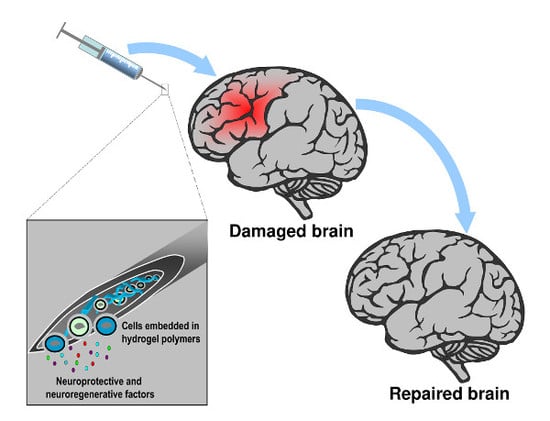
Hydrogels-Assisted Cell Engraftment for Repairing the Stroke-Damaged Brain: Chimera or Reality
Abstract
:1. Introduction
2. Clinical Scenario and Challenges to Solve
3. Therapeutic Intervention after Stroke: What Have Two Decades of Cell Therapy Research Taught Us?
3.1. Stem Cells for Neuroprotection and Repair of the Injured Brain
3.2. Mesenchymal Stem Cells
3.3. Optimal Time Window and Best Administration Route
3.4. Clinical Scenario: Where Are We Now?
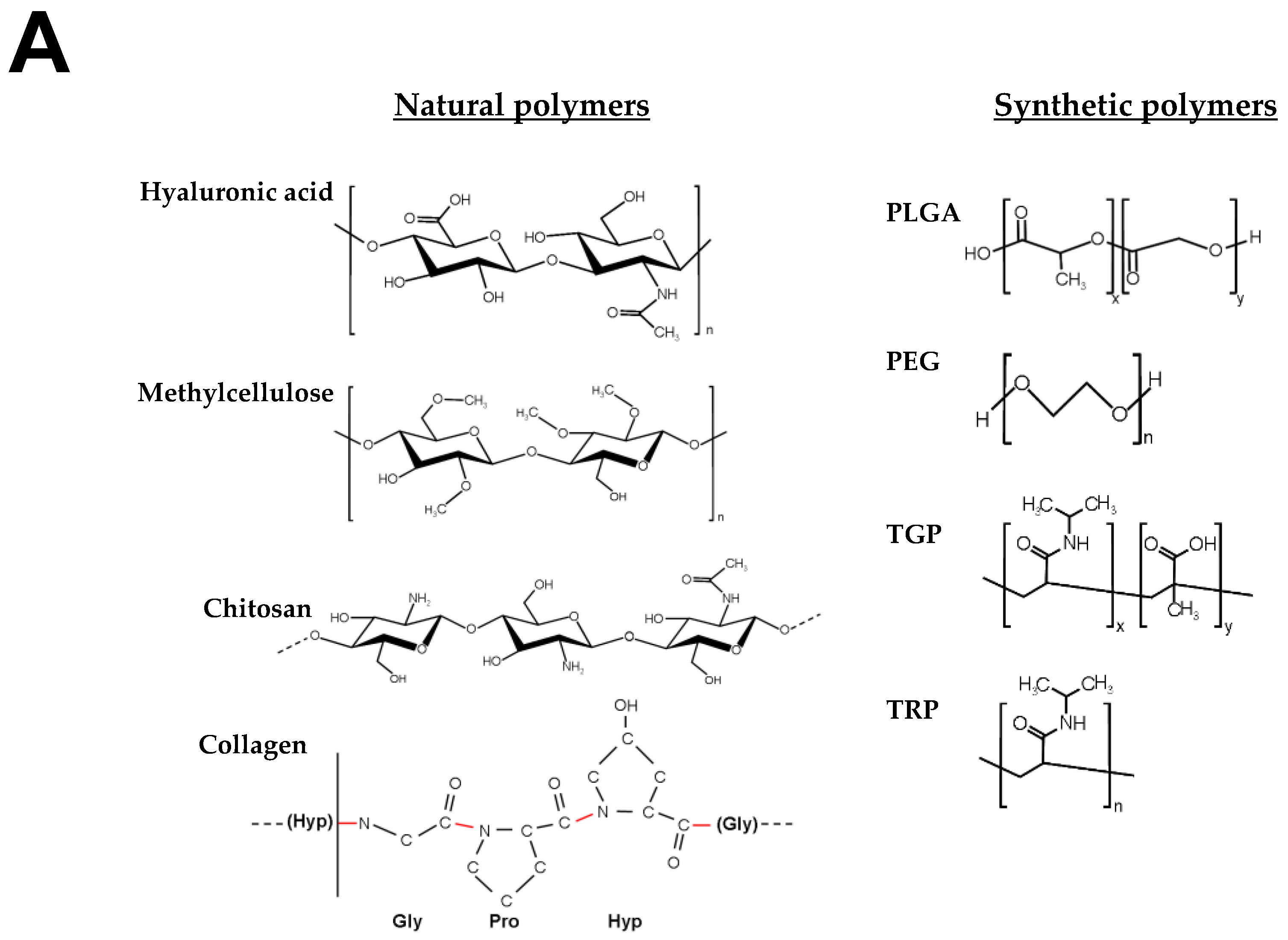
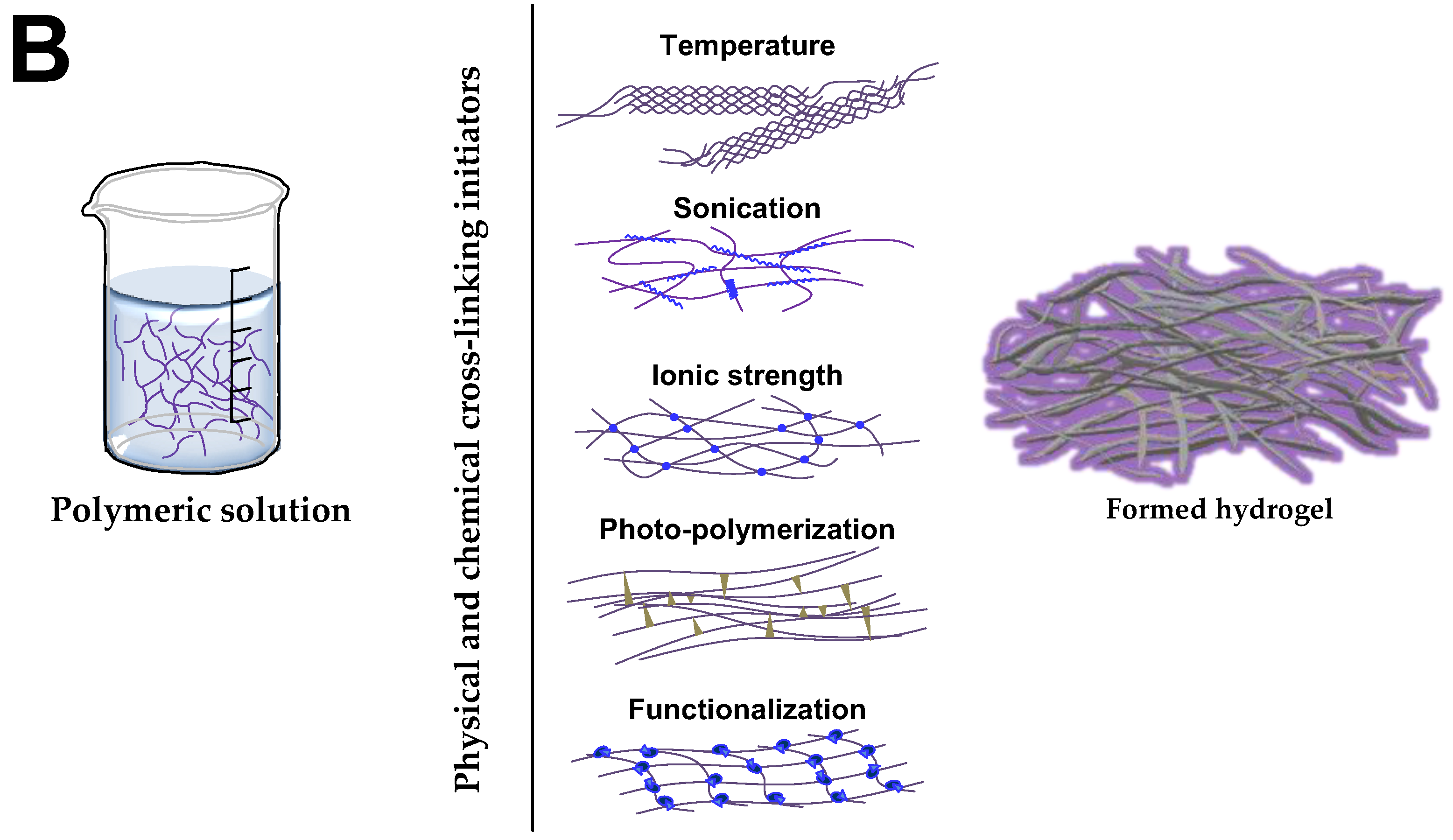
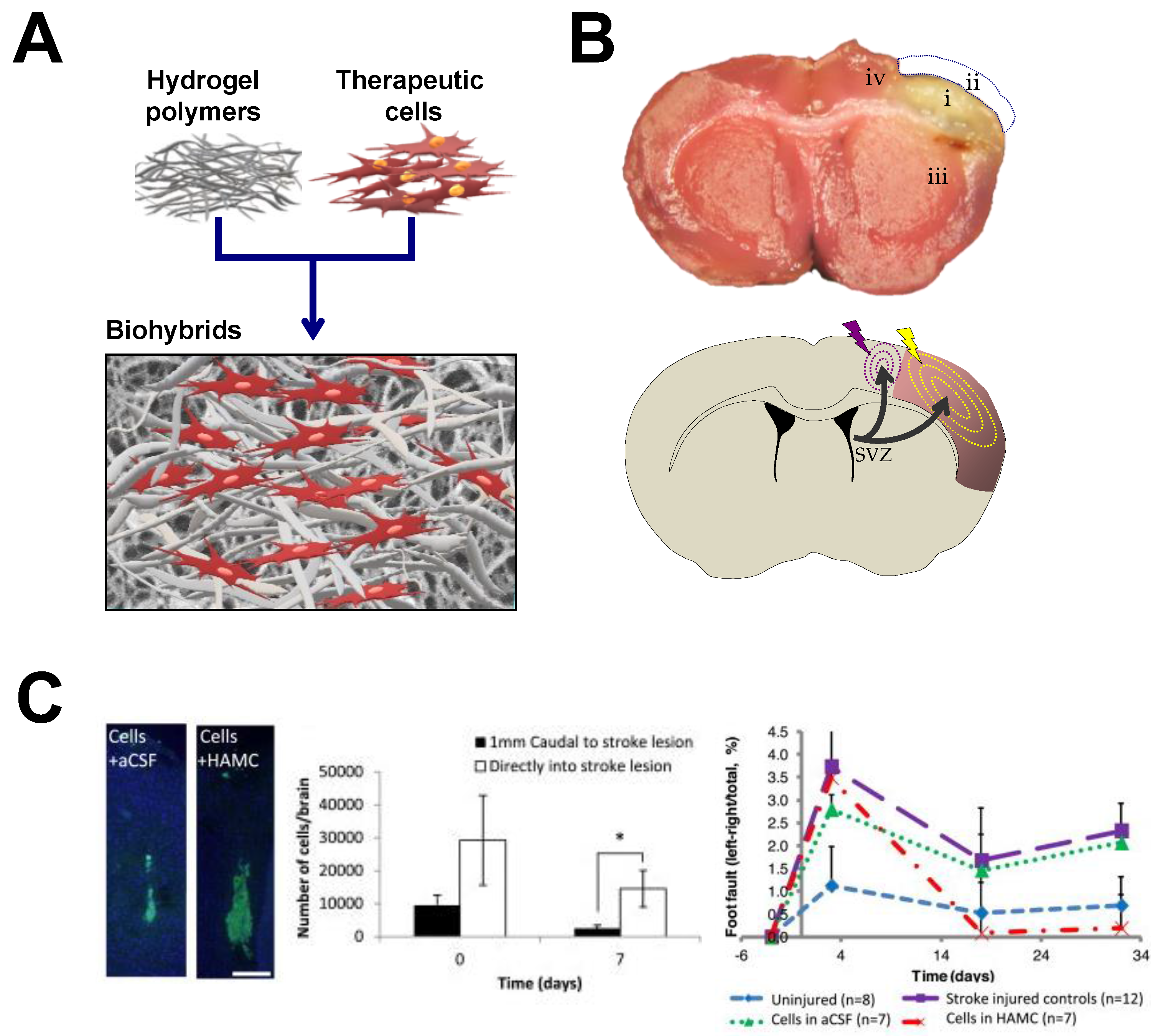
4. Hydrogels-Assisted Cell Therapies for Brain Stroke
4.1. Hydrogels for Brain Repair
4.2. Toxicity and Adverse Concerns
5. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Boltze, J.; Arnold, A.; Walczak, P.; Jolkkonen, J.; Cui, L.; Wagner, D.C. The Dark Side of the Force—Constraints and Complications of Cell Therapies for Stroke. Front. Neurol. 2015, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Feng, M.; Wei, J.; Han, Q.; Zhao, H.; Li, G.; Zhu, Z.; Xing, H.; An, Y.; Qin, C.; et al. Transplantation of Flk-1+ human bone marrow-derived mesenchymal stem cells promotes angiogenesis and neurogenesis after cerebral ischemia in rats. Eur. J. Neurosci. 2011, 34, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Andres, R.H.; Horie, N.; Slikker, W.; Keren-Gill, H.; Zhan, K.; Sun, G.; Manley, N.C.; Pereira, M.P.; Sheikh, L.A.; McMillan, E.L.; et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 2011, 134, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Darsalia, V.; Kallur, T.; Kokaia, Z. Survival, migration and neuronal differentiation of human fetal striatal and cortical neural stem cells grafted in stroke-damaged rat striatum. Eur. J. Neurosci. 2007, 26, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Bliss, T.M.; Shah, A.K.; Sun, G.H.; Ma, M.; Foo, W.C.; Masel, J.; Yenari, M.A.; Weissman, I.L.; Uchida, N.; et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc. Natl. Acad. Sci. USA 2004, 101, 11839–11844. [Google Scholar] [CrossRef] [PubMed]
- Mora-Lee, S.; Sirerol-Piquer, M.S.; Gutierrez-Perez, M.; Gomez-Pinedo, U.; Roobrouck, V.D.; Lopez, T.; Casado-Nieto, M.; Abizanda, G.; Rabena, M.T.; Verfaille, C.; et al. Therapeutic effects of hMAPC and hMSC transplantation after stroke in mice. PLoS ONE 2012, 7, e43683. [Google Scholar] [CrossRef] [PubMed]
- Tuladhar, A.; Morshead, C.M.; Shoichet, M.S. Circumventing the blood-brain barrier: Local delivery of cyclosporin A stimulates stem cells in stroke-injured rat brain. J. Control. Release 2015, 215, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Z.; Rege, S.V.; Wang, M.; Si, G.; Zhou, Y.; Wang, S.; Griffin, J.H.; Goldman, S.A.; Zlokovic, B.V. 3K3A-activated protein C stimulates postischemic neuronal repair by human neural stem cells in mice. Nat. Med. 2016, 22, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Hoag, H. Drug delivery: Brain food. Nature 2014, 510, S6–S7. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.J.; Vulicb, K.; Shoichet, M.S. Design of biomaterials to enhance stem cell survival when transplanted into the damaged central nervous system. Soft Matter 2010, 6, 4988–4998. [Google Scholar] [CrossRef]
- Khaing, Z.Z.; Thomas, R.C.; Geissler, S.A.; Schmidt, C.E. Advanced biomaterials for repairing the nervous system: What can hydrogels do for the brain? Materialstoday 2014, 17, 332–340. [Google Scholar] [CrossRef]
- Lim, T.C.; Spector, M. Biomaterials for Enhancing CNS Repair. Transl. Stroke Res. 2017, 8, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Anitua, E.; Pedraz, J.L.; Emerich, D.F. Biomaterials for promoting brain protection, repair and regeneration. Nat. Rev. Neurosci. 2009, 10, 682–692. [Google Scholar] [CrossRef] [PubMed]
- GBD-2013-study. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- O’Mahony, P.G.; Thomson, R.G.; Dobson, R.; Rodgers, H.; James, O.F. The prevalence of stroke and associated disability. J. Public Health Med. 1999, 21, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthi, R.V.; Feigin, V.L.; Forouzanfar, M.H.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.M.; Truelsen, T.; et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet Glob. Health 2013, 1, e259–e281. [Google Scholar] [CrossRef]
- Smajlovic, D. Strokes in young adults: Epidemiology and prevention. Vasc. Health Risk Manag. 2015, 11, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Brott, T.; Bogousslavsky, J. Treatment of acute ischemic stroke. N. Engl. J. Med. 2000, 343, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef]
- Campbell, B.C.; Mitchell, P.J. Endovascular therapy for ischemic stroke. N. Engl. J. Med. 2015, 372, 2365–2366. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Davalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- Emberson, J.; Lees, K.R.; Lyden, P.; Blackwell, L.; Albers, G.; Bluhmki, E.; Brott, T.; Cohen, G.; Davis, S.; Donnan, G.; et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014, 384, 1929–1935. [Google Scholar] [CrossRef]
- George, P.M.; Steinberg, G.K. Novel Stroke Therapeutics: Unraveling Stroke Pathophysiology and Its Impact on Clinical Treatments. Neuron 2015, 87, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, D.; Imeri, F.; Gatti, R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: A systematic review. J. Physiother. 2015, 61, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.E.; George, S.; Thomas, S.; Deutsch, J.E.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2015, CD008349. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Ruland, S.; Weinand, M.; Lowry, D.; Dafer, R.; Bakay, R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: A multicenter feasibility study of safety and efficacy. J. Neurosurg. 2008, 108, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.M.; Harvey, R.L.; Kissela, B.M.; Winstein, C.J.; Lutsep, H.L.; Parrish, T.B.; Cramer, S.C.; Venkatesan, L. Epidural Electrical Stimulation for Stroke Rehabilitation: Results of the Prospective, Multicenter, Randomized, Single-Blinded Everest Trial. Neurorehabil. Neural Repair 2016, 30, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Jaillard, A.; Martin, C.D.; Garambois, K.; Lebas, J.F.; Hommel, M. Vicarious function within the human primary motor cortex? A longitudinal fMRI stroke study. Brain 2005, 128, 1122–1138. [Google Scholar] [CrossRef] [PubMed]
- Nudo, R.J.; Wise, B.M.; SiFuentes, F.; Milliken, G.W. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 1996, 272, 1791–1794. [Google Scholar] [CrossRef] [PubMed]
- Krucoff, M.O.; Rahimpour, S.; Slutzky, M.W.; Edgerton, V.R.; Turner, D.A. Enhancing Nervous System Recovery through Neurobiologics, Neural Interface Training, and Neurorehabilitation. Front. Neurosci. 2016, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V.; Tajima, Y.; Trojanowski, J.Q.; Lee, V.M.; Sanberg, P.R. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp. Neurol. 1998, 149, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Chopp, M. Intracerebral transplantation of bone marrow with BDNF after MCAo in rat. Neuropharmacology 2000, 39, 711–716. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, L.; Lu, M.; Zhang, X.; Chopp, M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J. Neurol. Sci. 2001, 189, 49–57. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, L.; Zhang, Z.; Lu, D.; Lu, M.; Chopp, M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001, 32, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chopp, M.; Chen, J.; Wang, L.; Gautam, S.C.; Xu, Y.X.; Zhang, Z. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J. Cereb. Blood Flow MeTable 2000, 20, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.Y.; Lee, J.S.; Lee, P.H.; Lee, G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005, 57, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V. Preliminary Reports of Stereotaxic Stem Cell Transplants in Chronic Stroke Patients. Mol. Ther. 2016, 24, 1710–1711. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V. Age of PISCES: Stem-cell clinical trials in stroke. Lancet 2016, 388, 736–738. [Google Scholar] [CrossRef]
- Kondziolka, D.; Steinberg, G.K.; Wechsler, L.; Meltzer, C.C.; Elder, E.; Gebel, J.; Decesare, S.; Jovin, T.; Zafonte, R.; Lebowitz, J.; et al. Neurotransplantation for patients with subcortical motor stroke: A phase 2 randomized trial. J. Neurosurg. 2005, 103, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D.; Wechsler, L.; Goldstein, S.; Meltzer, C.; Thulborn, K.R.; Gebel, J.; Jannetta, P.; DeCesare, S.; Elder, E.M.; McGrogan, M.; et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 2000, 55, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Freeman, T.B.; Snow, B.J.; Vingerhoets, F.J.; Mufson, E.J.; Sanberg, P.R.; Hauser, R.A.; Smith, D.A.; Nauert, G.M.; Perl, D.P.; et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N. Engl. J. Med. 1995, 332, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hong, J.M.; Moon, G.J.; Lee, P.H.; Ahn, Y.H.; Bang, O.Y. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010, 28, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Sawle, G.; Widner, H.; Rothwell, J.C.; Bjorklund, A.; Brooks, D.; Brundin, P.; Frackowiak, R.; Marsden, C.D.; Odin, P.; et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson’s disease. Ann. Neurol. 1994, 35, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Savitz, S.I.; Dinsmore, J.; Wu, J.; Henderson, G.V.; Stieg, P.; Caplan, L.R. Neurotransplantation of fetal porcine cells in patients with basal ganglia infarcts: A preliminary safety and feasibility study. Cerebrovasc. Dis. 2005, 20, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.K.; Kondziolka, D.; Wechsler, L.R.; Lunsford, L.D.; Coburn, M.L.; Billigen, J.B.; Kim, A.S.; Johnson, J.N.; Bates, D.; King, B.; et al. Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study. Stroke 2016, 47, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L.O.; Simmons, E.L.; Marks, E.K.; Eldredge, J.H. Recovery from radiation injury. Science 1951, 113, 510–511. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, E.; Uphoff, D.; Reid, T.R.; Shelton, E. Modification of irradiation injury in mice and guinea pigs by bone marrow injections. J. Natl. Cancer Inst. 1951, 12, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.D.; Lochte, H.L., Jr.; Lu, W.C.; Ferrebee, J.W. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N. Engl. J. Med. 1957, 257, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Panes, J. Stem Cell Therapy for Perianal Fistulas in Crohn’s Disease. Gastroenterol Hepatol (N.Y.) 2016, 12, 637–640. [Google Scholar]
- Hirsch, T.; Rothoeft, T.; Teig, N.; Bauer, J.W.; Pellegrini, G.; De Rosa, L.; Scaglione, D.; Reichelt, J.; Klausegger, A.; Kneisz, D.; et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 2017, 551, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Abou-El-Enein, M.; Elsanhoury, A.; Reinke, P. Overcoming Challenges Facing Advanced Therapies in the EU Market. Cell Stem Cell 2016, 19, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Gervois, P.; Wolfs, E.; Ratajczak, J.; Dillen, Y.; Vangansewinkel, T.; Hilkens, P.; Bronckaers, A.; Lambrichts, I.; Struys, T. Stem Cell-Based Therapies for Ischemic Stroke: Preclinical Results and the Potential of Imaging-Assisted Evaluation of Donor Cell Fate and Mechanisms of Brain Regeneration. Med. Res. Rev. 2016, 36, 1080–1126. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.S.; Sena, E.S.; Egan, K.J.; Antonic, A.; Koblar, S.A.; Howells, D.W.; Macleod, M.R. Stem cell-based therapy for experimental stroke: A systematic review and meta-analysis. Int. J. Stroke 2012, 7, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Bernstock, J.D.; Peruzzotti-Jametti, L.; Ye, D.; Gessler, F.A.; Maric, D.; Vicario, N.; Lee, Y.J.; Pluchino, S.; Hallenbeck, J.M. Neural stem cell transplantation in ischemic stroke: A role for preconditioning and cellular engineering. J. Cereb. Blood Flow MeTable 2017, 37, 2314–2319. [Google Scholar] [CrossRef] [PubMed]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Ramos-Cabrer, P.; Justicia, C.; Wiedermann, D.; Hoehn, M. Stem cell mediation of functional recovery after stroke in the rat. PLoS ONE 2010, 5, e12779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguchi, A.; Soma, T.; Tanaka, H.; Kanda, T.; Nishimura, H.; Yoshikawa, H.; Tsukamoto, Y.; Iso, H.; Fujimori, Y.; Stern, D.M.; et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J. Clin. Invest. 2004, 114, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Astrup, J.; Symon, L.; Branston, N.M.; Lassen, N.A. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke 1977, 8, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.H.; Corbett, D. Plasticity during stroke recovery: From synapse to behaviour. Nat. Rev. Neurosci. 2009, 10, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Rallu, M.; Corbin, J.G.; Fishell, G. Parsing the prosencephalon. Nat. Rev. Neurosci. 2002, 3, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Sawada, M.; Sawamoto, K. Mechanisms of neuronal migration in the adult brain. J. Neurochem. 2017, 141, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Obernier, K.; Cebrian-Silla, A.; Thomson, M.; Parraguez, J.I.; Anderson, R.; Guinto, C.; Rodas Rodriguez, J.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Adult Neurogenesis Is Sustained by Symmetric Self-Renewal and Differentiation. Cell Stem Cell 2018, 22, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Reumers, V.; Deroose, C.M.; Krylyshkina, O.; Nuyts, J.; Geraerts, M.; Mortelmans, L.; Gijsbers, R.; Van den Haute, C.; Debyser, Z.; Baekelandt, V. Noninvasive and quantitative monitoring of adult neuronal stem cell migration in mouse brain using bioluminescence imaging. Stem Cells 2008, 26, 2382–2390. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Kim, M.; Park, K.I.; Jeong, S.W.; Park, H.K.; Jung, K.H.; Lee, S.T.; Kang, L.; Lee, K.; Park, D.K.; et al. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004, 1016, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Riess, P.; Zhang, C.; Saatman, K.E.; Laurer, H.L.; Longhi, L.G.; Raghupathi, R.; Lenzlinger, P.M.; Lifshitz, J.; Boockvar, J.; Neugebauer, E.; et al. Transplanted neural stem cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury. Neurosurgery 2002, 51, 1043–1052, discussion 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.M.; Zhao, Y.Y.; Chen, S.D.; Zhang, W.H.; Lou, L.; Jin, X. Functional recovery after transplantation of neural stem cells modified by brain-derived neurotrophic factor in rats with cerebral ischaemia. J. Int. Med. Res. 2011, 39, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Hattiangady, B.; Shuai, B.; Cai, J.; Coksaygan, T.; Rao, M.S.; Shetty, A.K. Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging hippocampus. Stem Cells 2007, 25, 2104–2117. [Google Scholar] [CrossRef] [PubMed]
- Augestad, I.L.; Nyman, A.K.G.; Costa, A.I.; Barnett, S.C.; Sandvig, A.; Haberg, A.K.; Sandvig, I. Effects of Neural Stem Cell and Olfactory Ensheathing Cell Co-transplants on Tissue Remodelling After Transient Focal Cerebral Ischemia in the Adult Rat. Neurochem. Res. 2017, 42, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Harms, K.M.; Li, L.; Cunningham, L.A. Murine neural stem/progenitor cells protect neurons against ischemia by HIF-1alpha-regulated VEGF signaling. PLoS ONE 2010, 5, e9767. [Google Scholar] [CrossRef] [PubMed]
- Roitbak, T.; Li, L.; Cunningham, L.A. Neural stem/progenitor cells promote endothelial cell morphogenesis and protect endothelial cells against ischemia via HIF-1alpha-regulated VEGF signaling. J. Cereb. Blood Flow MeTable 2008, 28, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.; Guzman, R.; Daadi, M.; Steinberg, G.K. Cell transplantation therapy for stroke. Stroke 2007, 38, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.A. Stem and Progenitor Cell-Based Therapy of the Central Nervous System: Hopes, Hype, and Wishful Thinking. Cell Stem Cell 2016, 18, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Cui, L.; Snider, B.J.; Rivkin, M.; Yu, S.S.; Lee, C.S.; Adams, L.D.; Gottlieb, D.I.; Johnson, E.M., Jr.; Yu, S.P.; et al. Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia. Neurobiol. Dis. 2005, 19, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, D.; Qi, M.; Kim, D.H.; Kitamura, Y.; Inden, M.; Tsuchiya, D.; Takata, K.; Taniguchi, T.; Yoshimoto, K.; Shimohama, S.; et al. Improvement of focal ischemia-induced rat dopaminergic dysfunction by striatal transplantation of mouse embryonic stem cells. Neurosci. Lett. 2006, 407, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Solter, D. From teratocarcinomas to embryonic stem cells and beyond: A history of embryonic stem cell research. Nat. Rev. Genet. 2006, 7, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Honmou, O.; Onodera, R.; Sasaki, M.; Waxman, S.G.; Kocsis, J.D. Mesenchymal stem cells: Therapeutic outlook for stroke. Trends Mol. Med. 2012, 18, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Janowski, M.; Walczak, P.; Date, I. Intravenous route of cell delivery for treatment of neurological disorders: A meta-analysis of preclinical results. Stem Cells Dev. 2010, 19, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chopp, M. Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci. Lett. 2009, 456, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chao, J.; Shi, Y. Modeling neurological diseases using iPSC-derived neural cells : IPSC modeling of neurological diseases. Cell Tissue Res. 2017, 371, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Yuasa, S.; Oda, M.; Egashira, T.; Yae, K.; Kusumoto, D.; Nakata, H.; Tohyama, S.; Hashimoto, H.; Kodaira, M.; et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell 2010, 7, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tat, P.A.; Sumer, H.; Jones, K.L.; Upton, K.; Verma, P.J. The efficient generation of induced pluripotent stem (iPS) cells from adult mouse adipose tissue-derived and neural stem cells. Cell Transplant. 2010, 19, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Chang, C.M.; Tsai, S.K.; Chang, Y.L.; Chou, S.J.; Huang, S.S.; Tai, L.K.; Chen, Y.C.; Ku, H.H.; Li, H.Y.; et al. Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem. Cells Dev. 2010, 19, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Lv, L.; Ji, H.; Yang, X.; Zhu, W.; Cai, L.; Gu, X.; Chai, C.; Huang, S.; Sun, J.; et al. Induction of pluripotent stem cells transplantation therapy for ischemic stroke. Mol. Cell Biochem. 2011, 354, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Liao, W.; Feng, N.H.; Lou, Y.L.; Niu, X.; Zhang, A.J.; Wang, Y.; Deng, Z.F. Human induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurologic function in a rat model of middle cerebral artery occlusion. Stem Cell Res. Ther. 2013, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Yamashita, T.; Ohta, Y.; Deguchi, K.; Nagotani, S.; Zhang, X.; Ikeda, Y.; Matsuura, T.; Abe, K. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J. Cereb. Blood Flow MeTable 2010, 30, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Lamm, N.; Ben-David, U.; Golan-Lev, T.; Storchova, Z.; Benvenisty, N.; Kerem, B. Genomic Instability in Human Pluripotent Stem Cells Arises from Replicative Stress and Chromosome Condensation Defects. Cell Stem Cell 2016, 18, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Lister, R.; Pelizzola, M.; Kida, Y.S.; Hawkins, R.D.; Nery, J.R.; Hon, G.; Antosiewicz-Bourget, J.; O’Malley, R.; Castanon, R.; Klugman, S.; et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 2011, 471, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Nakano-Doi, A.; Nakagomi, T.; Fujikawa, M.; Nakagomi, N.; Kubo, S.; Lu, S.; Yoshikawa, H.; Soma, T.; Taguchi, A.; Matsuyama, T. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem Cells 2010, 28, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Shyu, W.C.; Lin, S.Z.; Chiang, M.F.; Su, C.Y.; Li, H. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. J. Neurosci. 2006, 26, 3444–3453. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P. “Mesenchymal” stem cells. Annu. Rev. Cell Dev. Biol. 2014, 30, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Nieto, D.; Li, L.; Kohler, A.; Ghiaur, G.; Ishikawa, E.; Sengupta, A.; Madhu, M.; Arnett, J.L.; Santho, R.A.; Dunn, S.K.; et al. Connexin-43 in the osteogenic BM niche regulates its cellular composition and the bidirectional traffic of hematopoietic stem cells and progenitors. Blood 2012, 119, 5144–5154. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Z.G.; Li, Y.; Wang, L.; Xu, Y.X.; Gautam, S.C.; Lu, M.; Zhu, Z.; Chopp, M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ. Res. 2003, 92, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Horie, N.; Pereira, M.P.; Niizuma, K.; Sun, G.; Keren-Gill, H.; Encarnacion, A.; Shamloo, M.; Hamilton, S.A.; Jiang, K.; Huhn, S.; et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells 2011, 29, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, S.; Horie, N.; Satoh, K.; Fukuda, Y.; Nishida, N.; Nagata, I. Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke 2013, 44, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yu, F.; Lei, T.; Gao, H.; Li, P.; Sun, Y.; Huang, H.; Mu, Q. Bone marrow mesenchymal stem cell therapy in ischemic stroke: Mechanisms of action and treatment optimization strategies. Neural Regen. Res. 2016, 11, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Chopp, M. Adult bone marrow transplantation after stroke in adult rats. Cell Transplant. 2001, 10, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, F.; Klein, O.; Klose, J.; Priller, J. Mesenchymal stromal cells rescue cortical neurons from apoptotic cell death in an in vitro model of cerebral ischemia. Cell Mol. Neurobiol. 2012, 32, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, F.; Ladhoff, J.; Huck, J.; Grohmann, M.; Blazej, K.; Oersal, A.; Baeva, N.; Seifert, M.; Priller, J. Immune effects of mesenchymal stromal cells in experimental stroke. J. Cereb. Blood Flow MeTable 2012, 32, 1578–1588. [Google Scholar] [CrossRef] [PubMed]
- Shinozuka, K.; Dailey, T.; Tajiri, N.; Ishikawa, H.; Kaneko, Y.; Borlongan, C.V. Stem cell transplantation for neuroprotection in stroke. Brain Sci. 2013, 3, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Kopen, G.C.; Prockop, D.J.; Phinney, D.G. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc. Natl. Acad. Sci. USA 1999, 96, 10711–10716. [Google Scholar] [CrossRef] [PubMed]
- Lepski, G.; Jannes, C.E.; Strauss, B.; Marie, S.K.; Nikkhah, G. Survival and neuronal differentiation of mesenchymal stem cells transplanted into the rodent brain are dependent upon microenvironment. Tissue Eng. Part A 2010, 16, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Dolado, M.; Pardal, R.; Garcia-Verdugo, J.M.; Fike, J.R.; Lee, H.O.; Pfeffer, K.; Lois, C.; Morrison, S.J.; Alvarez-Buylla, A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 2003, 425, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Kokaia, Z.; Martino, G.; Schwartz, M.; Lindvall, O. Cross-talk between neural stem cells and immune cells: The key to better brain repair? Nat. Neurosci. 2012, 15, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.; Lin, Y.C.; Yuen, C.M.; Yen, C.H.; Kao, Y.H.; Sun, C.K.; Yip, H.K. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J. Transl. Med. 2010, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Meisel, R.; Zibert, A.; Laryea, M.; Gobel, U.; Daubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guan, Y.M.; Huang, H.L.; Wang, Q.S. Human umbilical cord blood mesenchymal stem cell transplantation suppresses inflammatory responses and neuronal apoptosis during early stage of focal cerebral ischemia in rabbits. Acta Pharmacol. Sin. 2014, 35, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Burns, T.C.; Verfaillie, C.M.; Low, W.C. Stem cells for ischemic brain injury: A critical review. J. Comp. Neurol. 2009, 515, 125–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoehn, B.D.; Palmer, T.D.; Steinberg, G.K. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke 2005, 36, 2718–2724. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, B.; Kornyei, Z.; Ferenczi, S.; Fekete, R.; Kudlik, G.; Kovacs, K.J.; Madarasz, E.; Uher, F. Regulation of mouse microglia activation and effector functions by bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2014, 23, 2600–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Zhang, C.L.; Wang, L.; Lu, D.; Katakowski, M.; Gao, Q.; Shen, L.H.; Zhang, J.; Lu, M.; et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 2005, 49, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Hsuan, Y.C.; Lin, C.H.; Chang, C.P.; Lin, M.T. Mesenchymal stem cell-based treatments for stroke, neural trauma, and heat stroke. Brain Behav. 2016, 6, e00526. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Nagai, A.; Sheikh, A.M.; Shiota, Y.; Narantuya, D.; Watanabe, T.; Masuda, J.; Kobayashi, S.; Kim, S.U.; Yamaguchi, S. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J. Neurosci. Res. 2010, 88, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Ji, K.; Guo, L.; Wu, W.; Lu, H.; Shan, P.; Yan, C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 2014, 92, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Mohamad, O.; Gu, X.; Wei, L.; Yu, S.P. Restoration of intracortical and thalamocortical circuits after transplantation of bone marrow mesenchymal stem cells into the ischemic brain of mice. Cell Transplant. 2013, 22, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, A.; Kokaia, Z.; Lindvall, O. N-methyl-D-aspartate receptor-mediated increase of neurogenesis in adult rat dentate gyrus following stroke. Eur. J. Neurosci. 2001, 14, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Parent, J.M.; Vexler, Z.S.; Gong, C.; Derugin, N.; Ferriero, D.M. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann. Neurol. 2002, 52, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; McIntosh, K.; Chen, J.; Zhang, C.; Gao, Q.; Borneman, J.; Raginski, K.; Mitchell, J.; Shen, L.; Zhang, J.; et al. Allogeneic bone marrow stromal cells promote glial-axonal remodeling without immunologic sensitization after stroke in rats. Exp. Neurol. 2006, 198, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Zhang, R.L.; Cui, Y.; Chopp, M. Bone marrow stromal cells promote skilled motor recovery and enhance contralesional axonal connections after ischemic stroke in adult mice. Stroke 2011, 42, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Van Velthoven, C.T.; van de Looij, Y.; Kavelaars, A.; Zijlstra, J.; van Bel, F.; Huppi, P.S.; Sizonenko, S.; Heijnen, C.J. Mesenchymal stem cells restore cortical rewiring after neonatal ischemia in mice. Ann. Neurol. 2012, 71, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Verrilli, M.A.; Caviedes, A.; Cabrera, A.; Sandoval, S.; Wyneken, U.; Khoury, M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 2016, 320, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chopp, M.; Liu, X.S.; Katakowski, M.; Wang, X.; Tian, X.; Wu, D.; Zhang, Z.G. Exosomes Derived from Mesenchymal Stromal Cells Promote Axonal Growth of Cortical Neurons. Mol. Neurobiol. 2017, 54, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Liu, Z.; Wang, X.; Shang, X.; Cui, Y.; Zhang, Z.G.; Chopp, M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 2013, 31, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Mao, X.; Xie, L.; Greenberg, R.B.; Peng, B.; Moore, A.; Greenberg, M.B.; Greenberg, D.A. Delayed transplantation of human neural precursor cells improves outcome from focal cerebral ischemia in aged rats. Aging Cell 2010, 9, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Biernaskie, J.; Chernenko, G.; Corbett, D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J. Neurosci. 2004, 24, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Vu, Q.; Xie, K.; Eckert, M.; Zhao, W.; Cramer, S.C. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology 2014, 82, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Fernandez, M.; Rodriguez-Frutos, B.; Alvarez-Grech, J.; Vallejo-Cremades, M.T.; Exposito-Alcaide, M.; Merino, J.; Roda, J.M.; Diez-Tejedor, E. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience 2011, 175, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.L.; Kerkela, E.; Bakreen, A.; Nitzsche, F.; Andrzejewska, A.; Nowakowski, A.; Janowski, M.; Walczak, P.; Boltze, J.; Lukomska, B.; et al. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res. Ther. 2015, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Guo, L.; Wang, S.; Zhang, Y.; Cai, T.; Zhao, R.C.; Wu, Y. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem Cell Rev. 2014, 10, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.D.; Frey, W.H., 2nd; Craft, S.; Danielyan, L.; Hallschmid, M.; Schioth, H.B.; Benedict, C. Intranasal treatment of central nervous system dysfunction in humans. Pharm. Res. 2013, 30, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Frutos, B.; Otero-Ortega, L.; Gutierrez-Fernandez, M.; Fuentes, B.; Ramos-Cejudo, J.; Diez-Tejedor, E. Stem Cell Therapy and Administration Routes After Stroke. Transl. Stroke Res. 2016, 7, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Nih, L.R.; Carmichael, S.T.; Segura, T. Hydrogels for brain repair after stroke: An emerging treatment option. Curr. Opin. Biotechnol. 2016, 40, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Overman, J.J.; Clarkson, A.N.; Wanner, I.B.; Overman, W.T.; Eckstein, I.; Maguire, J.L.; Dinov, I.D.; Toga, A.W.; Carmichael, S.T. A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc. Natl. Acad. Sci. USA 2012, 109, E2230–E2239. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, J.; Ju, R.; Chen, Z.; Xu, Q. Comparison of intracerebral transplantation effects of different stem cells on rodent stroke models. Cell Biochem. Funct. 2015, 33, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Moshayedi, P.; Carmichael, S.T. Hyaluronan, neural stem cells and tissue reconstruction after acute ischemic stroke. Biomatter 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- De Keyser, J. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005, 58, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, A.; Srivastava, M.V.; Mohanty, S.; Bhatia, R.; Kumaran, S.S.; Bose, S. Stem cell therapy: A clinical trial of stroke. Clin. Neurol. Neurosurg. 2013, 115, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.Y.; Huang, F.J.; Zhao, M.; Xie, J.H.; Shi, J.; Wang, J.; Lin, X.Z.; Zuo, H.; Wang, Y.L.; Geng, T.C. A two-year follow-up study of cotransplantation with neural stem/progenitor cells and mesenchymal stromal cells in ischemic stroke patients. Cell Transplant. 2014, 23 (Suppl. 1), S65–S72. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.A.; Martins, M.P.; Araujo, M.D.; Klamt, C.; Vedolin, L.; Garicochea, B.; Raupp, E.F.; El Ammar, S.J.; Machado, D.C.; Costa, J.C.; et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012, 21 (Suppl. 1), S13–S21. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Monteagudo, C.; Hernandez-Ramirez, P.; Alvarez-Gonzalez, L.; Garcia-Maeso, I.; de la Cuetara-Bernal, K.; Castillo-Diaz, L.; Bringas-Vega, M.L.; Martinez-Aching, G.; Morales-Chacon, L.M.; Baez-Martin, M.M.; et al. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Restor. Neurol. Neurosci. 2009, 27, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.M.; Andres, R.H.; Steinberg, G.K. Optimizing the success of cell transplantation therapy for stroke. Neurobiol. Dis. 2010, 37, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, A.; Keck, C.A.; Koshkin, V.S.; LeBold, D.G.; Siman, R.; Snyder, E.Y.; McIntosh, T.K. Caspase-mediated cell death predominates following engraftment of neural progenitor cells into traumatically injured rat brain. Brain Res. 2005, 1065, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Ballios, B.G.; Cooke, M.J.; Donaldson, L.; Coles, B.L.; Morshead, C.M.; van der Kooy, D.; Shoichet, M.S. A Hyaluronan-Based Injectable Hydrogel Improves the Survival and Integration of Stem Cell Progeny following Transplantation. Stem Cell Reports 2015, 4, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Bible, E.; Chau, D.Y.; Alexander, M.R.; Price, J.; Shakesheff, K.M.; Modo, M. The support of neural stem cells transplanted into stroke-induced brain cavities by PLGA particles. Biomaterials 2009, 30, 2985–2994. [Google Scholar] [CrossRef] [PubMed]
- Dreifus, M.; Wichterle, O.; Lim, D. [Intra-cameral lenses made of hydrocolloidal acrylates]. Cesk. Oftalmol. 1960, 16, 154–159. [Google Scholar] [PubMed]
- Wichterle, O.; Lim, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Pradhan, S.; Clary, J.M.; Seliktar, D.; Lipke, E.A. A three-dimensional spheroidal cancer model based on PEG-fibrinogen hydrogel microspheres. Biomaterials 2017, 115, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Sepantafar, M.; Maheronnaghsh, R.; Mohammadi, H.; Rajabi-Zeleti, S.; Annabi, N.; Aghdami, N.; Baharvand, H. Stem cells and injectable hydrogels: Synergistic therapeutics in myocardial repair. Biotechnol. Adv. 2016, 34, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.E.; Anseth, K.S. Spatiotemporal hydrogel biomaterials for regenerative medicine. Chem. Soc. Rev. 2017, 46, 6532–6552. [Google Scholar] [CrossRef] [PubMed]
- Gulrez, S.K.H.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; Carpi, A., Ed.; InTech: Rijeka, Croatia, 2011; Chapter 5; pp. 117–150. ISBN 978-953-307-268-5. [Google Scholar]
- Zabow, G.; Dodd, S.J.; Koretsky, A.P. Shape-changing magnetic assemblies as high-sensitivity NMR-readable nanoprobes. Nature 2015, 520, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Georges, P.C.; Li, B.; Du, Y.; Kutzing, M.K.; Previtera, M.L.; Langrana, N.; Firestein, B.L. Cell Growth in Response to Mechanical Stiffness is Affected by Neuron- Astroglia Interactions. Open Neurosci. J. 2007, 107, 7–14. [Google Scholar] [CrossRef]
- Lampe, K.J.; Mooney, R.G.; Bjugstad, K.B.; Mahoney, M.J. Effect of macromer weight percent on neural cell growth in 2D and 3D nondegradable PEG hydrogel culture. J. Biomed. Mater. Res. A. 2010, 94, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Aurand, E.R.; Wagner, J.; Lanning, C.; Bjugstad, K.B. Building biocompatible hydrogels for tissue engineering of the brain and spinal cord. J. Funct. Biomater. 2012, 3, 839–863. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.B.; Brown, X.Q.; Jacot, J.G.; Dimilla, P.A.; Wong, J.Y. Neurite outgrowth and branching of PC12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity. J. Neural. Eng. 2007, 4, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.C.; Curran, G.L.; Glaser, K.J.; Rossman, P.J.; Huston, J., 3rd; Poduslo, J.F.; Jack, C.R., Jr.; Felmlee, J.P.; Ehman, R.L. Magnetic resonance elastography of the brain in a mouse model of Alzheimer’s disease: Initial results. Magn. Reson. Imaging 2012, 30, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Tang-Schomer, M.D.; White, J.D.; Tien, L.W.; Schmitt, L.I.; Valentin, T.M.; Graziano, D.J.; Hopkins, A.M.; Omenetto, F.G.; Haydon, P.G.; Kaplan, D.L. Bioengineered functional brain-like cortical tissue. Proc. Natl. Acad. Sci. USA 2014, 111, 13811–13816. [Google Scholar] [CrossRef] [PubMed]
- Georges, P.C.; Miller, W.J.; Meaney, D.F.; Sawyer, E.S.; Janmey, P.A. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys. J. 2006, 90, 3012–3018. [Google Scholar] [CrossRef] [PubMed]
- Levental, I.; Georges, P.C.; Janmey, P.A. Soft biological materials and their impact on cell function. Soft Matter 2007, 3, 299–306. [Google Scholar] [CrossRef]
- Seidlits, S.K.; Khaing, Z.Z.; Petersen, R.R.; Nickels, J.D.; Vanscoy, J.E.; Shear, J.B.; Schmidt, C.E. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 2010, 31, 3930–3940. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.F.; Franze, K.; Gautier, H.; Moshayedi, P.; Fawcett, J.; Franklin, R.J.; Karadottir, R.T.; Guck, J. Mechanical difference between white and gray matter in the rat cerebellum measured by scanning force microscopy. J. Biomech. 2010, 43, 2986–2992. [Google Scholar] [CrossRef] [PubMed]
- Anseth, K.S.; Metters, A.T.; Bryant, S.J.; Martens, P.J.; Elisseeff, J.H.; Bowman, C.N. In situ forming degradable networks and their application in tissue engineering and drug delivery. J. Control. Release 2002, 78, 199–209. [Google Scholar] [CrossRef]
- Miller, K.; Chinzei, K.; Orssengo, G.; Bednarz, P. Mechanical properties of brain tissue in-vivo: Experiment and computer simulation. J. Biomech. 2000, 33, 1369–1376. [Google Scholar] [CrossRef]
- Khaing, Z.Z.; Milman, B.D.; Vanscoy, J.E.; Seidlits, S.K.; Grill, R.J.; Schmidt, C.E. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J. Neural. Eng. 2011, 8, 046033. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Xu, Q.; Tian, W.; Cui, F.; Cai, Q.; Ma, J.; Lee, I.S. The repair of brain lesion by implantation of hyaluronic acid hydrogels modified with laminin. J. Neurosci. Methods 2005, 148, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Carmichael, S.T.; Lowry, W.E.; Segura, T. Hydrogel design of experiments methodology to optimize hydrogel for iPSC-NPC culture. Adv. Healthc. Mater. 2015, 4, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Lowry, W.E.; Carmichael, S.T.; Segura, T. Delivery of iPS-NPCs to the Stroke Cavity within a Hyaluronic Acid Matrix Promotes the Differentiation of Transplanted Cells. Adv. Funct. Mater. 2014, 24, 7053–7062. [Google Scholar] [CrossRef] [PubMed]
- Osanai, T.; Kuroda, S.; Yasuda, H.; Chiba, Y.; Maruichi, K.; Hokari, M.; Sugiyama, T.; Shichinohe, H.; Iwasaki, Y. Noninvasive transplantation of bone marrow stromal cells for ischemic stroke: Preliminary study with a thermoreversible gelation polymer hydrogel. Neurosurgery 2010, 66, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Tator, C.H.; Shoichet, M.S. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials 2006, 27, 2370–2379. [Google Scholar] [CrossRef] [PubMed]
- Nih, L.R.; Sideris, E.; Carmichael, S.T.; Segura, T. Injection of Microporous Annealing Particle (MAP) Hydrogels in the Stroke Cavity Reduces Gliosis and Inflammation and Promotes NPC Migration to the Lesion. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Somaa, F.A.; Wang, T.Y.; Niclis, J.C.; Bruggeman, K.F.; Kauhausen, J.A.; Guo, H.; McDougall, S.; Williams, R.J.; Nisbet, D.R.; Thompson, L.H.; et al. Peptide-Based Scaffolds Support Human Cortical Progenitor Graft Integration to Reduce Atrophy and Promote Functional Repair in a Model of Stroke. Cell Rep. 2017, 20, 1964–1977. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lim, E.; Back, S.; Na, H.; Park, Y.; Sun, K. Nerve regeneration following spinal cord injury using matrix metalloproteinase-sensitive, hyaluronic acid-based biomimetic hydrogel scaffold containing brain-derived neurotrophic factor. J. Biomed. Mater. Res. A 2010, 93, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Massensini, A.R.; Ghuman, H.; Saldin, L.T.; Medberry, C.J.; Keane, T.J.; Nicholls, F.J.; Velankar, S.S.; Badylak, S.F.; Modo, M. Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity. Acta Biomater. 2015, 27, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.C.; Tao, L.; Hsieh, F.Y.; Wei, Y.; Chiu, I.M.; Hsu, S.H. An Injectable, Self-Healing Hydrogel to Repair the Central Nervous System. Adv. Mater. 2015, 27, 3518–3524. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, L.; Mari-Buye, N.; Barios, J.A.; Madurga, R.; Elices, M.; Perez-Rigueiro, J.; Ramos, M.; Guinea, G.V.; Gonzalez-Nieto, D. Safety and tolerability of silk fibroin hydrogels implanted into the mouse brain. Acta Biomater. 2016, 45, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Yannas, I.V.; Burke, J.F. Design of an artificial skin. I. Basic design principles. J. Biomed. Mater. Res. 1980, 14, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, J.P.; Morse, M.A.; Saltzman, W.M.; Domb, A.J.; Perez-Atayde, A.; Langer, R. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J. Pediatr. Surg. 1988, 23, 3–9. [Google Scholar] [CrossRef]
- Lim, F.; Sun, A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980, 210, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Woerly, S.; Laroche, G.; Marchand, R.; Pato, J.; Subr, V.; Ulbrich, K. Intracerebral implantation of hydrogel-coupled adhesion peptides: Tissue reaction. J. Neural. Transplant. Plast. 1995, 5, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Woerly, S.; Marchand, R.; Lavallee, C. Intracerebral implantation of synthetic polymer/biopolymer matrix: A new perspective for brain repair. Biomaterials 1990, 11, 97–107. [Google Scholar] [CrossRef]
- Lesny, P.; De Croos, J.; Pradny, M.; Vacik, J.; Michalek, J.; Woerly, S.; Sykova, E. Polymer hydrogels usable for nervous tissue repair. J. Chem. Neuroanat. 2002, 23, 243–247. [Google Scholar] [CrossRef]
- Sykova, E.; Jendelova, P. In vivo tracking of stem cells in brain and spinal cord injury. Prog. Brain Res. 2007, 161, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Pakulska, M.M.; Ballios, B.G.; Shoichet, M.S. Injectable hydrogels for central nervous system therapy. Biomed. Mater. 2012, 7, 024101. [Google Scholar] [CrossRef] [PubMed]
- Ju, R.; Wen, Y.; Gou, R.; Wang, Y.; Xu, Q. The experimental therapy on brain ischemia by improvement of local angiogenesis with tissue engineering in the mouse. Cell Transplant. 2014, 23 (Suppl. 1), S83–S95. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Huh, N. Application of a thermo-reversible gelation polymer, mebiol gel, for stem cell culture and regenerative medicine. J. Stem Cells Regen. Med. 2010, 6, 10–14. [Google Scholar] [PubMed]
- Payne, S.L.; Anandakumaran, P.N.; Varga, B.V.; Morshead, C.M.; Nagy, A.; Shoichet, M.S. In Vitro Maturation of Human iPSC-Derived Neuroepithelial Cells Influences Transplant Survival in the Stroke-Injured Rat Brain. Tissue Eng. Part A 2017, 24. [Google Scholar] [CrossRef]
- Sun, W.; Incitti, T.; Migliaresi, C.; Quattrone, A.; Casarosa, S.; Motta, A. Viability and neuronal differentiation of neural stem cells encapsulated in silk fibroin hydrogel functionalized with an IKVAV peptide. J. Tissue Eng. Regen. Med. 2017, 11, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Thonhoff, J.R.; Lou, D.I.; Jordan, P.M.; Zhao, X.; Wu, P. Compatibility of human fetal neural stem cells with hydrogel biomaterials in vitro. Brain Res. 2008, 1187, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, J.; Jin, Y.; Luo, Z.; Yang, W.; Xie, H.; Huang, K.; Wang, L. A Neuroprotective Sericin Hydrogel As an Effective Neuronal Cell Carrier for the Repair of Ischemic Stroke. ACS Appl. Mater. Interfaces 2015, 7, 24629–24640. [Google Scholar] [CrossRef] [PubMed]
- Park, K.I.; Teng, Y.D.; Snyder, E.Y. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat. Biotechnol. 2002, 20, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Caicco, M.J.; Cooke, M.J.; Wang, Y.; Tuladhar, A.; Morshead, C.M.; Shoichet, M.S. A hydrogel composite system for sustained epi-cortical delivery of Cyclosporin A to the brain for treatment of stroke. J. Control. Release. 2013, 166, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Nguyen, C.; Chun, H.N.; Llorente, I.L.; Chiu, A.S.; Machnicki, M.; Zarembinski, T.I.; Carmichael, S.T. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J. Cereb. Blood Flow MeTable 2017, 37, 1030–1045. [Google Scholar] [CrossRef] [PubMed]
- Emerich, D.F.; Silva, E.; Ali, O.; Mooney, D.; Bell, W.; Yu, S.J.; Kaneko, Y.; Borlongan, C. Injectable VEGF hydrogels produce near complete neurological and anatomical protection following cerebral ischemia in rats. Cell Transplant. 2010, 19, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Tian, W.M.; Hou, S.P.; Xu, Q.Y.; Spector, M.; Cui, F.Z. An experimental test of stroke recovery by implanting a hyaluronic acid hydrogel carrying a Nogo receptor antibody in a rat model. Biomed. Mater. 2007, 2, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cooke, M.J.; Morshead, C.M.; Shoichet, M.S. Hydrogel delivery of erythropoietin to the brain for endogenous stem cell stimulation after stroke injury. Biomaterials 2012, 33, 2681–2692. [Google Scholar] [CrossRef] [PubMed]
- Hoban, D.B.; Newland, B.; Moloney, T.C.; Howard, L.; Pandit, A.; Dowd, E. The reduction in immunogenicity of neurotrophin overexpressing stem cells after intra-striatal transplantation by encapsulation in an in situ gelling collagen hydrogel. Biomaterials 2013, 34, 9420–9429. [Google Scholar] [CrossRef] [PubMed]
- Jendelova, P.; Kubinova, S.; Sandvig, I.; Erceg, S.; Sandvig, A.; Sykova, E. Current developments in cell- and biomaterial-based approaches for stroke repair. Expert Opin. Biol. Ther. 2016, 16, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.M.; Havenstrite, K.L.; Magnusson, K.E.; Sacco, A.; Leonardi, N.A.; Kraft, P.; Nguyen, N.K.; Thrun, S.; Lutolf, M.P.; Blau, H.M. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 2010, 329, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Madl, C.M.; LeSavage, B.L.; Dewi, R.E.; Dinh, C.B.; Stowers, R.S.; Khariton, M.; Lampe, K.J.; Nguyen, D.; Chaudhuri, O.; Enejder, A.; et al. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat. Mater. 2017, 16, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.W.; Spector, M. Development of hyaluronic acid-based scaffolds for brain tissue engineering. Acta Biomater. 2009, 5, 2371–2384. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.; Burdick, J. Cellular encapsulation in 3D hydrogels for tissue engineering. J. Vis. Exp. 2009, 32, 1590. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kluge, J.A.; Leisk, G.G.; Kaplan, D.L. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials 2008, 29, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Schlosshauer, B.; Muller, E.; Schroder, B.; Planck, H.; Muller, H.W. Rat Schwann cells in bioresorbable nerve guides to promote and accelerate axonal regeneration. Brain Res. 2003, 963, 321–326. [Google Scholar] [CrossRef]
- Peppas, N.A.; Khademhosseini, A. Make better, safer biomaterials. Nature 2016, 540, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Boisserand, L.S.; Kodama, T.; Papassin, J.; Auzely, R.; Moisan, A.; Rome, C.; Detante, O. Biomaterial Applications in Cell-Based Therapy in Experimental Stroke. Stem Cells Int. 2016, 2016, 6810562. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D.; Mey, J. Neural interactions with materials. Front Biosci (Landmark Ed.) 2009, 14, 769–795. [Google Scholar] [CrossRef] [PubMed]
- Potter, W.; Kalil, R.E.; Kao, W.J. Biomimetic material systems for neural progenitor cell-based therapy. Front. Biosci. 2008, 13, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R.; Carter, A.B.; Hager, L.E.; Price, E.M. In Vivo Neural Tissue Engineering: Cylindrical Biocompatible Hydrogels That Create New Neural Tracts in the Adult Mammalian Brain. Stem Cells Dev. 2016, 25, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, H.; Massensini, A.R.; Donnelly, J.; Kim, S.M.; Medberry, C.J.; Badylak, S.F.; Modo, M. ECM hydrogel for the treatment of stroke: Characterization of the host cell infiltrate. Biomaterials 2016, 91, 166–181. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.Z.; Tian, W.M.; Hou, S.P.; Xu, Q.Y.; Lee, I.S. Hyaluronic acid hydrogel immobilized with RGD peptides for brain tissue engineering. J. Mater. Sci. Mater. Med. 2006, 17, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V.; Skinner, S.J.; Geaney, M.; Vasconcellos, A.V.; Elliott, R.B.; Emerich, D.F. Intracerebral transplantation of porcine choroid plexus provides structural and functional neuroprotection in a rodent model of stroke. Stroke 2004, 35, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Lampe, K.J.; Kern, D.S.; Mahoney, M.J.; Bjugstad, K.B. The administration of BDNF and GDNF to the brain via PLGA microparticles patterned within a degradable PEG-based hydrogel: Protein distribution and the glial response. J. Biomed. Mater. Res. A 2011, 96, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, K.; Tsuru, K.; Hayashi, T.; Takaishi, M.; Nagahara, M.; Nagotani, S.; Sehara, Y.; Jin, G.; Zhang, H.; Hayakawa, S.; et al. Implantation of a new porous gelatin-siloxane hybrid into a brain lesion as a potential scaffold for tissue regeneration. J. Cereb. Blood Flow MeTable 2006, 26, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cao, B.; Feng, M.; Zhou, Q.; Sun, X.; Wu, S.; Jin, S.; Liu, H.; Lianhong, J. Combinated transplantation of neural stem cells and collagen type I promote functional recovery after cerebral ischemia in rats. Anat. Rec. (Hoboken) 2010, 293, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Bible, E.; Qutachi, O.; Chau, D.Y.; Alexander, M.R.; Shakesheff, K.M.; Modo, M. Neo-vascularization of the stroke cavity by implantation of human neural stem cells on VEGF-releasing PLGA microparticles. Biomaterials 2012, 33, 7435–7446. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Shichinohe, H.; Houkin, K.; Kuroda, S. Application of cell sheet technology to bone marrow stromal cell transplantation for rat brain infarct. J. Tissue Eng. Regen. Med. 2017, 11, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Moshayedi, P.; Nih, L.R.; Llorente, I.L.; Berg, A.R.; Cinkornpumin, J.; Lowry, W.E.; Segura, T.; Carmichael, S.T. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials 2016, 105, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Chan, A.; Morad, L.; Kornblum, H.I.; Fan, G.; Carmichael, S.T. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil. Neural Repair 2010, 24, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, K.; Sephel, G.C.; Weeks, B.; Sasaki, M.; Martin, G.R.; Kleinman, H.K.; Yamada, Y. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J. Biol. Chem. 1989, 264, 16174–16182. [Google Scholar] [PubMed]
- Ghuman, H.; Gerwig, M.; Nicholls, F.J.; Liu, J.R.; Donnelly, J.; Badylak, S.F.; Modo, M. Long-term retention of ECM hydrogel after implantation into a sub-acute stroke cavity reduces lesion volume. Acta Biomater. 2017, 63, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhu, Z.; Zhao, R.C.; Xiao, Z.; Wu, C.; Han, Q.; Chen, L.; Tong, W.; Zhang, J.; Gao, J.; et al. Transplantation of human mesenchymal stem cells loaded on collagen scaffolds for the treatment of traumatic brain injury in rats. Biomaterials 2013, 34, 5937–5946. [Google Scholar] [CrossRef] [PubMed]
- Tate, C.C.; Shear, D.A.; Tate, M.C.; Archer, D.R.; Stein, D.G.; LaPlaca, M.C. Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain. J. Tissue Eng. Regen. Med. 2009, 3, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Phng, L.K.; Gerhardt, H. Angiogenesis: A team effort coordinated by notch. Dev. Cell 2009, 16, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Nakaguchi, K.; Jinnou, H.; Kaneko, N.; Sawada, M.; Hikita, T.; Saitoh, S.; Tabata, Y.; Sawamoto, K. Growth factors released from gelatin hydrogel microspheres increase new neurons in the adult mouse brain. Stem Cells Int. 2012, 2012, 915160. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.J.; Rosenzweig, E.S.; McDonald, J.W., 3rd; Sakiyama-Elbert, S.E. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J. Control. Release 2006, 113, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Tesar, B.M.; Jiang, D.; Liang, J.; Palmer, S.M.; Noble, P.W.; Goldstein, D.R. The role of hyaluronan degradation products as innate alloimmune agonists. Am. J. Transplant. 2006, 6, 2622–2635. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Tuohy, T.M.; Chen, H.; Wallingford, N.; Craig, A.; Struve, J.; Luo, N.L.; Banine, F.; Liu, Y.; Chang, A.; et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med. 2005, 11, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Cargill, R.; Kohama, S.G.; Struve, J.; Su, W.; Banine, F.; Witkowski, E.; Back, S.A.; Sherman, L.S. Astrocytes in aged nonhuman primate brain gray matter synthesize excess hyaluronan. Neurobiol. Aging 2012, 33, 830.e13–830.e24. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, A.P.; McInnis, C.; Gupta, K.B.; Snow, W.W.; Love, D.F.; Mason, D.W.; Ferrell, T.M.; Staas, J.K.; Tice, T.R. The fate of biodegradable microspheres injected into rat brain. Neurosci. Lett. 2002, 323, 85–88. [Google Scholar] [CrossRef]
- Skop, N.B.; Calderon, F.; Cho, C.H.; Gandhi, C.D.; Levison, S.W. Improvements in biomaterial matrices for neural precursor cell transplantation. Mol. Cell. Ther. 2014, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Woung, L.C.; Yen, J.C.; Tseng, P.C.; Chiou, S.H.; Sung, Y.J.; Liu, K.T.; Cheng, Y.H. Thermosensitive chitosan-based hydrogels for sustained release of ferulic acid on corneal wound healing. Carbohydr. Polym. 2016, 135, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Foo, C.T.; Rossetti, F.; Textor, M.; Vunjak-Novakovic, G.; Kaplan, D.L.; Merkle, H.P.; Meinel, L. Silk fibroin as an organic polymer for controlled drug delivery. J. Control. Release 2006, 111, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, L.; Pérez-Rigueiro, J.; Martinez-Murillo, R.; Panetsos, F.; Ramos, M.; Guinea, G.V.; González-Nieto, D. (Center for Biomedical Technology, Madrid, Spain). Personal Communication, 2017. [Google Scholar]
- Flanagan, L.A.; Rebaza, L.M.; Derzic, S.; Schwartz, P.H.; Monuki, E.S. Regulation of human neural precursor cells by laminin and integrins. J. Neurosci. Res. 2006, 83, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Aurand, E.R.; Wagner, J.L.; Shandas, R.; Bjugstad, K.B. Hydrogel formulation determines cell fate of fetal and adult neural progenitor cells. Stem Cell Res. 2014, 12, 11–23. [Google Scholar] [CrossRef] [PubMed]


| Organ/tissue | Measured property | Measured value | References |
| Brain (rat and mouse) | Elastic modulus (Compressive) | E = 50 kPa | [170] |
| Brain (rat) | Shear storage modulus | G′ = 0.33 kPa | [171] |
| Brain (rat) | Shear storage modulus | G′ = 0.6 kPa | [172] |
| Brain (swine) | Elastic modulus | E = 3.2 kPa | [176] |
| Brain (mouse) | Shear modulus | E = 25 kPa | [169] |
| Brain (rat) | Bulk elastic modulus | E = 5.5 kPa | [173] |
| Brain (rat) | Bulk elastic modulus | E = 5.7 kPa | [177] |
| Cerebellum (rat) | Bulk elastic modulus | E = 0.3–0.45 kPa | [174] |
| Biomaterial hydrogel | Measured property | Measured value | References |
| PEG | Elastic modulus | E = 1–10 kPa | [166] |
| HA-Laminin | Shear Storage/Loss modulus | G′ (Storage) = 0.6 kPa G′′ (Loss)= 0.2 kPa | [178] |
| HA | Bulk elastic modulus | E = 7–8 kPa | [177] |
| HA | Shear Storage/Loss modulus | G′ = 0.33–0.84 kPa G′′ = 0.001–0.01 kPa | [179] |
| HA with MPP degradable | Shear Storage/Loss modulus | G′ = 0.33 kPa G′′ = 0.0057 kPa | [180] |
| HA with MPP nondegradable | Shear Storage/Loss modulus | G′ = 0.29 kPa G′′ = 0.0024 kPa | [180] |
| TGP (Mebiol gel) | Shear Storage/Loss modulus | G′ ~ 0.8 kPa (~37 °C) G′′ ~ 0.2 kPa (~37 °C) | [181] |
| HAMC | Shear Storage/Loss modulus | G′ < 0.1 kPa G′′ < 0.1 kPa | [182] |
| Microporous HA | Elastic modulus | E = 1.5 kPa | [183] |
| Laminin peptide sequence IKVAV | Shear Storage/Loss modulus | G′ = 0.8–1.0 kPa G′′ = 0.1–0.4 kPa | [184] |
| HA-IKVAV-MPP | Shear Storage/Loss modulus | G′ = 0.47–1.6 kPa G′′ < 0.1 kPa | [185] |
| ECM-UBM | Shear Storage/Loss modulus | G′ = 0.07–0.46 kPa G′′ < 0.01–0.06 kPa | [186] |
| Chitosan | Shear Storage/Loss modulus | G′ = 0.8–1.5 kPa G′′ = 0.001–0.01 kPa | [187] |
| Alginate | Shear Storage/Loss modulus | G′ = 0.8–1.5 kPa G′′ = 0.4–0.5 kPa | [187] |
| Silk fibroin | Elastic modulus | E = 6–30 kPa | [188] |
| Stroke model, specie | Biomaterial, cell population, site of implantation | Therapeutic effects | References |
|---|---|---|---|
| CCAO, mouse | PGA, nSCs, infarct cavity | Increasing axonal rewiring, reduction of inflammation and glial scar formation | [204] |
| MCAO, mouse | TGP, mSCs, brain surface | Increasing engraftment of transplanted cells, increasing neuronal differentiation, no functional improvement | [181] |
| MCAO, mouse | HA alone, infarct cavity | Reduction of inflammation and glial scar, enhanced perilesional vascularization, stimulation of endogenous neurogenesis | [183] |
| MCAO, rat | PLGA, nSCs, infarct cavity | Cavity size reduction, increasing engraftment of transplanted cells, de novo tissue formation | [156] |
| MCAO, rat | Matrigel, eSCs-nPCs, infarct cavity | Reduction in lesion size, increasing survival of transplanted cells, neuronal and astroglial differentiation, neuronal migration, improvement of behavioral outcome | [135] |
| MCAO, rat | Col I , nSCs, infarct cavity | Increasing survival of transplanted cells, neuronal differentiation, increasing synaptogenesis, improvement of behavioral outcome | [229] |
| MCAO, rat | PLGA-VEGF, nSCs, infarct cavity | Hipervascularization linked with astrocytic differentiation, limited neuronal commitment | [230] |
| MCAO, rat | TRP, mSCs, brain surface | Improvement of motor function | [231] |
| PTS, mouse | HA, iPSCs –nPCs, infarct cavity | Enhancing survival of transplanted cells, reduction of post-stroke inflammation, increasing neuronal and astrocytic differentiation | [180,232] |
| PTS, mouse | HA-heparin-Col, eSCs-nPCs, infarct cavity | Increasing survival of transplanted cells, reduction of inflammation and glial scar | [233] |
| ET-1, mouse | HAMC, nSCs, infarct cavity | Increasing survival of transplanted cells, astrocytic differentiation, limited neuronal and oligodendrocyte commitment, improvement of behavioral outcome | [155] |
| ET-1, rat | Self-assembling IKVAV peptide (laminin epitope), eSCs-nPCs, infarct cavity | Increasing survival of transplanted cells, tissue regeneration, cell adhesion and axonal growth, improvement of behavioral outcome | [184] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Nieto, D.; Fernández-García, L.; Pérez-Rigueiro, J.; Guinea, G.V.; Panetsos, F. Hydrogels-Assisted Cell Engraftment for Repairing the Stroke-Damaged Brain: Chimera or Reality. Polymers 2018, 10, 184. https://doi.org/10.3390/polym10020184
González-Nieto D, Fernández-García L, Pérez-Rigueiro J, Guinea GV, Panetsos F. Hydrogels-Assisted Cell Engraftment for Repairing the Stroke-Damaged Brain: Chimera or Reality. Polymers. 2018; 10(2):184. https://doi.org/10.3390/polym10020184
Chicago/Turabian StyleGonzález-Nieto, Daniel, Laura Fernández-García, José Pérez-Rigueiro, Gustavo V. Guinea, and Fivos Panetsos. 2018. "Hydrogels-Assisted Cell Engraftment for Repairing the Stroke-Damaged Brain: Chimera or Reality" Polymers 10, no. 2: 184. https://doi.org/10.3390/polym10020184