Reversible Protonic Doping in Poly(3,4-Ethylenedioxythiophene)
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Film Preparation
2.3. Characterization
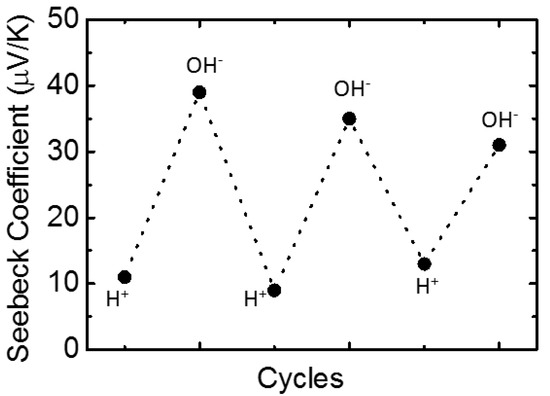
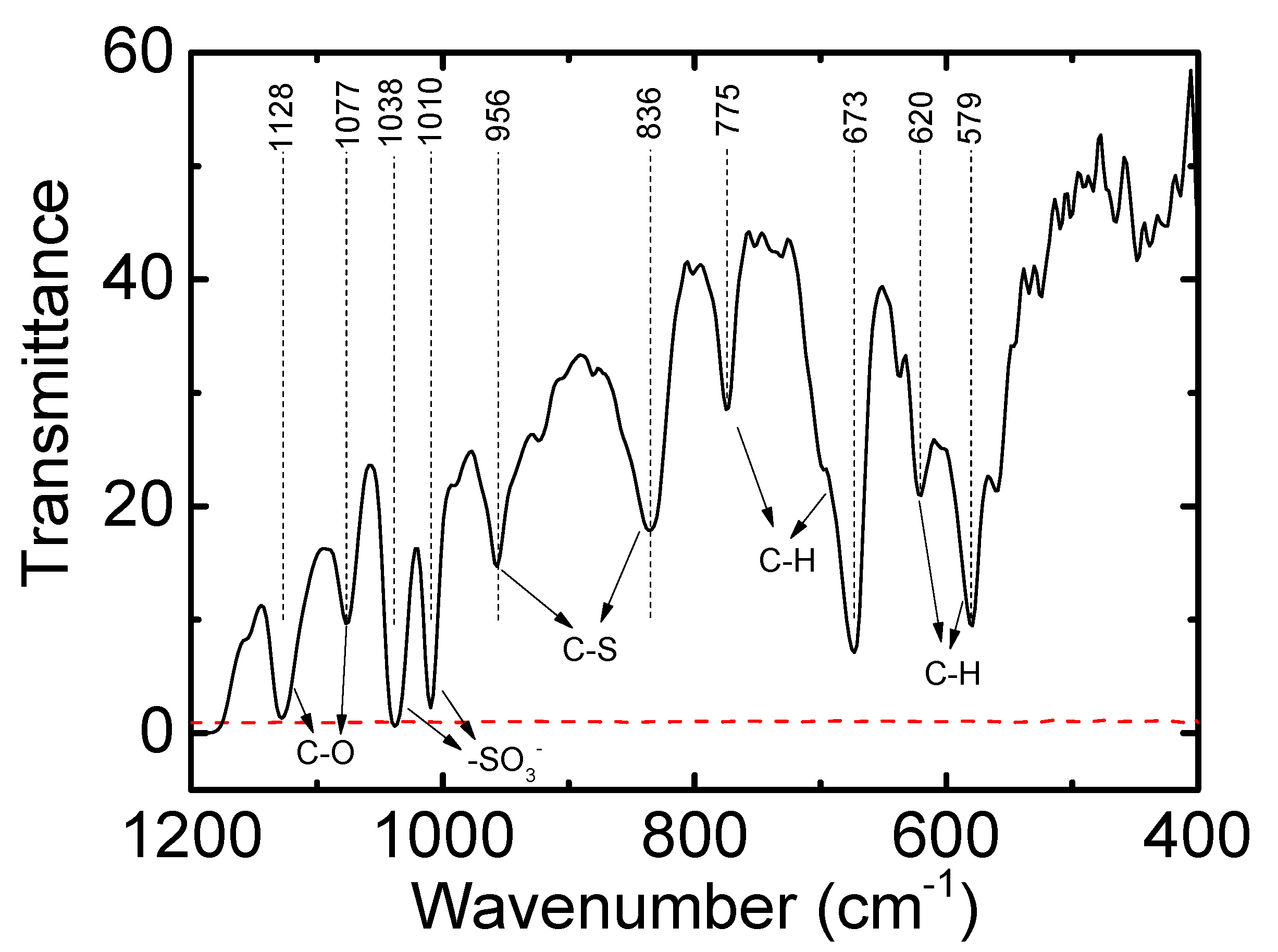
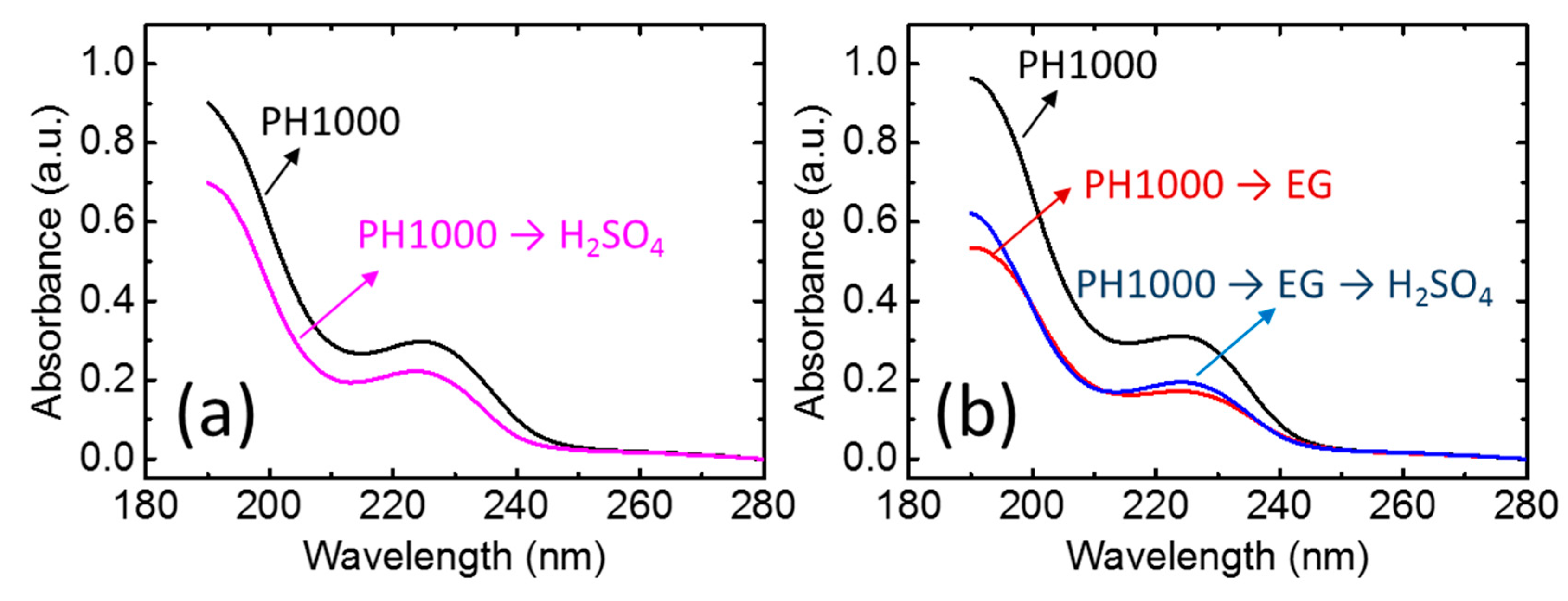
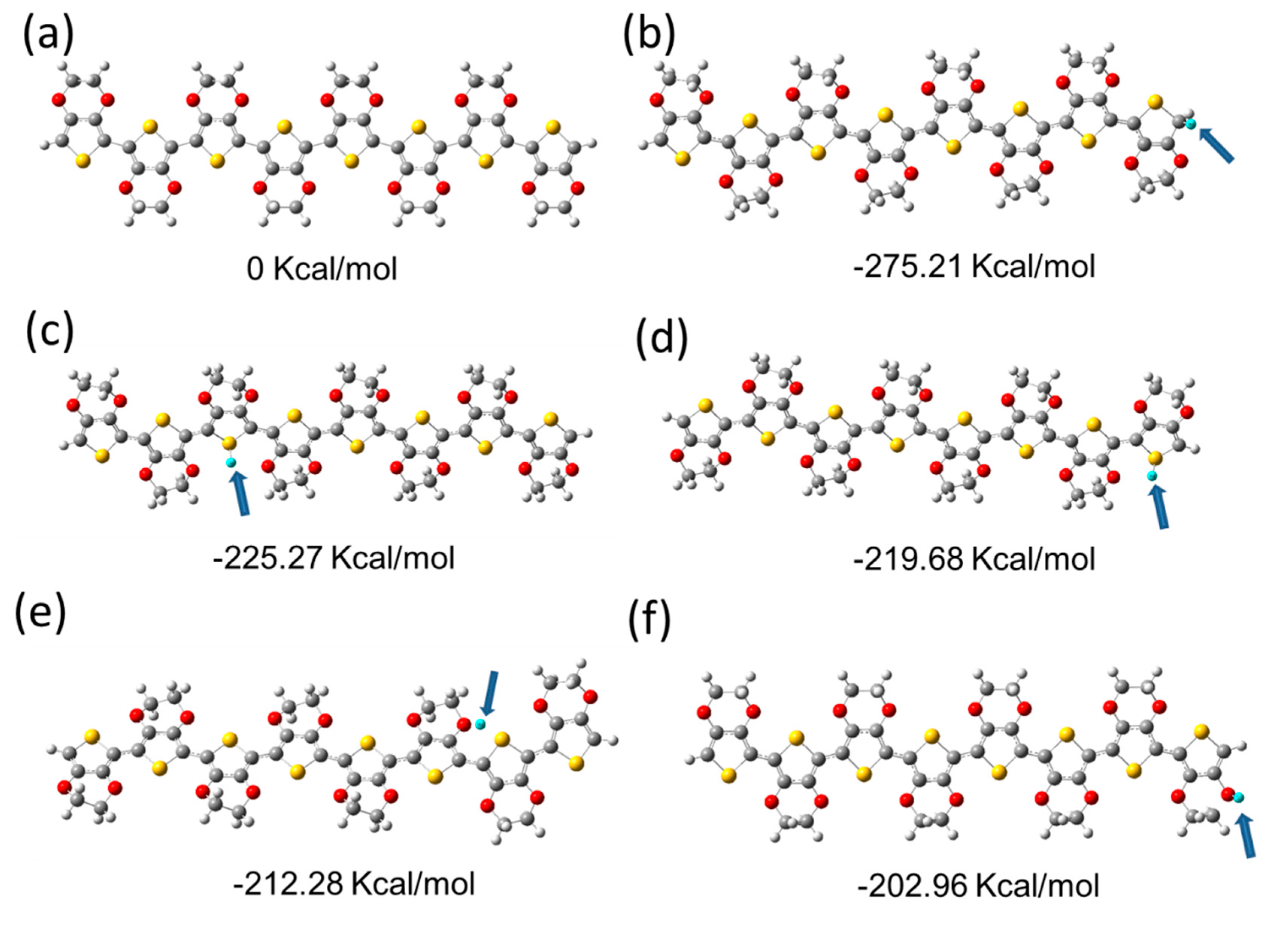
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Forrest, S.R.; Thompson, M.E. Introduction: Organic Electronics and Optoelectronics. Chem. Rev. 2007, 107, 923–925. [Google Scholar] [CrossRef]
- Elschner, A.; Lovenich, W.; Merker, U.; Reuter, K. PEDOT: Principles and Applications of an Intrinsically Con-Ductive Polymer; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Winther-Jensen, B.; Winther-Jensen, O.; Forsyth, M.; MacFarlane, D.R. High Rates of Oxygen Reduction over a Vapor Phase–Polymerized PEDOT Electrode. Science 2008, 321, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Bubnova, O.; Khan, Z.U.; Malti, A.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X. Optimization of the thermoelectric figure of merit in the conducting polymer poly(3,4-ethylenedioxythiophene). Nat. Mater. 2011, 10, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Mukaida, M.; Kirihara, K.; Naitoh, Y.; Ishida, T. Recent Progress on PEDOT-Based Thermoelectric Materials. Materials 2015, 8, 732–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Z.U.; Bubnova, O.; Jafari, M.J.; Brooke, R.; Liu, X.; Gabrielsson, R.; Ederth, T.; Evans, D.R.; Andreasen, J.W.; Fahlman, M.; et al. Acido-basic control of the thermoelectric properties of poly(3,4-ethylenedioxythiophene)tosylate (PEDOT-Tos) thin films. J. Mater. Chem. C 2015, 3, 10616–10623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, F.; Pengcheng, L.; Donghe, D.; Jianyong, O. Significantly Enhanced Thermoelectric Properties of PEDOT:PSS Films through Sequential Post-Treatments with Common Acids and Bases. Adv. Energy Mater. 2017, 7, 1602116. [Google Scholar]
- Kim, G.H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Bubnova, O.; Khan, Z.U.; Wang, H.; Braun, S.; Evans, D.R.; Fabretto, M.; Hojati-Talemi, P.; Dagnelund, D.; Arlin, J.-B.; Geerts, Y.H.; et al. Semi-metallic polymers. Nat. Mater. 2014, 13, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Park, K.S.; Baek, J.; Oh, H.S.; Koo Lee, Y.-E.; Sung, M.M. Single-Crystal Poly(3,4-ethylenedioxythiophene) Nanowires with Ultrahigh Conductivity. Nano Lett. 2014, 14, 3321–3327. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kee, S.; Lee, S.H.; Lee, B.H.; Kahng, Y.H.; Jo, Y.-R.; Kim, B.-J.; Lee, K. Highly Conductive PEDOT:PSS Nanofibrils Induced by Solution-Processed Crystallization. Adv. Mater. 2014, 26, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Mengistie, D.A.; Ibrahem, M.A.; Wang, P.-C.; Chu, C.-W. Highly Conductive PEDOT:PSS Treated with Formic Acid for ITO-Free Polymer Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Mitraka, E.; Jafari, M.J.; Vagin, M.; Liu, X.; Fahlman, M.; Ederth, T.; Berggren, M.; Jonsson, M.P.; Crispin, X. Oxygen-induced doping on reduced PEDOT. J. Mater. Chem. A 2017, 5, 4404–4412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDiarmid, A.G. “Synthetic Metals”: A Novel Role for Organic Polymers (Nobel Lecture). Angew. Chem. Int. Ed. 2001, 40, 2581–2590. [Google Scholar] [CrossRef]
- Lüssem, B.; Riede, M.; Leo, K. Doping of organic semiconductors. Phys. Status Solidi A 2013, 210, 9–43. [Google Scholar] [CrossRef]
- Chiang, J.-C.; MacDiarmid, A.G. ‘Polyaniline’: Protonic acid doping of the emeraldine form to the metallic regime. Synth. Met. 1986, 13, 193–205. [Google Scholar] [CrossRef]
- Yu, H.-H.; Xu, B.; Swager, T.M. A Proton-Doped Calix[4]arene-Based Conducting Polymer. J. Am. Chem. Soc. 2003, 125, 1142–1143. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.E.; Lee, K.S.; Garcia, A.; Tarver, J.; Gomez, E.D.; Baldwin, K.; Sun, Y.; Meng, H.; Nguyen, T.-Q.; Loo, Y.-L. Directly patternable, highly conducting polymers for broad applications in organic electronics. Proc. Natl. Acad. Sci. USA 2010, 107, 5712–5717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aasmundtveit, K.E.; Samuelsen, E.J.; Pettersson, L.A.A.; Inganäs, O.; Johansson, T.; Feidenhans’l, R. Structure of thin films of poly(3,4-ethylenedioxythiophene). Synth. Met. 1999, 101, 561–564. [Google Scholar] [CrossRef]
- Podzorov, V.; Menard, E.; Rogers, J.A.; Gershenson, M.E. Hall Effect in the Accumulation Layers on the Surface of Organic Semiconductors. Phys. Rev. Lett. 2005, 95, 226601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeya, J.; Yamagishi, M.; Tominari, Y.; Hirahara, R.; Nakazawa, Y.; Nishikawa, T.; Kawase, T.; Shimoda, T.; Ogawa, S. Very high-mobility organic single-crystal transistors with in-crystal conduction channels. Appl. Phys. Lett. 2007, 90, 102120. [Google Scholar] [CrossRef]
- Wei, Q.; Mukaida, M.; Naitoh, Y.; Ishida, T. Morphological Change and Mobility Enhancement in PEDOT:PSS by Adding Co-solvents. Adv. Mater. 2013, 25, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Mukaida, M.; Kirihara, K.; Naitoh, Y.; Ishida, T. Thermoelectric Power Enhancement of PEDOT:PSS in High Humidity Conditions. Appl. Phys. Express 2014, 7, 031601. [Google Scholar] [CrossRef]
- Wei, Q.; Mukaida, M.; Kirihara, K.; Naitoh, Y.; Ishida, T. Polymer Thermoelectric Modules Screen-Printed on Paper. RSC Adv. 2014, 4, 28802–28806. [Google Scholar] [CrossRef]
- Reyes-Reyes, M.; Cruz-Cruz, I.; López-Sandoval, R. Enhancement of the Electrical Conductivity in PEDOT:PSS Films by the Addition of Dimethyl Sulfate. J. Phys. Chem. C 2010, 114, 20220–20224. [Google Scholar] [CrossRef]
- Xia, Y.; Ouyang, J. Significant Conductivity Enhancement of Conductive Poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) Films through a Treatment with Organic Carboxylic Acids and Inorganic Acids. ACS Appl. Mater. Interfaces 2010, 2, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Sun, K.; Ouyang, J. Solution-Processed Metallic Conducting Polymer Films as Transparent Electrode of Optoelectronic Devices. Adv. Mater. 2012, 24, 2436–2440. [Google Scholar] [CrossRef] [PubMed]
- Yuta, H.; Keisuke, I.; Hiroyasu, M.; Akihiko, F.; Terukazu, N.; Satoshi, I.; Takahiko, S. Mesoscopic 2D Charge Transport in Commonplace PEDOT:PSS Films. Adv. Electron. Mater. 2018, 4, 1700490. [Google Scholar] [Green Version]
- Wei, Q.; Mukaida, M.; Kirihara, K.; Ishida, T. Experimental Studies on the Anisotropic Thermoelectric Properties of Conducting Polymer Films. ACS Macro Lett. 2014, 3, 948–952. [Google Scholar] [CrossRef]
- Saxena, N.; Keilhofer, J.; Maurya, A.K.; Fortunato, G.; Overbeck, J.; Müller-Buschbaum, P. Facile Optimization of Thermoelectric Properties in PEDOT:PSS Thin Films through Acido-Base and Redox Dedoping Using Readily Available Salts. ACS Appl. Energy Mater. 2018, 1, 336–342. [Google Scholar] [CrossRef]
- Welch, G.C.; Bazan, G.C. Lewis Acid Adducts of Narrow Band Gap Conjugated Polymers. J. Am. Chem. Soc. 2011, 133, 4632–4644. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.L.; Yu, Y. Synthesis of .beta.-Methoxy, Methyl-Capped .alpha.-Oligothiophenes. J. Org. Chem. 1995, 60, 6813–6819. [Google Scholar] [CrossRef]
- Zhang, Y.; Tajima, K.; Hirota, K.; Hashimoto, K. Synthesis of All-Conjugated Diblock Copolymers by Quasi-Living Polymerization and Observation of Their Microphase Separation. J. Am. Chem. Soc. 2008, 130, 7812–7813. [Google Scholar] [CrossRef] [PubMed]
- Yueh, K.C.; Bumsu, L.; Martin, H.; Iain, M.; Eric, G.; Vitaly, P. Doping of Conjugated Polythiophenes with Alkyl Silanes. Adv. Funct. Mater. 2009, 19, 1906–1911. [Google Scholar]
- Patel, S.N.; Glaudell, A.M.; Kiefer, D.; Chabinyc, M.L. Increasing the Thermoelectric Power Factor of a Semiconducting Polymer by Doping from the Vapor Phase. ACS Macro Lett. 2016, 5, 268–272. [Google Scholar] [CrossRef]
- Han, C.C.; Elsenbaumer, R.L. Protonic acids: Generally applicable dopants for conducting polymers. Synth. Met. 1989, 30, 123–131. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Horii, T.; Okuzaki, H. Effect of pH on Structure and Conductivity of PEDOT/PSS. Trans. Mat. Res. Soc. Jpn. 2012, 37, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Coehoorn, R.; Pasveer, W.F.; Bobbert, P.A.; Michels, M.A.J. Charge-carrier concentration dependence of the hopping mobility in organic materials with Gaussian disorder. Phys. Rev. B 2005, 72, 155206. [Google Scholar] [CrossRef]



| Sample Name | Electrical Conductivity (S/cm) |
|---|---|
| PH1000 | 2.5 ± 1.2 |
| PH1000 → H2SO4 | 2118 ± 102 |
| PH1000 → EG | 971 ± 166 |
| PH1000 → EG → H2SO4 | 1968 ± 150 |
| Sample Name | Electrical Conductivity (S/cm) |
|---|---|
| PH1000 → Li2SO4 | 3.7 ± 0.5 |
| PH1000 → EG → Li2SO4 | 950 ± 120 |
| PH1000 → Na2SO4 | 2.4 ± 0.3 |
| PH1000 → EG → Na2SO4 | 932 ± 77 |
| PH1000 → K2SO4 | 2.4 ± 0.1 |
| PH1000 → EG → K2SO4 | 998 ± 167 |
| Sample Name | Electrical Conductivity (S/cm) | Seebeck Coefficient (μV/K) |
|---|---|---|
| As prepared (PH1000–EG) | 1012 | 18 |
| 1 M H2SO4 treated | 2025 | 10 |
| 1 M KOH treated | 53 | 35 |
| 5 M KOH treated | 0.9 | 142 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Mukaida, M.; Kirihara, K.; Lyu, L.; Wei, Q. Reversible Protonic Doping in Poly(3,4-Ethylenedioxythiophene). Polymers 2018, 10, 1065. https://doi.org/10.3390/polym10101065
He S, Mukaida M, Kirihara K, Lyu L, Wei Q. Reversible Protonic Doping in Poly(3,4-Ethylenedioxythiophene). Polymers. 2018; 10(10):1065. https://doi.org/10.3390/polym10101065
Chicago/Turabian StyleHe, Shuzhong, Masakazu Mukaida, Kazuhiro Kirihara, Lingyun Lyu, and Qingshuo Wei. 2018. "Reversible Protonic Doping in Poly(3,4-Ethylenedioxythiophene)" Polymers 10, no. 10: 1065. https://doi.org/10.3390/polym10101065
APA StyleHe, S., Mukaida, M., Kirihara, K., Lyu, L., & Wei, Q. (2018). Reversible Protonic Doping in Poly(3,4-Ethylenedioxythiophene). Polymers, 10(10), 1065. https://doi.org/10.3390/polym10101065