Self-Assembled Nanostructures of Red Fluorescent Amphiphilic Block Copolymers as Both Imaging Probes and Drug Carriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
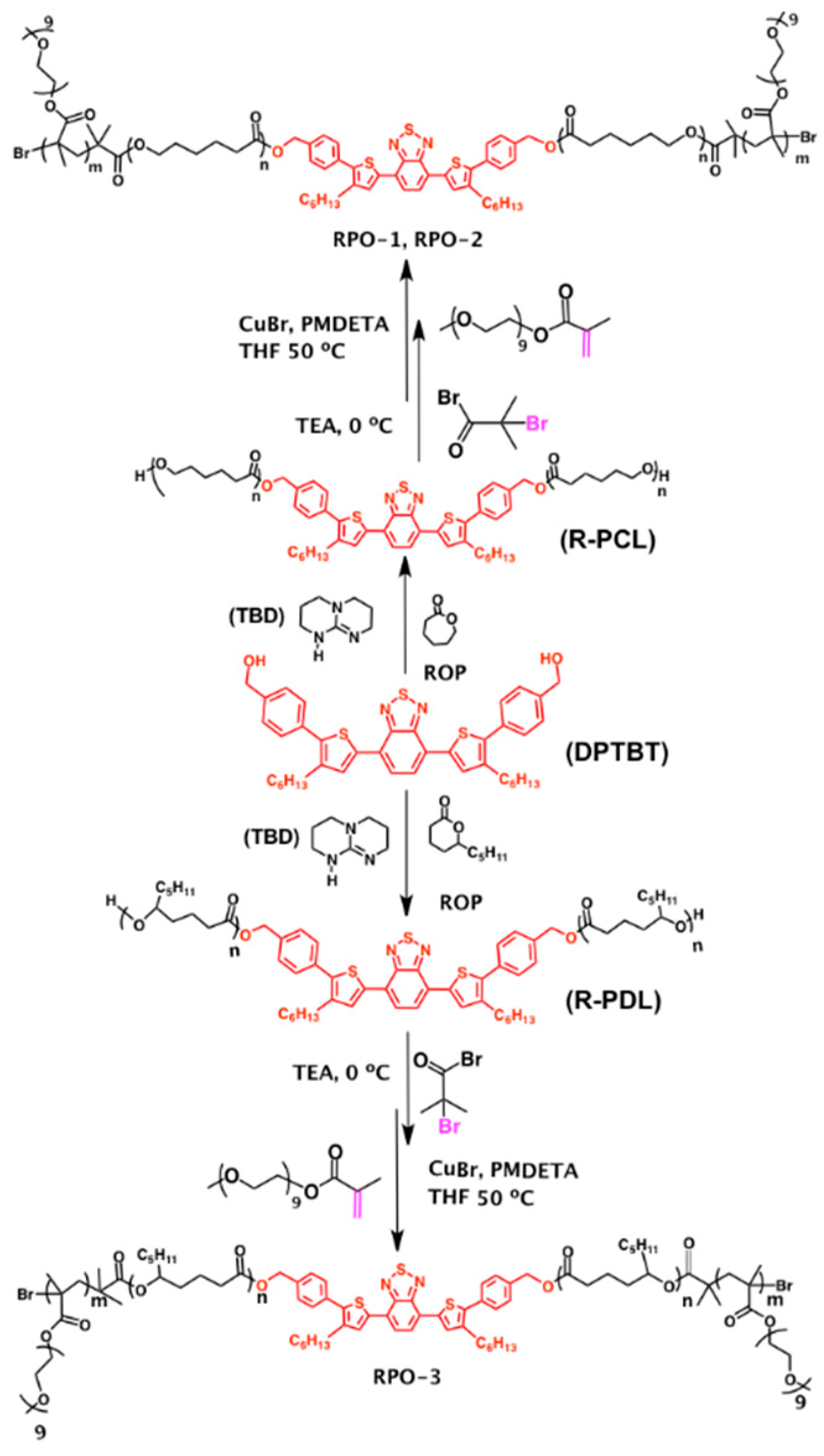
2.2. Synthesis of Red Fluorescent Homopolymers: Polycaprolactone (R-PCL) and Polyedecalactone (R-PDL)
2.3. Synthesis of Red-Fluorescent Amphiphilic Block Copolymers (RPO-1–3)
2.4. Preparation of RPO Micellar Structures
2.5. Encapsulation of Doxorubicin (DOX) into RPO-3 Nanoparticles (NPs)
2.6. In Vitro DOX Release with Near-Infrared (NIR) Laser Irradiation
2.7. In Vitro Cellular Uptake of RPO-3 Micelles and DOX@RPO-3 Composite Micelles
2.8. Cytotoxicity of RPO-3 and DOX@RPO-3 NPs
3. Results and Discussion
3.1. Polymer Synthesis and Structural Characterization
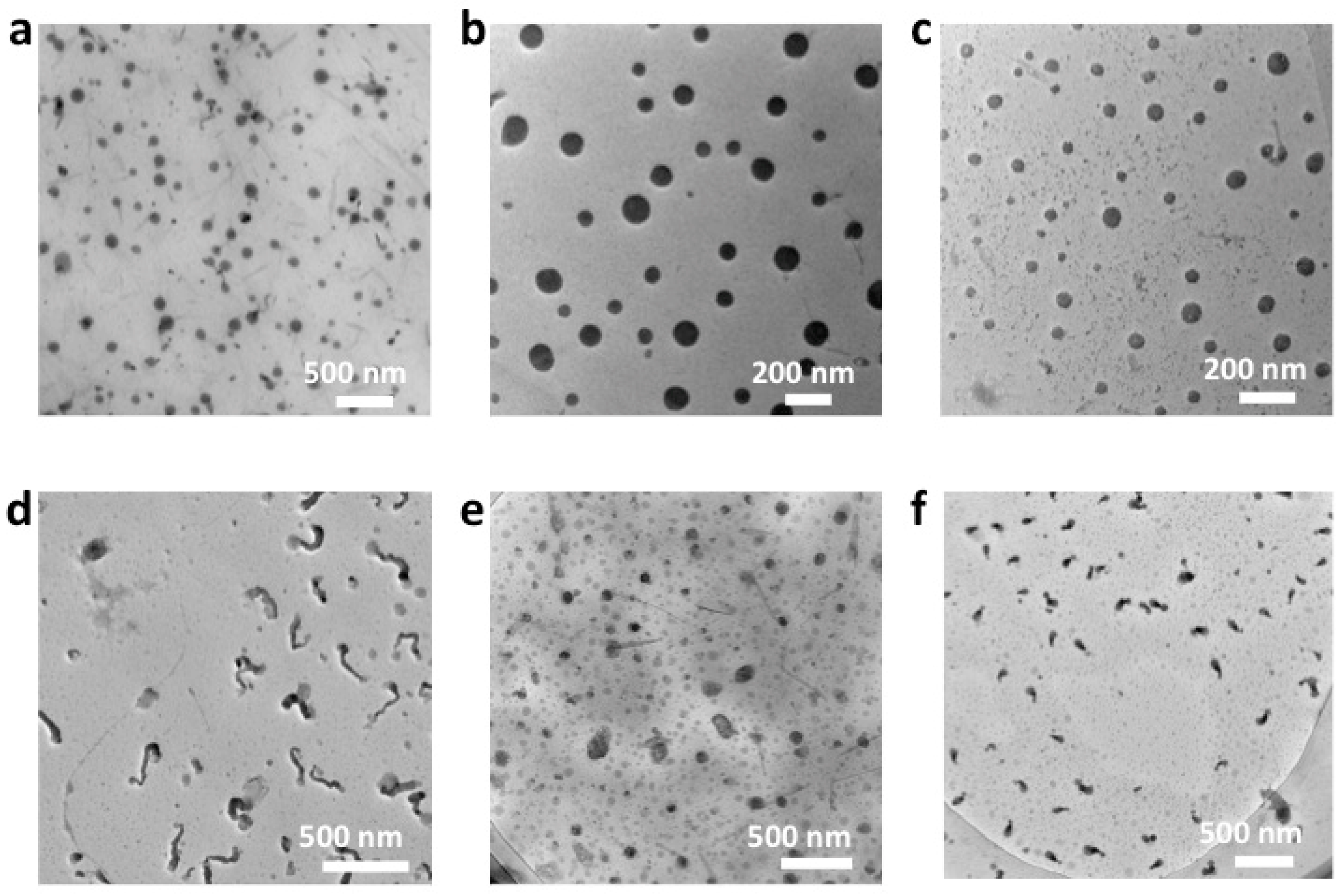
3.2. Self-Assembly of RPO-(1–3) in Aqueous Solutions
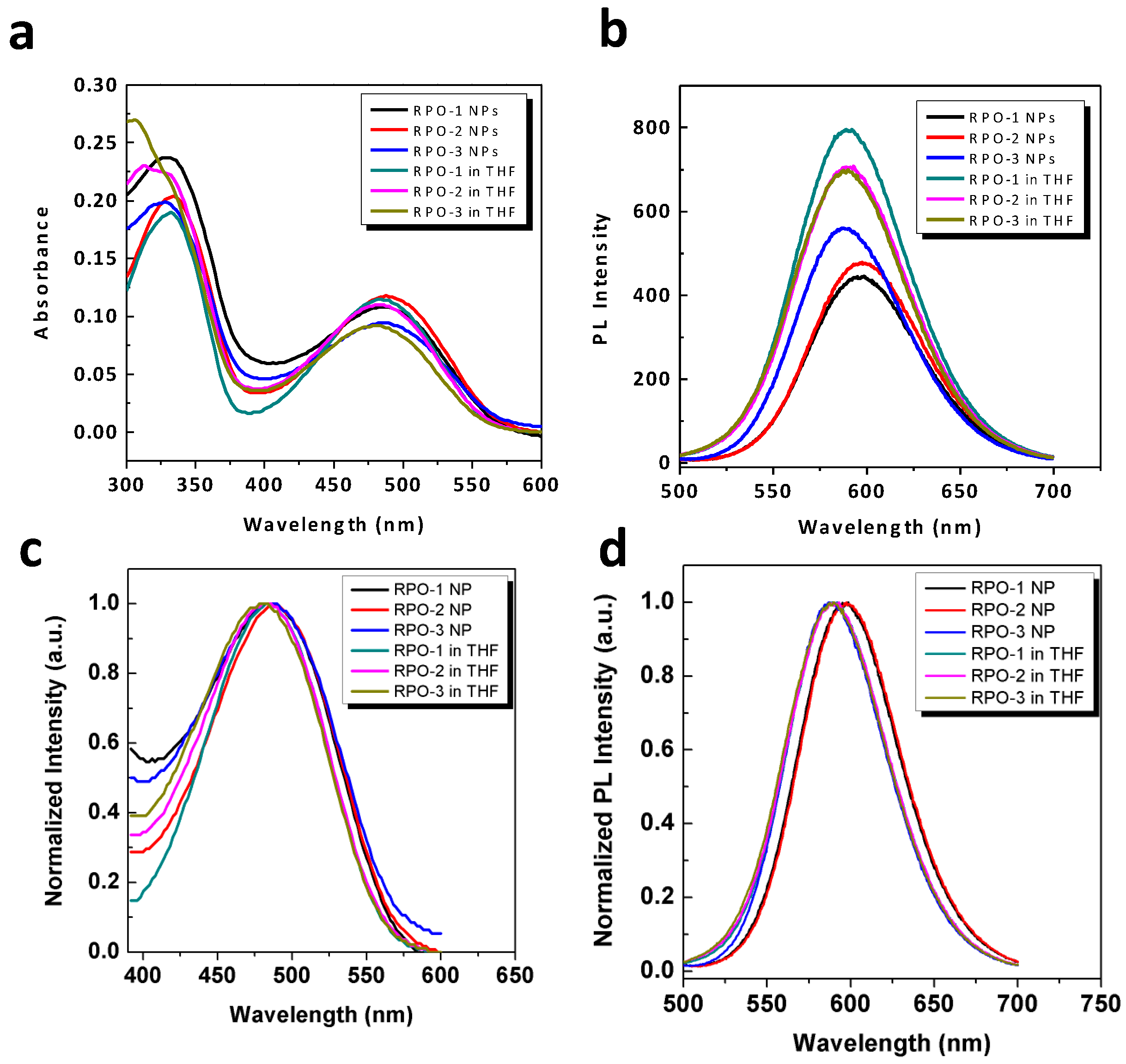
3.3. Optical Properties of RPO-(1–3) and Their Self-Assembled Nanostructures in Water
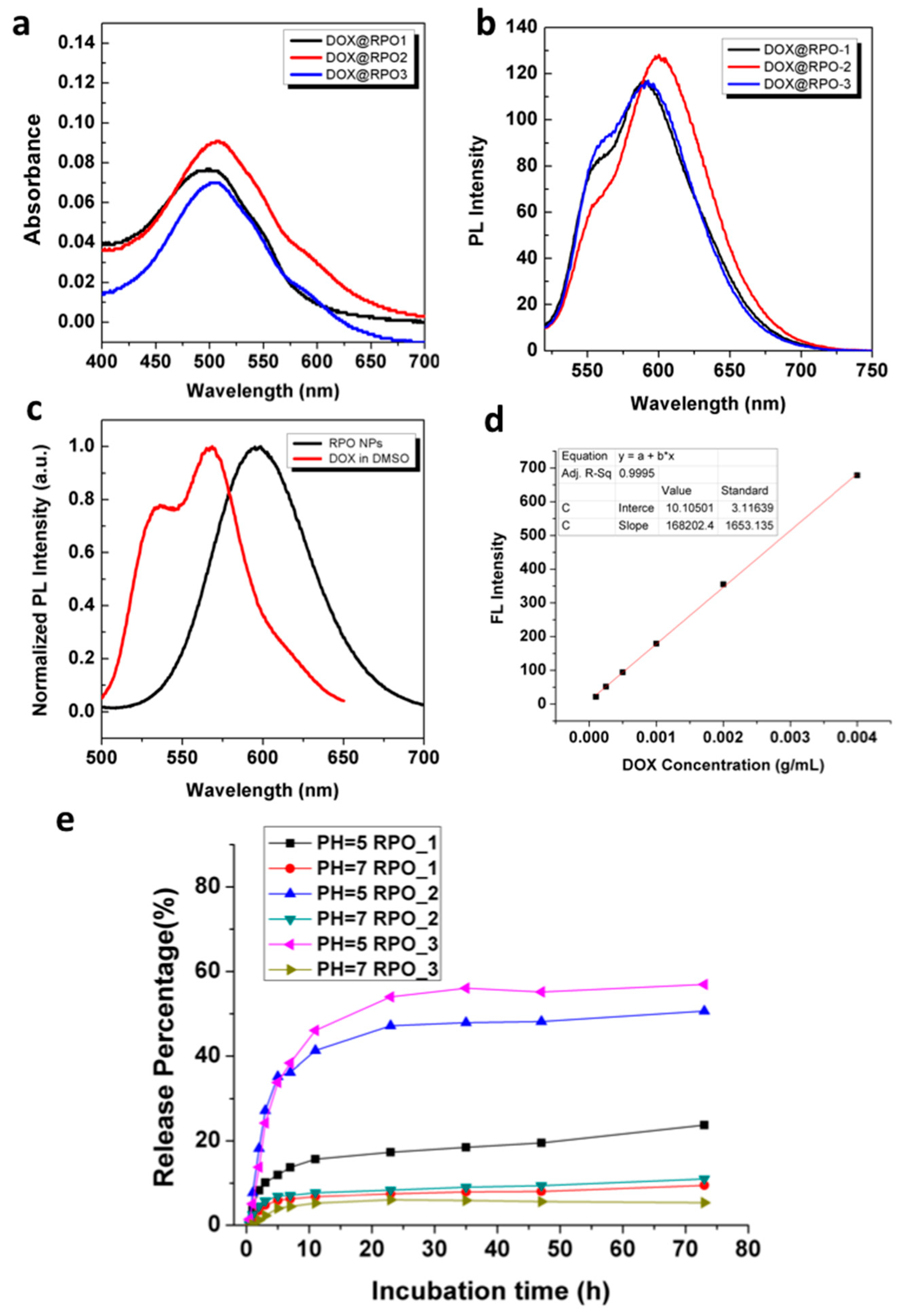
3.4. Encapsulation of Anticancer Drugs into RPO Micellar Structures and Controlled Release
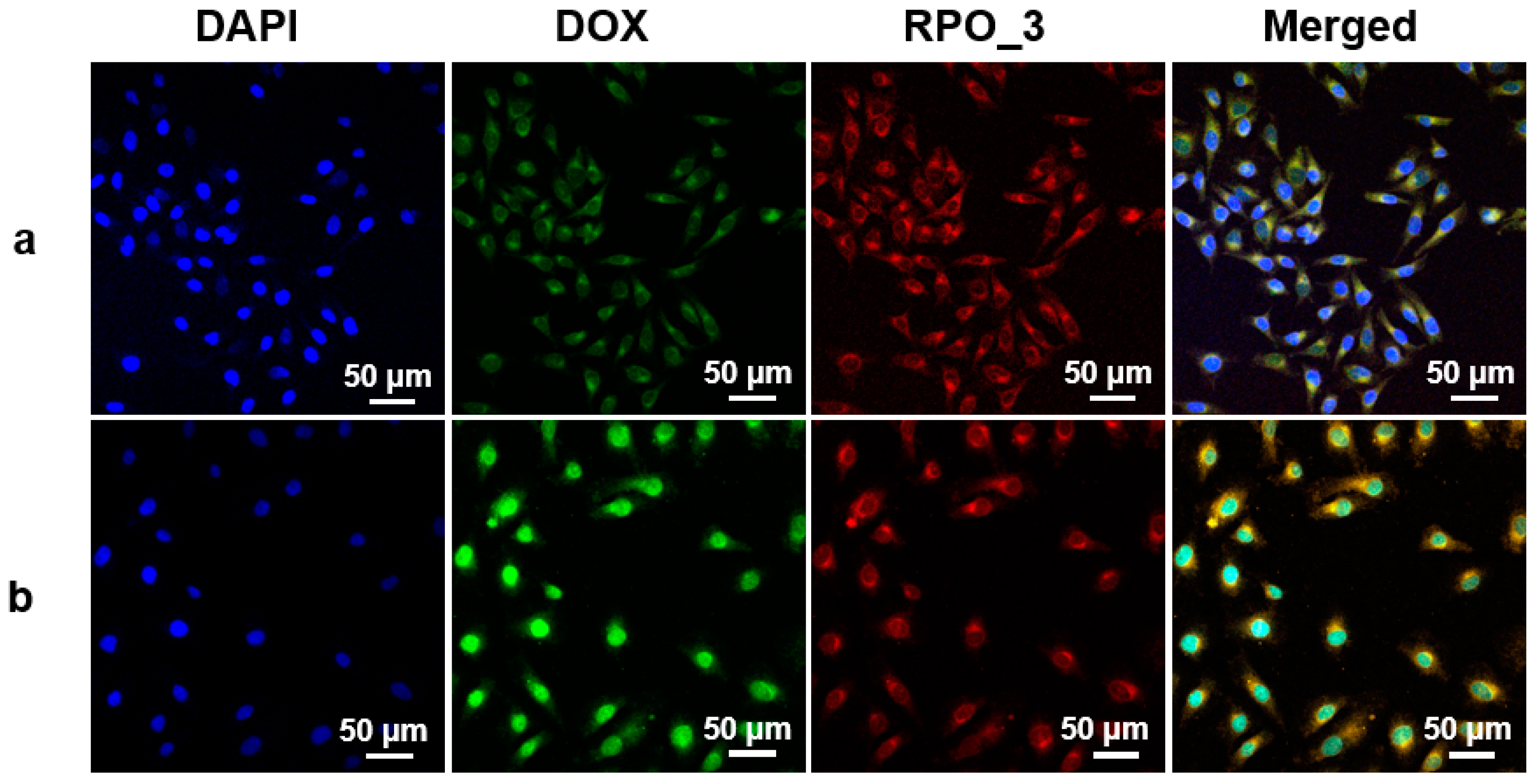
3.5. Cellular Internalization of DOX@RPO-3 Nanoparticles and Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yoo, J.-W.; Irvine, D.J.; Discher, D.E.; Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011, 10, 521. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- Chandna, P.; Khandare, J.J.; Ber, E.; Rodriguez-Rodriguez, L.; Minko, T. Multifunctional Tumor-Targeted Polymer-Peptide-Drug Delivery System for Treatment of Primary and Metastatic Cancers. Pharm. Res. 2010, 27, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Huang, S.; Yang, C.; Wang, M. Unimolecular Micelles of Amphiphilic Cyclodextrin-Core Star-Like Copolymers with Covalent pH-Responsive Linkage of Anticancer Prodrugs. Mol. Pharm. 2017, 14, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lv, L.-P.; Jiang, S.; Landfester, K.; Crespy, D. Advanced stimuli-responsive polymer nanocapsules with enhanced capabilities for payloads delivery. Polym. Chem. 2015, 6, 4197–4205. [Google Scholar] [CrossRef] [Green Version]
- Staff, R.H.; Gallei, M.; Landfester, K.; Crespy, D. Hydrophobic Nanocontainers for Stimulus-Selective Release in Aqueous Environments. Macromolecules 2014, 47, 4876–4883. [Google Scholar] [CrossRef]
- Staff, R.H.; Gallei, M.; Mazurowski, M.; Rehahn, M.; Berger, R.; Landfester, K.; Crespy, D. Patchy Nanocapsules of Poly(vinylferrocene)-Based Block Copolymers for Redox-Responsive Release. ACS Nano 2012, 6, 9042–9049. [Google Scholar] [CrossRef] [PubMed]
- Sahle, F.F.; Gulfam, M.; Lowe, T.L. Design strategies for physical-stimuli-responsive programmable nanotherapeutics. Drug Discov. Today 2018, 23, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Savić, R.; Azzam, T.; Eisenberg, A.; Maysinger, D. Assessment of the Integrity of Poly(caprolactone)-b-poly(ethylene oxide) Micelles under Biological Conditions: A Fluorogenic-Based Approach. Langmuir 2006, 22, 3570–3578. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kumar, S.; Lee, A.; Felorzabihi, N.; Shen, L.; Zhao, F.; Froimowicz, P.; Scholes, G.D.; Winnik, M.A. Nanoscale Co-organization of Quantum Dots and Conjugated Polymers Using Polymeric Micelles As Templates. J. Am. Chem. Soc. 2008, 130, 9481–9491. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Eisenberg, A. Selective Localization of Preformed Nanoparticles in Morphologically Controllable Block Copolymer Aggregates in Solution. Acc. Chem. Res. 2012, 45, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Ahmed, F.; Bhasin, N.; Discher, D.E. Visualizing Worm Micelle Dynamics and Phase Transitions of a Charged Diblock Copolymer in Water. J. Phys. Chem. B 2005, 109, 3772–3779. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-I.; Peng, C.-L.; Lee, P.-C.; Tsai, M.-H.; Lin, C.-Y.; Shih, Y.-H.; Wei, M.-F.; Luo, T.-Y.; Shieh, M.-J. Traceable Self-Assembly of Laser-Triggered Cyanine-Based Micelle for Synergistic Therapeutic Effect. Adv. Healthc. Mater. 2015, 4, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cui, Z.; Yu, P.; Guo, C.; Feng, B.; Jiang, T.; Wang, S.; Yin, Q.; Zhong, D.; Yang, X.; et al. pH- and NIR Light-Responsive Micelles with Hyperthermia-Triggered Tumor Penetration and Cytoplasm Drug Release to Reverse Doxorubicin Resistance in Breast Cancer. Adv. Funct. Mater. 2015, 25, 2489–2500. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, K.; Huang, G.; Hensley, C.; Huang, X.; Ma, X.; Zhao, T.; Sumer, B.D.; DeBerardinis, R.J.; Gao, J. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat. Mater. 2013, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, K.; Wang, S.; Wang, Y.; Wang, M. Highly Fluorescent Polycaprolactones with Tunable Light Emission Wavelengths across Visible to NIR Spectral Window. Adv. Mater. Interfaces 2016, 3, 1600259. [Google Scholar] [CrossRef]
- Huang, S.; Liu, S.; Wang, K.; Yang, C.; Luo, Y.; Zhang, Y.; Cao, B.; Kang, Y.; Wang, M. Highly fluorescent and bioresorbable polymeric nanoparticles with enhanced photostability for cell imaging. Nanoscale 2015, 7, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Luo, Y.; Huang, S.; Yang, H.; Liu, B.; Wang, M. Highly fluorescent polycaprolactones decorated with di(thiophene-2-yl)-diketopyrrolopyrrole: A covalent strategy of tuning fluorescence properties in solid states. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1032–1042. [Google Scholar] [CrossRef]
- Diao, H.J.; Wang, K.; Long, H.Y.; Wang, M.; Chew, S.Y. Highly Fluorescent and Photostable Polymeric Nanofibers as Scaffolds for Cell Interfacing and Long-Term Tracking. Adv. Healthc. Mater. 2016, 5, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, X.; Huang, S.; Wang, M. Tunable Förster Resonance Energy Transfer in Colloidal Nanoparticles Composed of Polycaprolactone-Tethered Donors and Acceptors: Enhanced Near-Infrared Emission and Compatibility for In Vitro and In Vivo Bioimaging. Adv. Funct. Mater. 2018, 28, 1705226. [Google Scholar] [CrossRef]
- Lebouille, J.G.J.L.; Stepanyan, R.; Slot, J.J.M.; Cohen Stuart, M.A.; Tuinier, R. Nanoprecipitation of polymers in a bad solvent. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 225–235. [Google Scholar] [CrossRef]
- Aschenbrenner, E.; Bley, K.; Koynov, K.; Makowski, M.; Kappl, M.; Landfester, K.; Weiss, C.K. Using the Polymeric Ouzo Effect for the Preparation of Polysaccharide-Based Nanoparticles. Langmuir 2013, 29, 8845–8855. [Google Scholar] [CrossRef] [PubMed]
- Musyanovych, A.; Landfester, K. Polymer Micro- and Nanocapsules as Biological Carriers with Multifunctional Properties. Macromol. Biosci. 2014, 14, 458–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijlard, A.-C.; Wald, S.; Crespy, D.; Taden, A.; Wurm, F.R.; Landfester, K. Functional Colloidal Stabilization. Adv. Mater. Interfaces 2017, 4, 1600443. [Google Scholar] [CrossRef]
- Yang, C.; Liu, H.; Zhang, Y.; Xu, Z.; Wang, X.; Cao, B.; Wang, M. Hydrophobic-Sheath Segregated Macromolecular Fluorophores: Colloidal Nanoparticles of Polycaprolactone-Grafted Conjugated Polymers with Bright Far-Red/Near-Infrared Emission for Biological Imaging. Biomacromolecules 2016, 17, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Huang, S.; Wang, X.; Wang, M. Theranostic unimolecular micelles of highly fluorescent conjugated polymer bottlebrushes for far red/near infrared bioimaging and efficient anticancer drug delivery. Polym. Chem. 2016, 7, 7455–7468. [Google Scholar] [CrossRef]
- Zhang, Y.; Jeon, M.; Rich, L.J.; Hong, H.; Geng, J.; Zhang, Y.; Shi, S.; Barnhart, T.E.; Alexandridis, P.; Huizinga, J.D.; et al. Non-invasive, Multimodal Functional Imaging of the Intestine with Frozen Micellar Naphthalocyanines. Nat. Nanotechnol. 2014, 9, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.K.; Kakde, D.; Purdie, L.; Irvine, D.J.; Howdle, S.M.; Mantovani, G.; Alexander, C. New biomaterials from renewable resources—Amphiphilic block copolymers from δ-decalactone. Polym. Chem. 2015, 6, 7196–7210. [Google Scholar] [CrossRef]
- Kakde, D.; Taresco, V.; Bansal, K.K.; Magennis, E.P.; Howdle, S.M.; Mantovani, G.; Irvine, D.J.; Alexander, C. Amphiphilic block copolymers from a renewable ε-decalactone monomer: Prediction and characterization of micellar core effects on drug encapsulation and release. J. Mater. Chem. B 2016, 4, 7119–7129. [Google Scholar] [CrossRef]
- Martello, M.T.; Burns, A.; Hillmyer, M. Bulk Ring-Opening Transesterification Polymerization of the Renewable δ-Decalactone Using an Organocatalyst. ACS Macro Lett. 2012, 1, 131–135. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Kang, Y.; Wang, M. Glutathione-Responsive Polymeric Micelles Formed by a Biodegradable Amphiphilic Triblock Copolymer for Anticancer Drug Delivery and Controlled Release. ACS Biomater. Sci. Eng. 2015, 1, 585–592. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Liu, H.; Yang, C.; Kang, Y.; Wang, M. Unimolecular micelles of amphiphilic cyclodextrin-core star-like block copolymers for anticancer drug delivery. Chem. Commun. 2015, 51, 15768–15771. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, K.; Yang, C.; Huang, S.; Wang, M. Multifunctional polymeric micelles loaded with doxorubicin and poly(dithienyl-diketopyrrolopyrrole) for near-infrared light-controlled chemo-phototherapy of cancer cells. Colloids Surf. B 2017, 157, 398–406. [Google Scholar] [CrossRef] [PubMed]
- De Gracia Lux, C.; McFearin, C.L.; Joshi-Barr, S.; Sankaranarayanan, J.; Fomina, N.; Almutairi, A. Single UV or Near IR Triggering Event Leads to Polymer Degradation into Small Molecules. ACS Macro Lett. 2012, 1, 922–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Eisenberg, A. Multiple Morphologies of “Crew-Cut” Aggregates of Polystyrene-b-poly(acrylic acid) Block Copolymers. Science 1995, 268, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.S.; Corbierre, M.K.; Eisenberg, A. 1998 E.W.R. Steacie Award Lecture Asymmetric amphiphilic block copolymers in solution: A morphological wonderland. Can. J. Chem. 1999, 77, 1311–1326. [Google Scholar] [CrossRef]
- Debele, T.A.; Lee, K.-Y.; Hsu, N.-Y.; Chiang, Y.-T.; Yu, L.-Y.; Shen, Y.-A.; Lo, C.-L. A pH sensitive polymeric micelle for co-delivery of doxorubicin and α-TOS for colon cancer therapy. J. Mater. Chem. B 2017, 5, 5870–5880. [Google Scholar] [CrossRef]

| Entry No. | Polymer Structure | Yield | Mn1 | Mw/Mn |
|---|---|---|---|---|
| R-PCL-1 | PCL54-dye-PCL54 | 70% | 13,000 | 1.5 |
| R-PCL-2 | PCL41-dye-PCL41 | 68% | 10,000 | 1.7 |
| R-PDL | PDL22-dye-PDL22 | 77% | 8,000 | 1.7 |
| RPO-1 | POEGMA62-b-PCL54-dye-PCL54-b-POEGMA62 | 71% | 50,000 | 1.9 |
| RPO-2 | POEGMA17-b-PCL41-dye-PCL41-b-POEGMA17 | 67% | 20,000 | 2.1 |
| RPO-3 | POEGMA25-b-PDL22-dye-PDL22-b-POEGMA25 | 40% | 23,000 | 3.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Wei, X.; Wang, M. Self-Assembled Nanostructures of Red Fluorescent Amphiphilic Block Copolymers as Both Imaging Probes and Drug Carriers. Polymers 2018, 10, 1120. https://doi.org/10.3390/polym10101120
Huang S, Wei X, Wang M. Self-Assembled Nanostructures of Red Fluorescent Amphiphilic Block Copolymers as Both Imaging Probes and Drug Carriers. Polymers. 2018; 10(10):1120. https://doi.org/10.3390/polym10101120
Chicago/Turabian StyleHuang, Shuo, Xin Wei, and Mingfeng Wang. 2018. "Self-Assembled Nanostructures of Red Fluorescent Amphiphilic Block Copolymers as Both Imaging Probes and Drug Carriers" Polymers 10, no. 10: 1120. https://doi.org/10.3390/polym10101120






