Multiscale Interface Effect on Homogeneous Dielectric Structure of ZrO2/Teflon Nanocomposite for Electrowetting Application
Abstract
:1. Introduction
2. Experimental and Simulated Methods
2.1. Materials
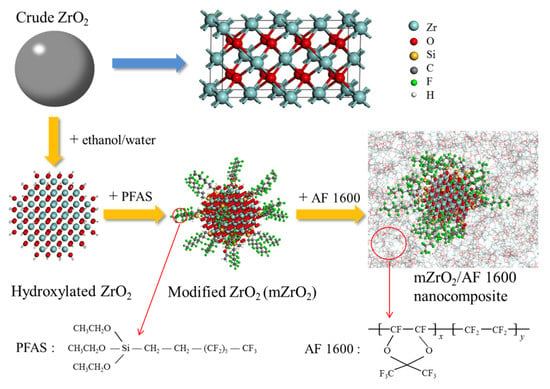
2.2. Surface Modification of ZrO2 Nanoparticles
2.3. Characterization of ZrO2 Nanoparticles
2.4. Compatibility Test of ZrO2 with AF 1600
2.5. Preparation on mZrO2@AF 1600 Layer
2.6. Characterization of mZrO2@AF 1600 Layer
2.7. Simulated Method
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mugele, F.; Baret, J.-C. Electrowetting: from basics to applications. J. Phys. Condens. Matter 2005, 17, R705–R774. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Chen, J.; Zhu, Z.; Zhang, Q.; Yang, H.; Wang, Q. Significantly enhanced dielectric and hydrophobic properties of SiO2@MgO/PMMA composite films. RSC Adv. 2018, 8, 4032–4038. [Google Scholar] [CrossRef]
- Hayes, R.A.; Feenstra, B.J. Video-speed electronic paper based on electrowetting. Nature 2003, 425, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Brien, R.; Njus, Z.; Kalwa, U.; Pandey, S. Motorized actuation system to perform droplet operations on printed plastic sheets. Lab Chip. 2016, 16, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, T.; Dong, C.; Jia, Y.; Mak, P.I.; Vai, M.I.; Rui, P.M. Adhesion promoter for a multi-dielectric-layer on a digital microfluidic chip. RSC Adv. 2015, 5, 48626–48630. [Google Scholar] [CrossRef] [Green Version]
- Terrab, S.; Watson, A.M.; Roath, C.; Gopinath, J.T.; Bright, V.M. Adaptive electrowetting lens-prism element. Opt. Express. 2015, 23, 25838–25845. [Google Scholar] [CrossRef] [PubMed]
- Ozbay, B.N.; Losacco, J.T.; Cormack, R.; Weir, R.; Bright, V.M.; Gopinath, J.T.; Restrepo, D.; Gibson, E.A. Miniaturized fiber-coupled confocal fluorescence microscope with an electrowetting variable focus lens using no moving parts. Opt. Lett. 2015, 40, 2553–2556. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Castelletto, S.; Rosengarten, G. Fabrication of solar beam steering electrowetting devices—present status and future prospects. J. Phys. D Appl. Phys. 2017, 50, 403001. [Google Scholar] [CrossRef] [Green Version]
- Nelson, W.C.; Kim, C.-J.C. Droplet Actuation by Electrowetting-on-Dielectric (EWOD): A Review. J. Adhes. Sci. Technol. 2012, 26, 1747–1771. [Google Scholar] [CrossRef]
- Liu, H.; Dharmatilleke, S.; Maurya, D.K.; Tay, A.A.O. Dielectric materials for electrowetting-on-dielectric actuation. Microsyst. Technol. 2010, 16, 449–460. [Google Scholar] [CrossRef]
- Li, Z.; Haigh, A.; Soutis, C.; Gibson, A.; Sloan, R. Dielectric constant of a three-dimensional woven glass fibre composite: Analysis and measurement. Compos. Struct. 2017, 180, 853–861. [Google Scholar] [CrossRef]
- Bormashenko, E.; Pogreb, R.; Bormashenko, Y.; Aharoni, H.; Shulzinger, E.; Grinev, R.; Rozenman, D.; Rozenman, Z. Progress in low voltage reversible electrowetting with lubricated polymer honeycomb substrates. RSC Adv. 2015, 5, 32491–32496. [Google Scholar] [CrossRef]
- Lapierre, F.; Brunet, P.; Coffinier, Y.; Thomy, V.; Blossey, R.; Boukherroub, R. Simulations of theoretically informed coarse grain models of polymeric systems. Faraday Discuss. 2010, 146, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.H.; Chung, L.Y.; Yao, D.J. Improving the dielectric properties of an electrowetting-on-dielectric microfluidic device with a low-pressure chemical vapor deposited Si3N4 dielectric layer. Biomicrofluidics 2015, 9, 022403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mats, L.; Bramwell, A.; Dupont, J.; Liu, G.; Oleschuk, R. Electrowetting on superhydrophobic natural (Colocasia) and synthetic surfaces based upon fluorinated silica nanoparticles. Microelectron. Eng. 2015, 148, 91–97. [Google Scholar] [CrossRef]
- Ghazzal, M.N.; Joseph, M.; Kebaili, H.; De Coninck, J.; Gaigneaux, E.M. Tuning the selectivity and sensitivity of mesoporous dielectric multilayers by modifiying the hydrophobic–hydrophilic balance of the silica layer. J. Mater. Chem. 2012, 22, 22526–22532. [Google Scholar] [CrossRef]
- Kudr, J.; Richtera, L.; Nejdl, L.; Xhaxhiu, K.; Vitek, P.; Rutkay-Nedecky, B.; Hynek, D.; Kopel, P.; Adam, V.; Kizek, R. Improved electrochemical detection of zinc ions using electrode modified with electrochemically reduced graphene oxide. Materials 2016, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xia, J.; Lei, W.; Wang, B.P. Electrowetting on ZnO nanowires. Appl. Phys. A 2010, 99, 931–934. [Google Scholar] [CrossRef]
- Papadopoulou, E.L.; Pagkozidis, A.; Barberoglou, M.; Fotakis, C.; Stratakis, E. Electrowetting properties of ZnO and TiO2 nanostructured thin films. J. Phys. Chem. C 2010, 114, 10249–10253. [Google Scholar] [CrossRef]
- Bienia, M.; Catherine Quilliet, A.; Vallade, M. Modification of drop shape controlled by electrowetting. Langmuir 2003, 19, 9328–9333. [Google Scholar] [CrossRef]
- Welters, W.J.J.; Fokkink, L.G.J. Fast electrically switchable capillary effects. Langmuir 1998, 14, 1535–1538. [Google Scholar] [CrossRef]
- Banpurkar, A.; Sawane, Y.B.; Wadhai, S.M.; Murade, C.; Siretanu, I.; van den Ende, D.; Mugele, F. Using one halogen bond to change the nature of a second bond in ternary complexes with P⋯Cl and F⋯Cl halogen bonds. Faraday Discuss. 2017, 199, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Burger, B.; Rabot, R. Design of low hysteresis electrowetting systems in non-aqueous media by the addition of low HLB amphiphilic compounds. Colloids Surfaces A 2016, 510, 129–134. [Google Scholar] [CrossRef]
- Tamaddoni, N.; Taylor, G.; Hepburn, T.; Michael, K.S.; Sarles, S.A. Reversible, voltage-activated formation of biomimetic membranes between triblock copolymer-coated aqueous droplets in good solvents. Soft Matter 2016, 12, 5096–5109. [Google Scholar] [CrossRef] [PubMed]
- Kopp, D.; Zappe, H. Tubular astigmatism-tunable fluidic lens. Opt. Lett. 2016, 41, 2735–2738. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Zhou, Y.; Kong, X.Y.; Xie, G.; Li, P.; Zhang, Z.; Wen, L.; Jiang, L. Electrostatic-charge- and Electric-field-induced smart gating for water transportation. ACS Nano 2016, 10, 9703–9709. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, N.N. SU-8 as hydrophobic and dielectric thin film in electrowetting-on-dielectric based microfluidics device. J. Nanotech. 2012, 312784. [Google Scholar] [CrossRef]
- Facchetti, A.M.; Yoon, H.; Marks, T.J. Gate Dielectrics for organic field-effect transistors: new opportunities for organic electronics. Adv. Mater. 2005, 17, 1705–1725. [Google Scholar] [CrossRef]
- Koumoulos, E.P.; Charitidis, C.A.; Papageorgiou, D.P.; Papathanasiou, AG.; Boudouvis, A.G. Nanomechanical and nanotribological properties of hydrophobic fluorocarbon dielectric coating on tetraethoxysilane for electrowetting applications. Surf. Coat. Technol. 2012, 206, 3823–3831. [Google Scholar] [CrossRef]
- Khodayari, M.; Hahne, B.; Crane, N.B.; Volinsky, A.A. Floating electrode electrowetting on hydrophobic dielectric with an SiO2 layer. Appl. Phys. Lett. 2013, 102, 192907. [Google Scholar] [CrossRef]
- Schultz, A.; Chevalliot, S.; Kuiper, S.; Heikenfeld, J. Detailed analysis of defect reduction in electrowetting dielectrics through a two-layer ‘barrier’ approach. Thin Solid Films 2013, 534, 348–355. [Google Scholar] [CrossRef]
- Jiang, C.; Ma, H.; Hasko, D.G.; Nathan, A. Influence of polarization on contact angle saturation during electrowetting. Appl. Phys. Lett. 2016, 109, 211601. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Zhang, Q.; Gu, L.; Hu, Y.; Du, J.; Lin, Y.; Nan, C.W. Ultrahigh Energy Density of Polymer Nanocomposites Containing BaTiO3@TiO2 Nanofibers by Atomic-Scale Interface Engineering. Adv. Mater. 2015, 27, 819. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.M.; Yuan, J.K.; Yao, S.H.; Liao, R.J. Flexible nanodielectric materials with high permittivity for power energy storage. Adv. Mater. 2013, 25, 6334–6365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, B.-W.; Dong, L.; Liu, H.; Chen, W.; Shen, Y.; Nan, C.-W. Superior Energy Storage Performances of Polymer Nanocomposites via Modification of Filler/Polymer Interfaces. Adv. Mater. Interf. 2018, 5, 1800096. [Google Scholar] [CrossRef]
- Beaulieu, M.R.; Baral, J.K.; Hendricks, N.R.; Tang, Y.; Briseno, A.L.; Watkins, J.J. Solution processable high dielectric constant nanocomposites based on ZrO2 nanoparticles for flexible organic transistors. ACS Appl. Mater. Interfaces 2013, 5, 13096–13103. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Shen, Y.; Guan, Y.; Zhang, X.; Lin, Y.; Zhang, Q.; Nan, C.W. Topological-Structure Modulated Polymer Nanocomposites Exhibiting Highly Enhanced Dielectric Strength and Energy Density. Adv. Funct. Mater. 2014, 24, 3172–3178. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, J.; Wang, L.; Yuan, Q.; Niu, Y.; Chen, J.; Wang, Q.; Wang, H. Compositional tailoring effect on electric field distribution for significantly enhanced breakdown strength and restrained conductive loss in sandwich-structured ceramic/polymer nanocomposites. J. Mater. Chem. A 2017, 5, 4710–4718. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Characterization of the surface modification process of Al2O3, TiO2 and ZrO2 powders by PFAS molecules. Colloids Surfaces A 2014, 447, 14–22. [Google Scholar] [CrossRef]
- Li, Y.; Krentz, T.M.; Wang, L.; Benicewicz, B.C.; Schadler, L.S. Ligand engineering of polymer nanocomposites: From the simple to the complex. ACS Appl. Mater. Interfaces 2014, 6, 6005–6021. [Google Scholar] [CrossRef] [PubMed]
- Özden-Yenigün, E.; Bilge, K.; Sünbüloğlu, E.; Bozdağ, E.; Papila, M. High strain rate response of nanofiber interlayered structural composites. Compos. Struct. 2017, 168, 47–55. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Iijima, M.; Kamiya, H. Surface modification for improving the stability of nanoparticles in liquid media. KONA Powder Part. J. 2009, 27, 119–129. [Google Scholar] [CrossRef]
- Lemal, D.M. Perspective on fluorocarbon chemistry. J. Org. Chem. 2004, 69, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.J.; Wang, P.F.; Zhang, D.W.; Wang, J.T.; Wei, W.L. A novel structural amorphous fluoropolymer film with an ultra-low dielectric constant. Mater. Lett. 2001, 49, 154–159. [Google Scholar] [CrossRef]
- Maxwell-Garnett, J.C. Colours in metal glasses and in metallic films. Philos. Trans. R. Soc. A 1904, 203, 385–420. [Google Scholar] [CrossRef]
- Rahaman, M.; Chaki, T.K.; Khastgir, D. Consideration of interface polarization in the modeling of dielectric property for ethylene vinyl acetate (EVA)/polyaniline conductive composites prepared through in-situ polymerization of aniline in EVA matrix. Eur. Polym. J. 2012, 48, 1241–1248. [Google Scholar] [CrossRef]
- Tanaka, T.; Kozako, M.; Fuse, N.; Ohki, Y. Proposal of a multi-core model for polymer nanocomposite dielectrics. IEEE Trans. Dielect. Electr. Insul. 2005, 12, 669–681. [Google Scholar] [CrossRef]
- Casalini, R.; Roland, C.M. Local and global dynamics in polypropylene glycol/silica composites. Macromolecules 2016, 49, 3919–3924. [Google Scholar] [CrossRef]
- Forlani, F.; Minnaja, N. Thickness influence in breakdown phenomena of thin dielectric films. Phys. Status Solidi 1964, 4, 311–324. [Google Scholar] [CrossRef]
- Dawber, M.; Rabe, K.M.; Scott, J.F. Critical adsorption at the free surface of a smectic liquid crystal possessing a second-order phase transition. Rev. Mod. Phys. 2005, 77, 1083–1130. [Google Scholar] [CrossRef]
- Huang, J.; Shian, S.; Diebold, R.M.; Suo, Z.; Clarke, D.R. The thickness and stretch dependence of the electrical breakdown strength of an acrylic dielectric elastomer. Appl. Phys. Lett. 2012, 101, 122905. [Google Scholar] [CrossRef] [Green Version]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Feng, Y.; Liao, J.; Ding, W.; Shui, L.; Li, H.; Wang, Y.; Tang, B.; Umar, A.; Zhou, G. Multiscale Interface Effect on Homogeneous Dielectric Structure of ZrO2/Teflon Nanocomposite for Electrowetting Application. Polymers 2018, 10, 1119. https://doi.org/10.3390/polym10101119
Hou J, Feng Y, Liao J, Ding W, Shui L, Li H, Wang Y, Tang B, Umar A, Zhou G. Multiscale Interface Effect on Homogeneous Dielectric Structure of ZrO2/Teflon Nanocomposite for Electrowetting Application. Polymers. 2018; 10(10):1119. https://doi.org/10.3390/polym10101119
Chicago/Turabian StyleHou, Jiaxin, Yancong Feng, Jinglun Liao, Wenwen Ding, Lingling Shui, Hao Li, Yao Wang, Biao Tang, Ahmad Umar, and Guofu Zhou. 2018. "Multiscale Interface Effect on Homogeneous Dielectric Structure of ZrO2/Teflon Nanocomposite for Electrowetting Application" Polymers 10, no. 10: 1119. https://doi.org/10.3390/polym10101119