Crystallisation and Preliminary Crystallographic Analysis of Helicobacter pylori Periplasmic Binding Protein YckK
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gene Cloning and Overexpression
2.2. Purification
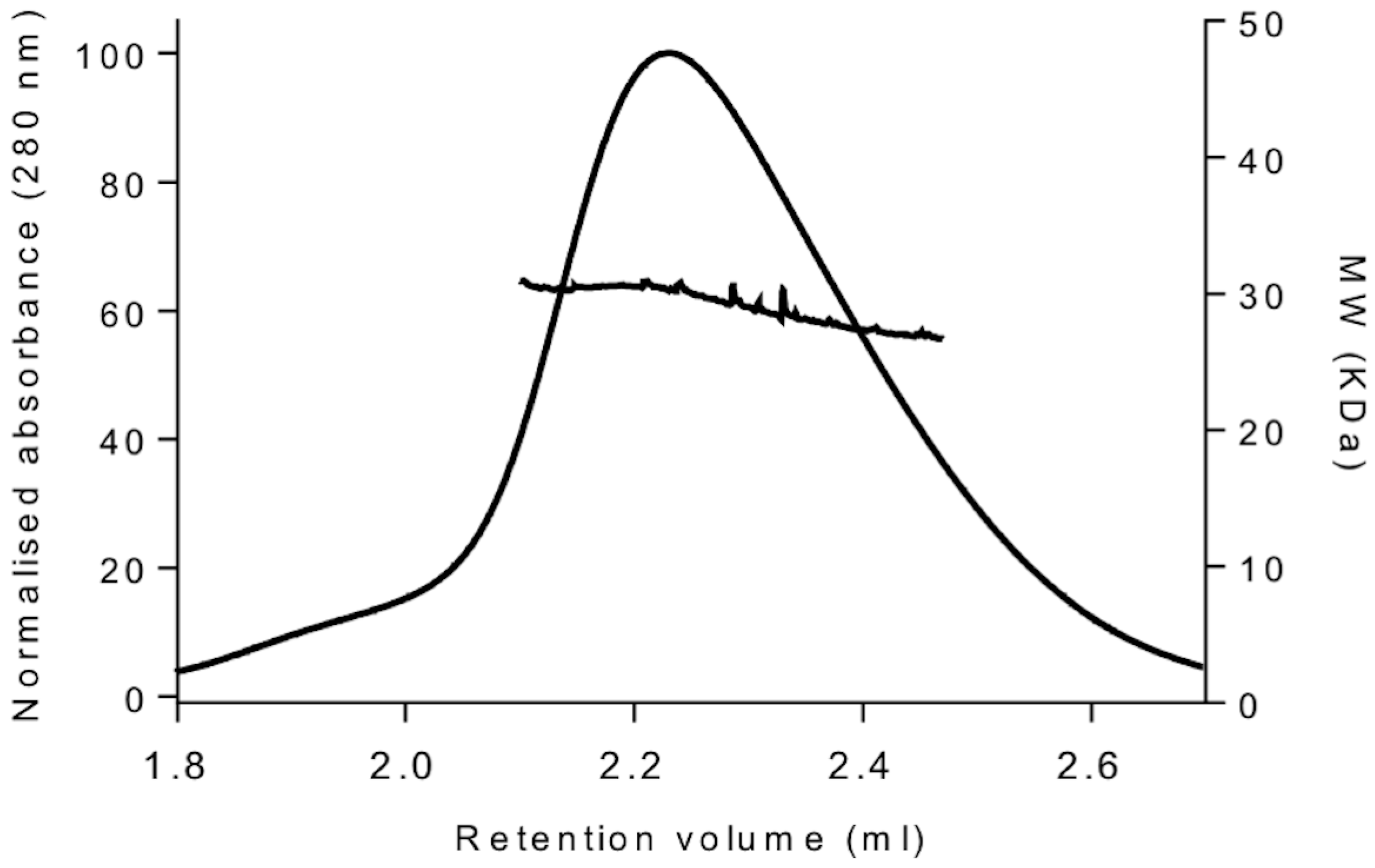
2.3. Size-Exclusion Chromatography Coupled to Multi-Angle Light Scattering (SEC-MALS) Analysis
2.4. Crystallisation, Preliminary X-ray Diffraction Data Collection, and Molecular Replacement
3. Results
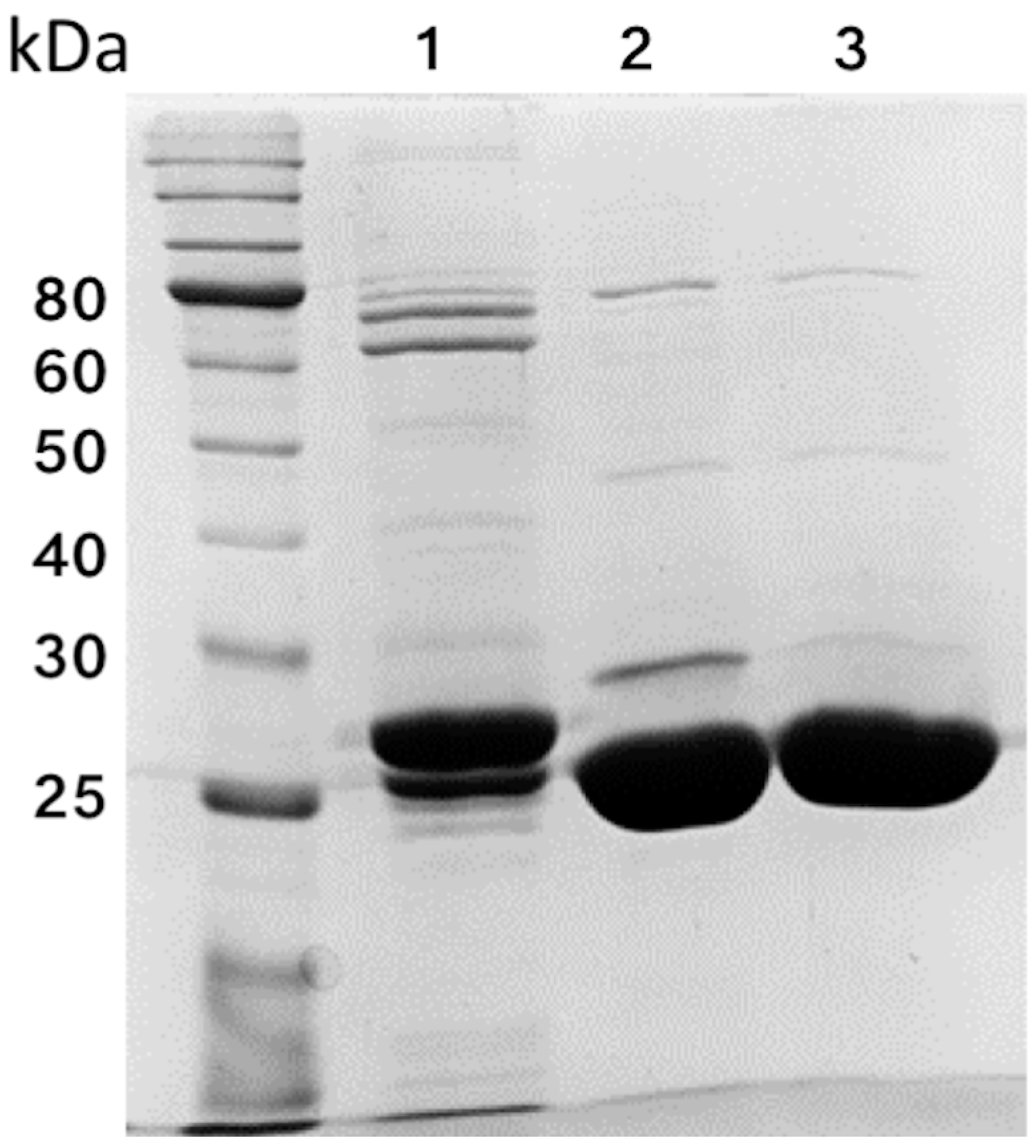
3.1. Overexpression and Purification
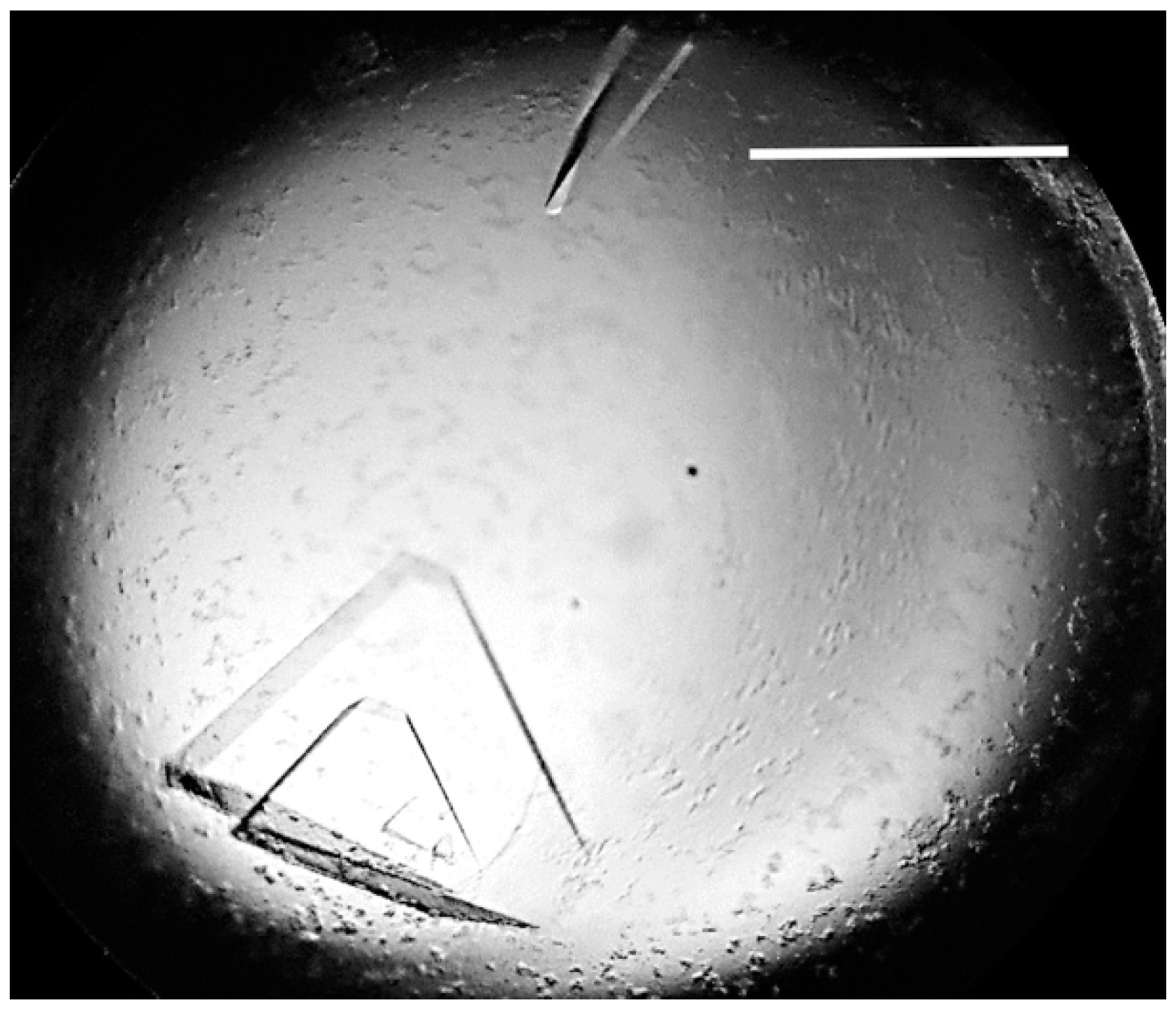
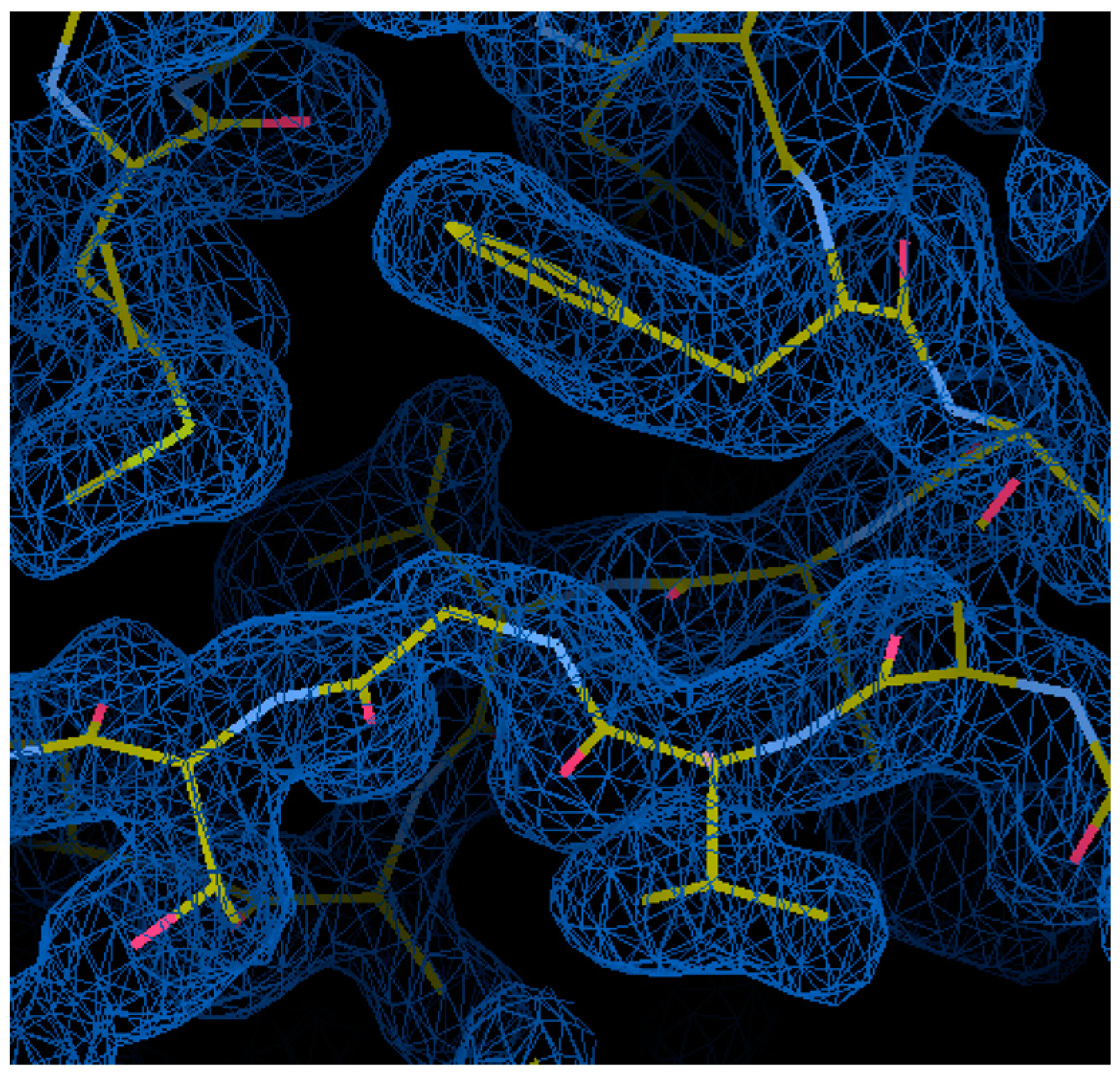
3.2. Crystallisation and Preliminary Crystallographic Analysis
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amieva, M.R.; El-Omar, E.M. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology 2008, 134, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Schutte, K.; Malfertheiner, P. Helicobacter pylori and other gastric microbiota in gastroduodenal pathologies. Dig Dis. 2016, 34, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, K.S. Optimal first-line treatment for Helicobacter pylori infection: Recent strategies. Gastroenterol. Res. Pract. 2016, 9086581. [Google Scholar]
- Quiocho, F.A.; Ledvina, P.S. Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis: Variation of common themes. Mol. Microbiol. 1996, 20, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Pear, M.R.; McCammon, J.A.; Quiocho, F.A. Hinge-bending in L-arabinose-binding protein. The “Venus’s-flytrap” model. J. Biol. Chem. 1982, 257, 1131–1133. [Google Scholar] [PubMed]
- Fischer, M.; Zhang, Q.; Hubbard, R.; Thomas, G. Caught in a TRAP: Substrate-binding proteins in secondary transport. Trends Microbiol. 2010, 18, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Draper, J.L.; Hansen, L.M.; Bernick, D.L.; Abedrabbo, S.; Underwood, J.G.; Kong, N.; Huang, B.C.; Weis, A.M.; Weimer, B.C.; van Vliet, A.H.M.; et al. Fallacy of the unique genome: Sequence diversity within single Helicobacter pylori strains. mBio 2017, 8, e02321-16. [Google Scholar] [CrossRef] [PubMed]
- Bulut, H.; Moniot, S.; Licht, A.; Scheffel, F.; Gathmann, S.; Saenger, W.; Schneider, E. Crystal structures of two solute receptors for L-cystine and L-cysteine, respectively, of the human pathogen Neisseria gonorrhoeae. J. Mol. Biol. 2012, 415, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Clifton, B.E.; Jackson, C.J. Ancestral protein reconstruction yields insights into adaptive evolution of binding specificity in solute-binding proteins. Cell Chem. Biol. 2016, 23, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Dattelbaum, J.; Staiano, M.; Berisio, R.; Vitagliano, L. A loose domain swapping organization confers a remarkable stability to the dimeric structure of the arginine binding protein from Thermotoga maritima. PLoS ONE 2014, 9, e96560. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, Y.; Hasebe, F.; Tomita, T.; Kuzuyama, T.; Nishiyama, M. Two ATP-binding cassette transporters involved in (S)-2-aminoethyl-cysteine uptake in Thermus thermophilus. J. Bacteriol. 2013, 195, 3845–3853. [Google Scholar] [CrossRef] [PubMed]
- Woon, A.P.; Tohidpour, A.; Alonso, H.; Saijo-Hamano, Y.; Kwok, T.; Roujeinikova, A. Conformational analysis of isolated domains of Helicobacter pylori CagA. PLoS ONE 2013, 8, e79367. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Battye, T.G.; Kontogiannis, L.; Johnson, O.; Powell, H.R.; Leslie, A.G. iMOSFLM: A new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.R.; Murshudov, G.N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.; McCoy, A. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 235–242. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Storoni, L.C.; Read, R.J. Likelihood-enhanced fast translation functions. Acta Crystallogr. D Biol. Crystallogr. 2005, 61, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.-W.; Kapral, G.J.; Grosse-Kunstleve, R.W. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.W. Solvent content in protein crystals. J. Mol. Biol. 1968, 33, 491–497. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Abate, F.; Malito, E.; Cozzi, R.; Lo Surdo, P.; Maione, D.; Bottomley, M. Apo, Zn2+-bound and Mn2+-bound structures reveal ligand-binding properties of SitA from the pathogen Staphylococcus pseudintermedius. Biosci. Rep. 2014, 34, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Giuroiu, I.; Chernishof, I.; Sawaya, M.R.; Chiang, J.; Gunsalus, R.P.; Arbing, M.A.; Perry, L.J. Apo and ligand-bound structures of ModA from the archaeon Methanosarcina acetivorans. Acta Crystallogr. F 2010, 66, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-J.; Kim, H.; Mikami, B.; Yu, Y.; Lee, H. Crystal structure of a putative oligopeptide-binding periplasmic protein from a hyperthermophile. Extremophiles 2016, 20, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Deka, R.; Neil, L.; Hagman, K.; Machius, M.; Tomchick, D.; Brautigam, C.; Norgard, M.V. Structural evidence that the 32-kilodalton lipoprotein (Tp32) of Treponema pallidum is an l-methionine-binding protein. J. Biol. Chem. 2004, 279, 55644–55650. [Google Scholar] [CrossRef] [PubMed]
- Culurgioni, S.; Tang, M.; Walsh, M.A. Structural characterization of the Streptococcus pneumoniae carbohydrate substrate-binding protein SP0092. Acta Crystallogr. F 2017, 73, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Schilling, C.H.; Covert, M.W.; Famili, I.; Church, G.M.; Edwards, J.S.; Palsson, B.O. Genome-scale metabolic model of Helicobacter pylori 26695. J. Bacteriol. 2002, 184, 4582–4593. [Google Scholar] [CrossRef]
- Stark, R.; Suleiman, M.S.; Hassan, I.; Greenman, J.; Millar, M. Amino acid utilisation and deamination of glutamine and asparagine by Helicobacter pylori. J. Med. Microbiol. 1997, 46, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Penn, C. Characteristics of Helicobacter pylori growth in a defined medium and determination of its amino acid requirements. Microbiology 1994, 140, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Worku, M.L.; Karim, Q.N.; Spencer, J.; Sidebotham, R.L. Chemotactic response of Helicobacter pylori to human plasma and bile. J. Med. Microbiol. 2004, 53, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Cerda, O.; Rivas, A.; Toledo, H. Helicobacter pylori strain ATCC700392 encodes a methyl-accepting chemotaxis receptor protein (MCP) for arginine and sodium bicarbonate. FEMS Microbiol. Lett. 2003, 224, 175–181. [Google Scholar] [CrossRef]
- McKellar, J.L.O.; Minnell, J.J.; Gerth, M.L. A high-throughput screen for ligand binding reveals the specificities of three amino acid chemoreceptors from Pseudomonas syringae pv. actinidiae. Mol. Microbiol. 2015, 96, 694–707. [Google Scholar] [CrossRef] [PubMed]
| Sample | Polydispersity | Molecular Weight (kDa) |
|---|---|---|
| YckKtag | 1.0 | 29.4 |
| BSA | 1.0 | 63.2 |
| Mosaicity (°) | 0.6 |
|---|---|
| No. of crystals | 1 |
| Temperature (K) | 100 |
| No. of images | 360 |
| Rotation per image (°) | 0.5 |
| Wavelength (Å) | 0.95 |
| Resolution range (Å) | 49.3–1.80 (1.83–1.80) |
| Completeness (%) | 95 (95) |
| Observed reflections | 171,880 (8674) |
| Unique reflections | 86,970 (4372) |
| Mean I/σ(I) | 8.9 (1.8) |
| Multiplicity | 2.0 (2.0) |
| Rmerge | 0.057 (0.327) |
| CC1/2 (%) | 99 (73) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Germantsis, D.P.; Machuca, M.A.; Ud-Din, A.I.S.; Roujeinikova, A. Crystallisation and Preliminary Crystallographic Analysis of Helicobacter pylori Periplasmic Binding Protein YckK. Crystals 2017, 7, 330. https://doi.org/10.3390/cryst7110330
Rahman MM, Germantsis DP, Machuca MA, Ud-Din AIS, Roujeinikova A. Crystallisation and Preliminary Crystallographic Analysis of Helicobacter pylori Periplasmic Binding Protein YckK. Crystals. 2017; 7(11):330. https://doi.org/10.3390/cryst7110330
Chicago/Turabian StyleRahman, Mohammad Mizanur, Daniel Phillip Germantsis, Mayra Alejandra Machuca, Abu Iftiaf Salah Ud-Din, and Anna Roujeinikova. 2017. "Crystallisation and Preliminary Crystallographic Analysis of Helicobacter pylori Periplasmic Binding Protein YckK" Crystals 7, no. 11: 330. https://doi.org/10.3390/cryst7110330
APA StyleRahman, M. M., Germantsis, D. P., Machuca, M. A., Ud-Din, A. I. S., & Roujeinikova, A. (2017). Crystallisation and Preliminary Crystallographic Analysis of Helicobacter pylori Periplasmic Binding Protein YckK. Crystals, 7(11), 330. https://doi.org/10.3390/cryst7110330






