Crystal Structure and Phase Transition of the C–H···F–H-Bonded Supramolecular Compound with 4-Nitroanilinium Based on 18-Crown-6
Abstract
:1. Introduction
2. Results and Discussion
2.1. Spectral Properties
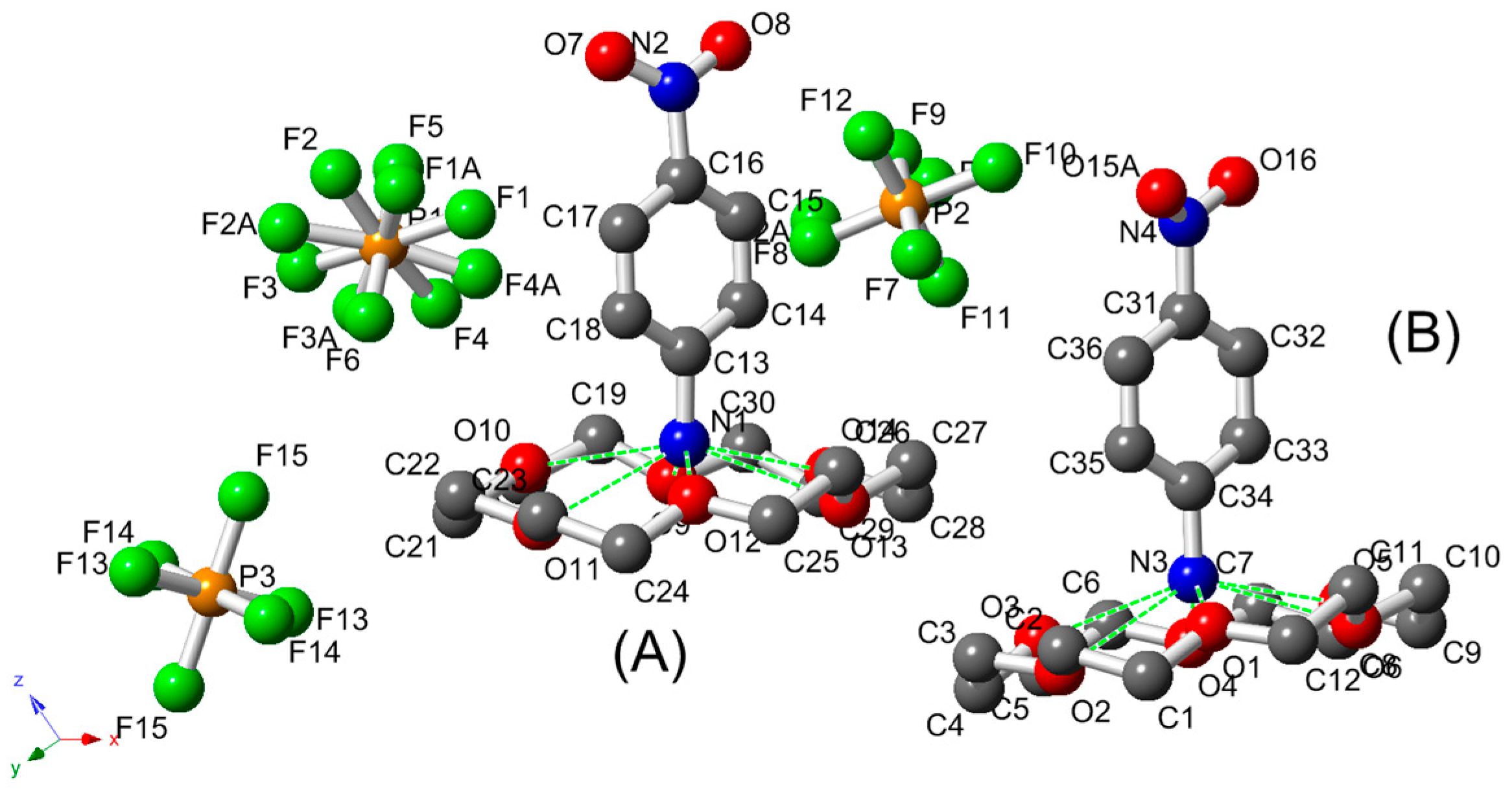
2.2. DSC and TG Analysis
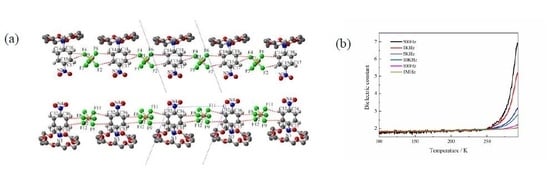
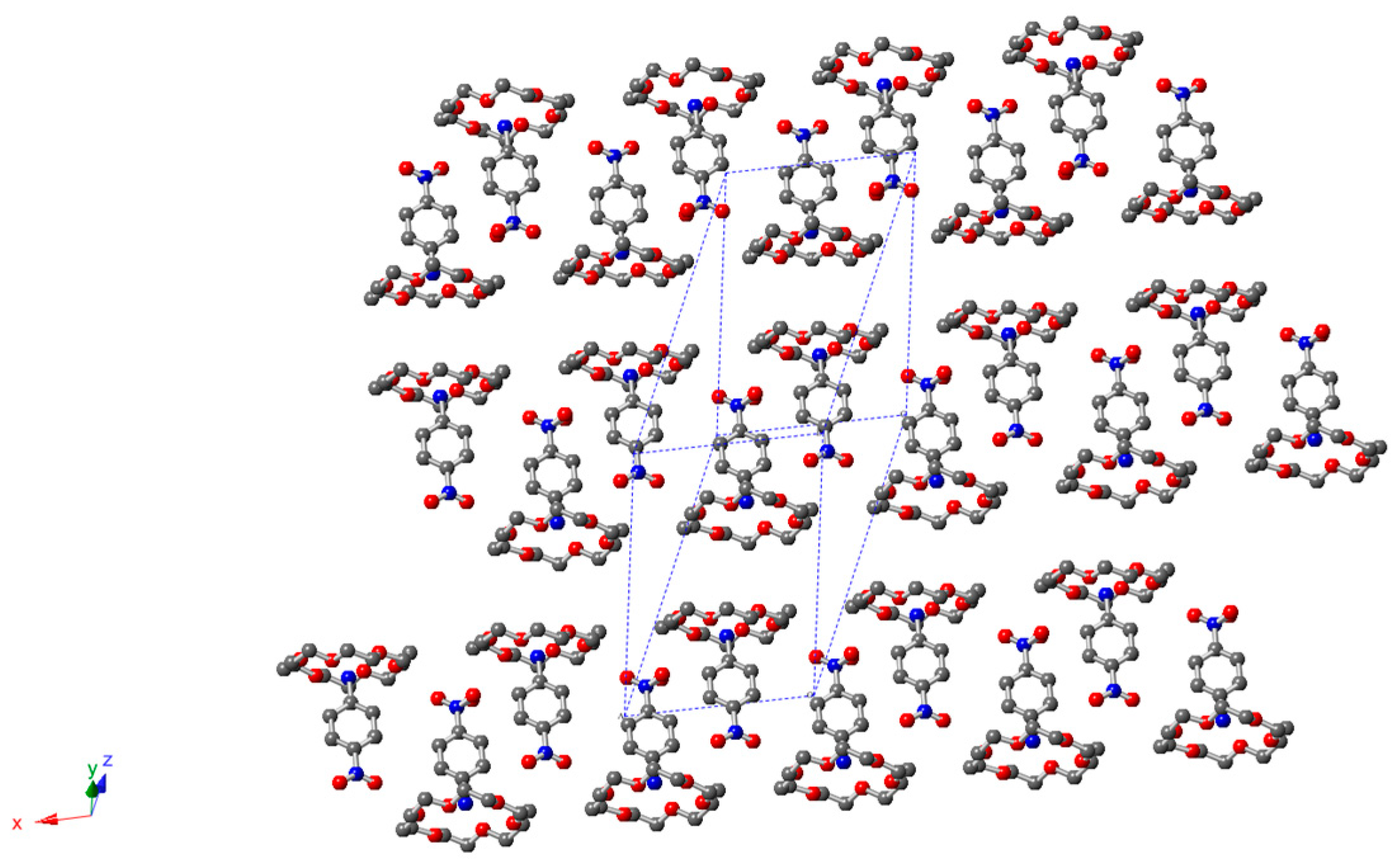
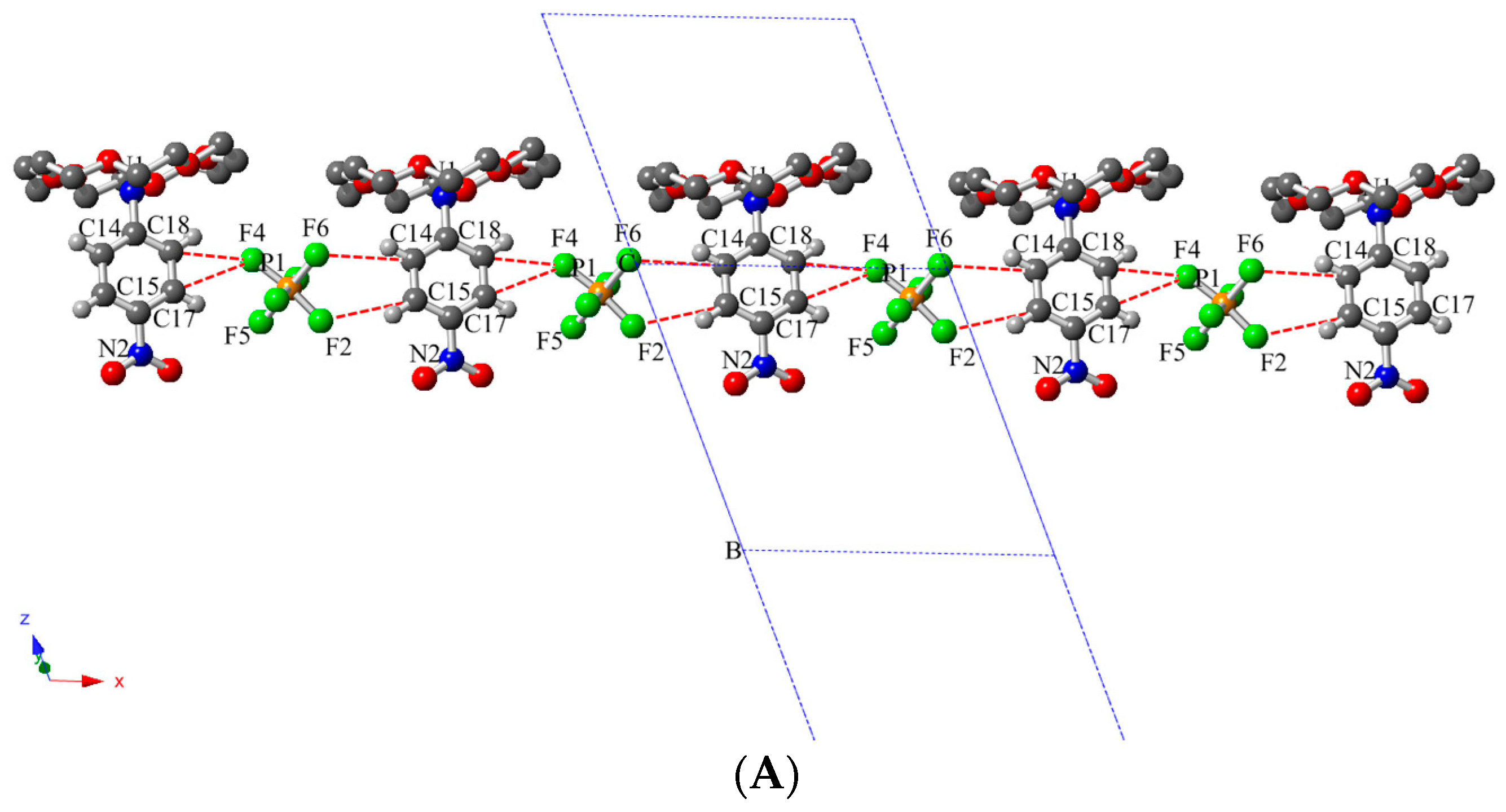
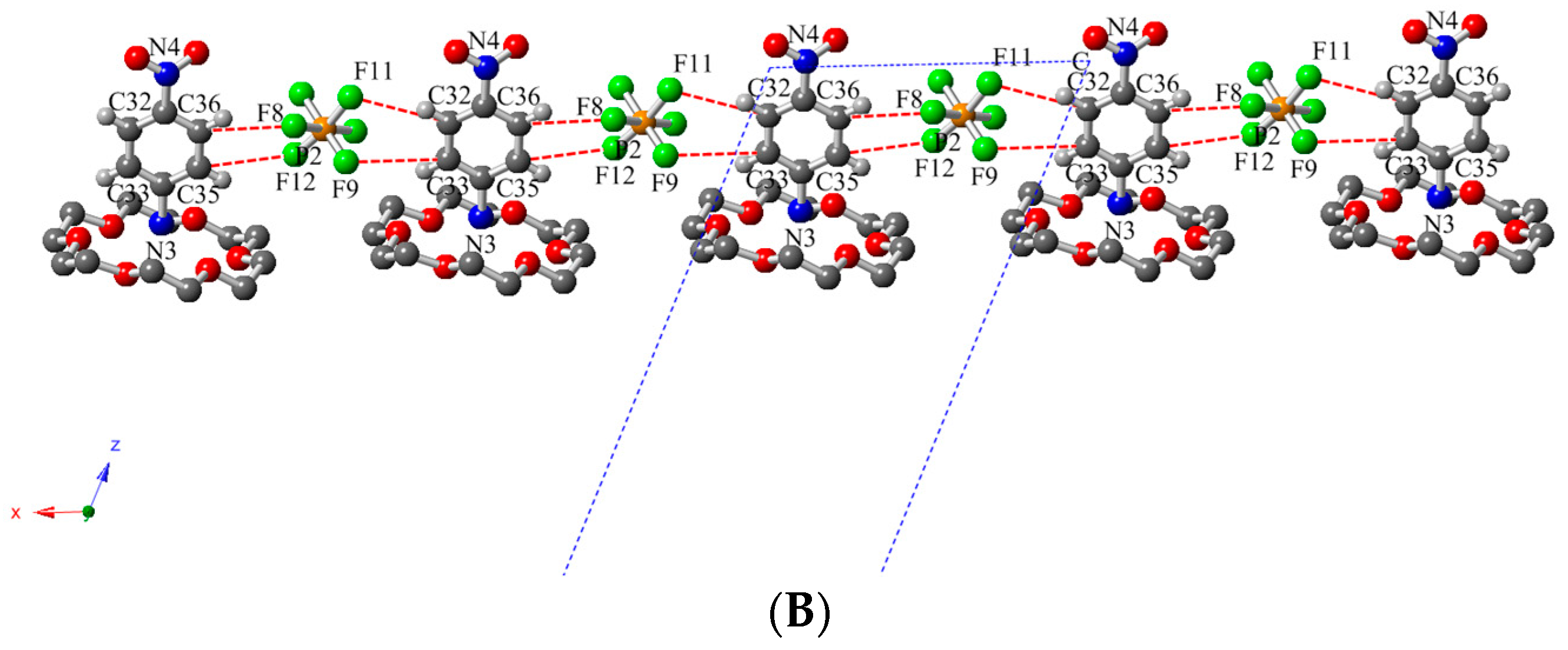
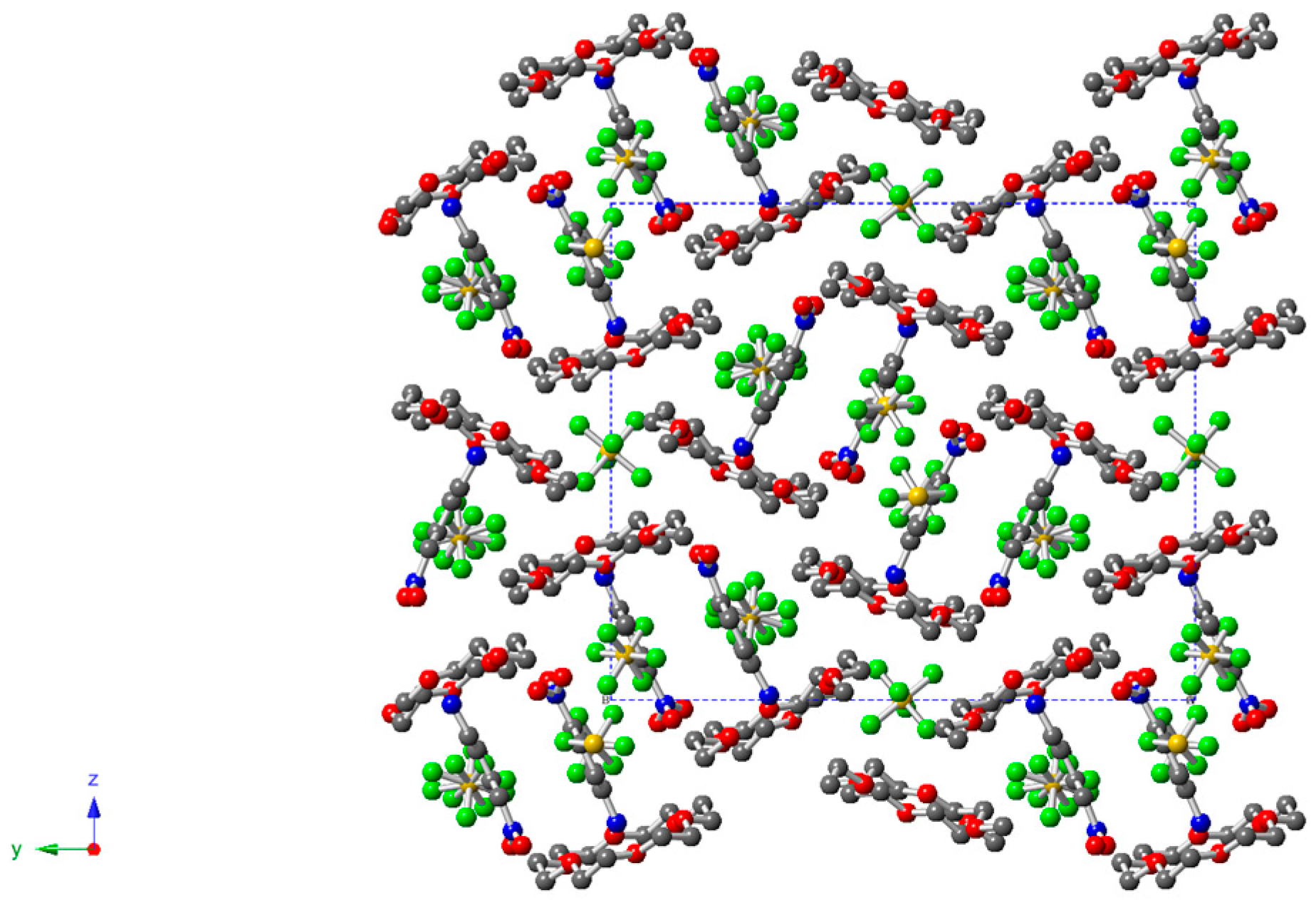
2.3. Description of Crystal Structure
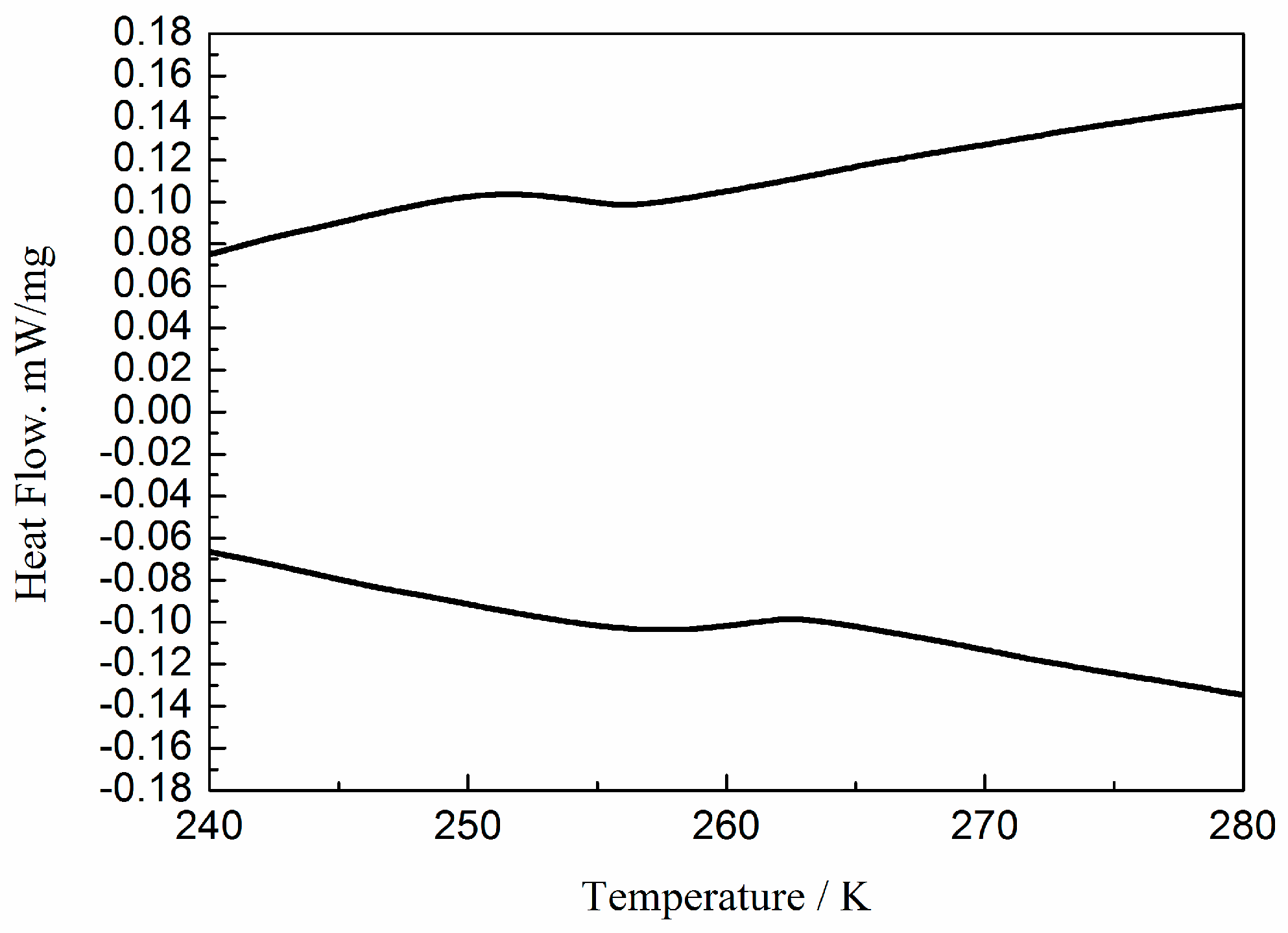
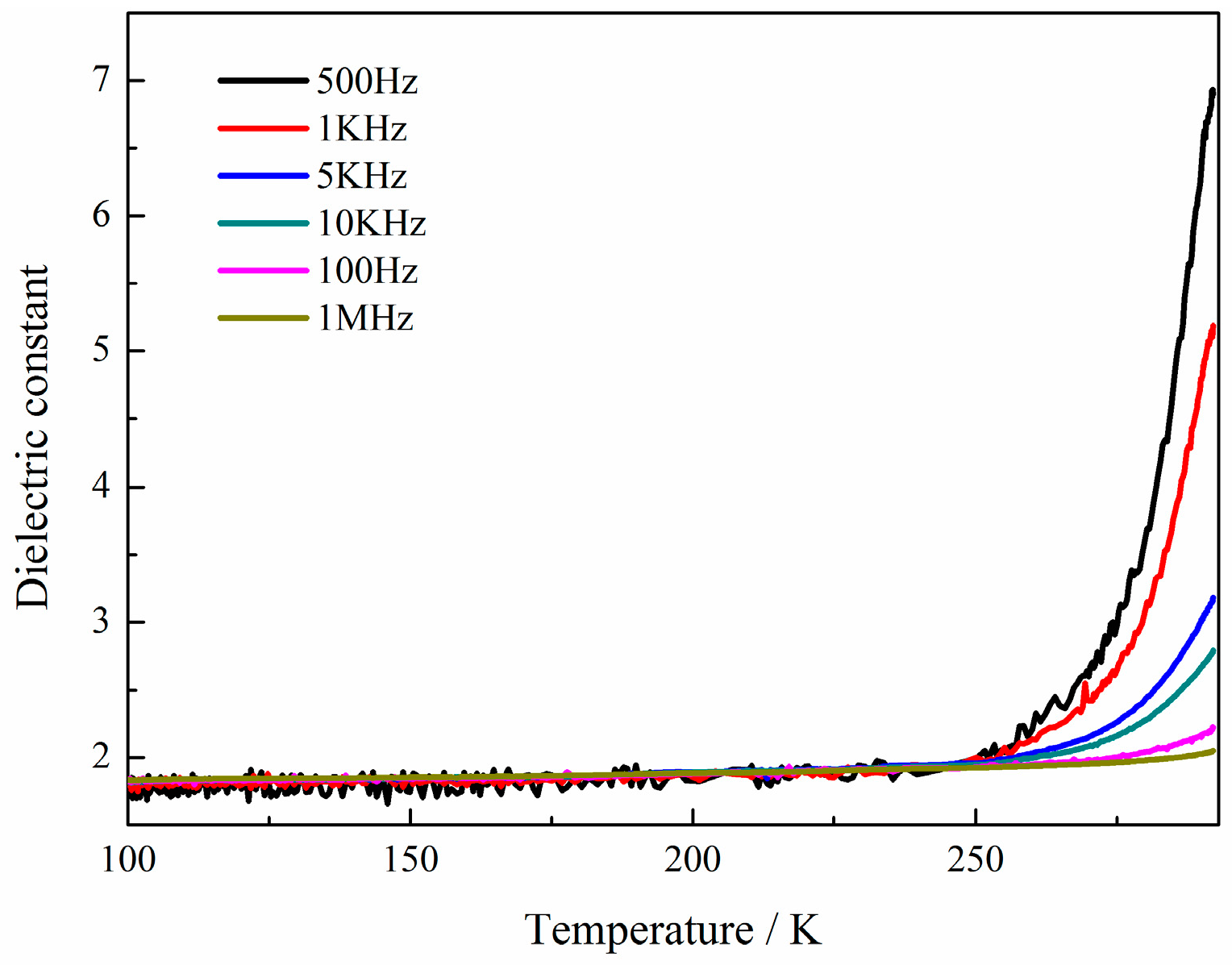
2.4. Dielectric Properties
3. Experimental
3.1. Material and Instruments
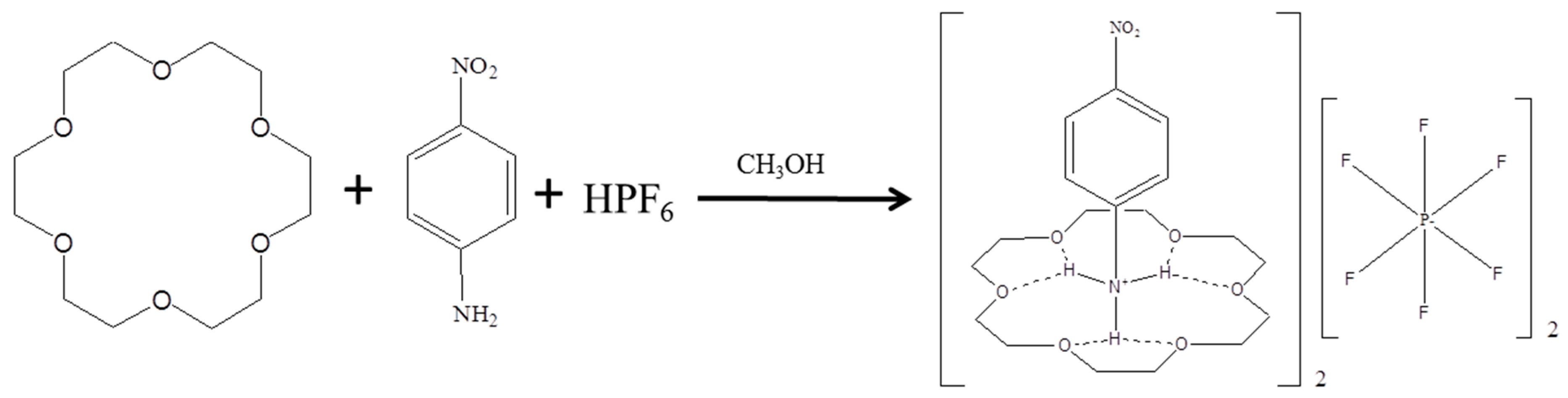
3.2. Preparation of (4-Nitroanilinium)(18-Crown-6)(PF6) (1)
3.3. Crystal Structure Determination
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Horiuchi, S.; Ishii, F.; Kumai, R.; Okimoto, Y.; Tachibana, H.; Nagaosa, N.; Tokura, Y. Ferroelectricity near room temperature in co-crystals of nonpolar organic molecules. Nat. Mater. 2005, 4, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Akutagawa, T.; Koshinaka, H.; Sato, D.; Takeda, S.; Noro, S.I.; Takahashi, H.; Kumai, R.; Tokura, Y.; Nakamura, T. Ferroelectricity and polarity control in solid-state flip-flop supramolecular rotators. Nat. Mater. 2009, 8, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.R.; Zhao, H.; Wang, X.S.; Qu, Z.R.; Xiong, R.G.; Xue, X.; Xue, Z.L.; You, X.Z. 2D Chiral Uranyl(v1) coordination polymers with second-Harmonic Generation Response and Ferroelectric Properties. Eur. J. Inorg. Chem. 2003, 2003, 3712–3715. [Google Scholar] [CrossRef]
- Ye, H.Y.; Ge, J.Z.; Tang, Y.Y.; Li, P.F.; Zhang, Y.; You, Y.M.; Xiong, R.G. Molecular Ferroelectric with Most Equivalent Polarization Directions Induced by the Plastic Phase Transition. J. Am. Chem. Soc. 2016, 138, 13175–13178. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Chen, T.; Luo, J.H.; Hong, M.C. Bis(imidazolium) L-Tartrate: A Hydrogen-Bonded Displacive-Type Molecular Ferroelectric Material. Angew. Chem. Int. Ed. 2012, 51, 3871–3876. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Kubo, K.; Noro, S.I.; Akutagawa, T.; Nakamura, T. Design of Crystalline Speces for Molecular Rotations in Crystals. Cryst. Growth Des. 2014, 14, 537–543. [Google Scholar] [CrossRef]
- Hoshino, N.; Yoshii, Y.; Aonuma, M.; Kubo, K.; Nakamura, T.; Akutagawa, T. Supramolecular Rotators of (Aniliniums)(18-crown-6) in Electrically Conductin [Ni(dmit)2] Crystals. Inorg. Chem. 2012, 51, 12968–12975. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Z.; Gu, Z.F.; Xiong, J.B.; Gao, J.X.; Liu, Y.; Wang, B.; Tan, Y.H. Unusual sequential reversible phase transitions containing switchable dielectric behaviors in cyclopentyl ammonium 18-crown-6 perchlorate. Chem. Mater. 2016, 28, 4476–4482. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Liu, Y.; Wang, J.F.; Yang, G.F. A novel proton transfer supramolecular compound induced by N–H-O hydrogen bond through 18-crown-6: Syntheses structure, and dielectric properties. Inorg. Chem. Commun. 2015, 61, 109–112. [Google Scholar] [CrossRef]
- Fu, D.W.; Dai, J.; Ge, J.Z.; Ye, H.Y.; Zhang, Y. Synthesis, structure and dielectric properties of the 2D K-tetrazole complex [K2(4-TPA)2(H2O)2]n. Inorg. Chim. Acta 2010, 363, 2584–2589. [Google Scholar] [CrossRef]
- Modec, B. The Solid state structure of pyridinium hydrogen squarate. J. Mol. Struct. 2015, 1099, 54–57. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, L.Z.; Gou, M.; Li, Y.H.; Fu, D.W.; Xiong, R.G. Ferroelectric Homochiral Organic Molecular Crystals. Cryst. Growth Des. 2010, 10, 1025–1027. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, X.; Cai, Y.; Yao, Y.F.; Zhang, W. A chemically triggered and thermally switched dielectric constant transition in a metal cyanide based crystal. Angew. Chem. Int. Ed. 2015, 54, 6206–6210. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Z.; Yu, Y.M.; Xiong, J.B.; Tan, Y.H.; Wen, H.R. Unusual high-temperature reversible phase transition behavior structure and dielectric ferroelectric properties of two new crown ether clathrates. J. Am. Chem. Soc. 2015, 137, 13345–13351. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shi, P.-P.; Ye, Q.; Wang, H.-T.; Wu, D.H.; Ye, H.Y.; Fu, D.W.; Zhang, Y. A switchable molecular dielectric with two sequential reversible phase transition: [(CH3)4P]4[Mn(SCN)6]. Inorg. Chem. 2015, 54, 10642–10647. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, S.; Ren, X.M.; Akutagawa, T.; Nakamura, T. Crystal structure and magnetic properties of [Ni(dmit)2] salts including (4-fluoroanilinium)([18]crown-6) and (4-methylanilinium)([18]crown-6) supramolecular cations. Polyhedron 2005, 24, 2844–2848. [Google Scholar] [CrossRef]
- Ohshima, Y.; Kubo, K.; Matsumoto, T.; Ye, H.Y.; Noro, S.I.; Akutagawa, T.; Nakamura, T. One-dimensional supramolecular columnar structure of trans-syn-trans-dicyclohexano[18]crown-6 and organic ammonium cations. CrystEngComm 2016, 18, 7959–7964. [Google Scholar] [CrossRef]
- Ge, J.Z.; Fu, X.Q.; Hang, T.; Ye, Q.; Xiong, R.G. Reversible phase transiton of the 1:1: Complexes of 18-crown-6 with 4-ethoxyanilinium perchlorate. Cryst. Growth Des. 2010, 10, 3632–3637. [Google Scholar] [CrossRef]
- Morimoto, M.; Irie, M. Photochemical control of dielectric peroperties based on intermolecular proton transfer in a hydrogen bonded diarylethene crystal. Chem. Commun. 2011, 47, 4186–4188. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Shi, P.-P.; Chen, Z.Q.; Akutagawa, T.; Noro, S.-I.; akamura, T. Flexible cis-cyclohexane-1,4-diammonium ion in magnetic [Ni(dmit)2] crystals. Eur. J. Inorg. Chem. 2012, 2012, 3732–3739. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Li, S.H.; Ye, Q.; Cai, H.L.; Deng, F.; Xiong, R.G.; Huang, S.D. Ferroelectricity induced by ordering of twisting motion in a molecular rotor. J. Am. Chem. Soc. 2012, 134, 11044–11049. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhu, Y.; Song, Y.L.; Hou, H.W.; Fan, Y.T. A novel inorganic organic tetragonal prism supramolecular compound [Fe(NCS)][(Hbpy)(Hbpy)(bpy)]: Crystal structure and non-linear optical properties. Inorg. Chem. Commun. 2002, 5, 166–170. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, C.-H.; Wang, Y.-F.; Li, S.-C.; Zhang, W. synthesis and structural phase transitions of copper(II) and iron(III) complexes containing [(C8H12NO)(18-crown-6)]+ supramolecular cations. Z. Anorg. Allg. Chem. 2014, 640, 1499–1505. [Google Scholar] [CrossRef]
- Ye, H.Y.; Cai, H.L.; Ge, J.Z.; Xiong, R.-G. Reversible structural phase transiton of pyridinium 4-carboxylic acid perchlorate. J. Appl. Cryst. 2010, 43, 1031–1035. [Google Scholar] [CrossRef]
- Han, X.B.; Hu, P.; Shi, C.; Zhang, W. Structure phase transitions and dielectric transitions in a1,4-diazabicyclo[2,2,2]octane (dabco) based organic crystal. J. Mol. Struct. 2017, 1127, 372–376. [Google Scholar] [CrossRef]
- Junk, P.C.; Raston, C.L. Hydrolytic stability of SnCl4 and GaCl3 in the formation of [cis-SnCl4(H2O)2] 18-crown-6 2H2O and [2,2,2]cryptand +2H+][GaCl4]. Inorg. Chim. Acta 2004, 357, 595–599. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Liu, Y.; Chen, Y.; Zhao, W.-Q.; Fang, W.-N. Synthesis, characterization, and phase transition of an inorganic-organic hybrid compound, [(3-nitroanilinium)(18-crown-6)] [IO4-](CH3OH). Chin. Chem. Lett. 2017, 28, 297–301. [Google Scholar] [CrossRef]
| Temperature | 100 K | 296 K |
| Chemical formula | C36H62F12N4O16P2 | C36H62F12N4O16P2 |
| Formula weight | 1096.8230 | 1096.8230 |
| Crystal size (mm3) | 0.21 × 0.2 × 0.19 | 0.21 × 0.20 × 0.19 |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/c | P21/c |
| a (Å) | 10.9077(9) | 11.067(3) |
| b (Å) | 23.296(2) | 23.778(6) |
| c (Å) | 21.5573(15) | 21.933(5) |
| α (°) | 90.00 | 90.00 |
| β (°) | 113.277(3) | 113.678(10) |
| γ (°) | 90.00 | 90.00 |
| V (Å3) | 5032.0(7) | 5286(2) |
| Z | 2 | 2 |
| Dcalc (g·cm−1) | 1.448 | 1.378 |
| F(000) | 2288.0 | 2288.0 |
| μ (mm−1) | 0.198 | 0.188 |
| Measured 2θ range (°) | 0.997/25.010 | 0.998/25.010 |
| Rint | 0.0954 | 0.0797 |
| R (I > 2σ(I)) [a] | 0.1205 | 0.1606 |
| WR (all data) [b] | 0.2093 | 0.1899 |
| GOF | 1.037 | 1.077 |
| D-H···A | d(D–H) | d(H···A) | d(D···A) | ∠DHA |
|---|---|---|---|---|
| 100 K | ||||
| N1–H1WC···O9 | 0.8268(5) | 2.4418(8) | 2.9409(10) | 119.759(4) |
| N1–H1WC···O10 | 0.8268(5) | 2.0648(7) | 2.8629(8) | 161.909(4) |
| N1–H1WB···O11 | 0.8239(6) | 2.5817(7) | 2.9768(9) | 110.866(5) |
| N1–H1WB···O12 | 0.8239(6) | 2.0148(8) | 2.7849(10) | 115.569(6) |
| N1–H1WA···O13 | 0.8209(6) | 2.6928(7) | 2.9289(9) | 98.478(6) |
| N1–H1WA···O14 | 0.8209(6) | 2.0289(8) | 2.8308(10) | 165.429(7) |
| N3–H3WA···O1 | 0.8228(5) | 2.0348(9) | 2.8518(2) | 172.059(7) |
| N3–H3WA···O2 | 0.8228(5) | 2.5728(10) | 2.9829(9) | 112.168(9) |
| N3–H3WB···O3 | 0.8228(9) | 2.0829(1) | 2.9049(2) | 175.079(9) |
| N3–H3WB···O4 | 0.8228(9) | 2.6228(7) | 2.9537(7) | 105.709(4) |
| N3–H3WC···O5 | 0.8218(4) | 2.0838(10) | 2.8979(3) | 170.569(7) |
| N3–H3WC···O6 | 0.8218(4) | 2.6148(5) | 2.9107(8) | 102.879(8) |
| 296 K | ||||
| N1–H1WA···O9 | 0.8895(6) | 1.9858(8) | 2.8549(3) | 165.028(9) |
| N1–H1WB···O10 | 0.8889(6) | 2.4589(3) | 2.9349(10) | 113.959(9) |
| N1–H1WB···O11 | 0.8889(6) | 1.9448(6) | 2.8127(9) | 165.195(8) |
| N1–H1WC···O12 | 0.8886(8) | 2.4569(6) | 2.9736(9) | 117.547(4) |
| N1–H1WC···O13 | 0.8886(8) | 2.0076(4) | 2.8726(8) | 164.038(6) |
| N1–H1WA···O14 | 0.8895(6) | 2.4437(5) | 2.9347(7) | 115.187(5) |
| N3–H3WA···O1 | 0.8886(7) | 2.0443(10) | 2.9106(8) | 164.398(6) |
| N3–H3WC···O2 | 0.8886(7) | 2.4427(9) | 2.9219(4) | 114.148(7) |
| N3–H3WC···O3 | 0.8886(7) | 1.9948(5) | 2.8676(7) | 166.616(6) |
| N3–H3WB···O4 | 0.8887(5) | 2.4958(4) | 2.9907(10) | 115.618(6) |
| N3–H3WB···O5 | 0.8887(5) | 2.0518(6) | 2.9307(8) | 169.146(8) |
| N3–H3WA···O6 | 0.8886(7) | 2.4429(4) | 2.9658(5) | 117.919(7) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.-l.; Liu, Y.; Wang, K.; Chen, Y.; Liu, Z.-q. Crystal Structure and Phase Transition of the C–H···F–H-Bonded Supramolecular Compound with 4-Nitroanilinium Based on 18-Crown-6. Crystals 2017, 7, 276. https://doi.org/10.3390/cryst7090276
Zhu C-l, Liu Y, Wang K, Chen Y, Liu Z-q. Crystal Structure and Phase Transition of the C–H···F–H-Bonded Supramolecular Compound with 4-Nitroanilinium Based on 18-Crown-6. Crystals. 2017; 7(9):276. https://doi.org/10.3390/cryst7090276
Chicago/Turabian StyleZhu, Chun-li, Yang Liu, Kun Wang, Yuan Chen, and Zun-qi Liu. 2017. "Crystal Structure and Phase Transition of the C–H···F–H-Bonded Supramolecular Compound with 4-Nitroanilinium Based on 18-Crown-6" Crystals 7, no. 9: 276. https://doi.org/10.3390/cryst7090276