; Sahgal, A.; Stepanov, E.V.; Luo, W. Nanoscale mechanics of solid-supported multilayered lipid films by force measurement. Thin Solid Films 2008, 516, 5039–5045. [Google Scholar] [CrossRef]
- Goertz, M.P.; Stottrup, B.L.; Houston, J.E.; Zhu, X.Y. Nanomechanical Contrasts of Gel and Fluid Phase Supported Lipid Bilayers. J. Phys. Chem. B 2009, 113, 9335–9339. [Google Scholar] [CrossRef] [PubMed]
- Leonenko, Z.V.; Finot, E.; Ma, H.; Dahms, T.; Cramb, D.T. Investigation of temperature-induced phase transitions in DOPC and DPPC phospholipid bilayers using temperature-controlled scanning force microscopy. Biophys. J. 2004, 86, 3783–3793. [Google Scholar] [CrossRef] [PubMed]



| T1 = 60 °C > Tm | T2 = 25 °C < Tm | ||||
|---|---|---|---|---|---|
| State | Distance/nm | Force/nN | State | Distance/nm | Force/nN |
| a | ≈H | 0 | a | ≈H | 0 |
| b | ≤1/2H | 2.93 ± 1.05 | - | - | - |
| c | 11.89 ± 1.15 | 2.53 ± 0.87 | c | 12.07 ± 1.12 | 1.42 ± 0.83 |
| d | 8.93 ± 0.98 | 6.88 ± 2.50 | d | 8.48 ± 0.89 | 3.23 ± 1.80 |
| e | 5.28 ± 0.68 | 9.58 ± 2.91 | e | 5.02 ± 0.83 | 6.33 ± 2.85 |
| Structure | Vesicle | Double-Bilayer Vesicle | Bilayer | |
|---|---|---|---|---|
| T1 = 60 °C > Tm | Frequencies | 1 | 1–2 | - |
| Force/nN | 9.58 ± 2.91 | 5.24 ± 2.25 | 2.08 ± 0.82 | |
| T2 = 25 °C < Tm | Frequencies | 3–34 | 4–12 | - |
| Force/nN | 6.33 ± 2.85 | 4.86 ± 2.11 | 2.19 ± 0.47 |
| Curves of Vesicle | Probability of Appearance | Curves of Double-Bilayer Vesicle | Probability of Appearance | |
|---|---|---|---|---|
| T1 = 60 °C > Tm | abc | 0 | c | 30.8% |
| abcd | 37.5% | cd | 23.1% | |
| abcde | 62.5% | cde | 46.1% | |
| T2 = 25 °C < Tm | ac | 55.2% | c | 53.0% |
| acd | 38.6% | cd | 42.4% | |
| acde | 6.2% | cde | 4.6% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Liu, Y.; Li, J.; Wang, H.; Wen, S. Investigation on the Nanomechanics of Liposome Adsorption on Titanium Alloys: Temperature and Loading Effects. Polymers 2018, 10, 383. https://doi.org/10.3390/polym10040383
Duan Y, Liu Y, Li J, Wang H, Wen S. Investigation on the Nanomechanics of Liposome Adsorption on Titanium Alloys: Temperature and Loading Effects. Polymers. 2018; 10(4):383. https://doi.org/10.3390/polym10040383
Chicago/Turabian StyleDuan, Yiqin, Yuhong Liu, Jinjin Li, Hongdong Wang, and Shizhu Wen. 2018. "Investigation on the Nanomechanics of Liposome Adsorption on Titanium Alloys: Temperature and Loading Effects" Polymers 10, no. 4: 383. https://doi.org/10.3390/polym10040383





