Formation of Metastable Crystals from Supercooled, Supersaturated, and Supercompressed Liquids: Role of Crystal-Liquid Interfacial Free Energy
Abstract
:1. Introduction
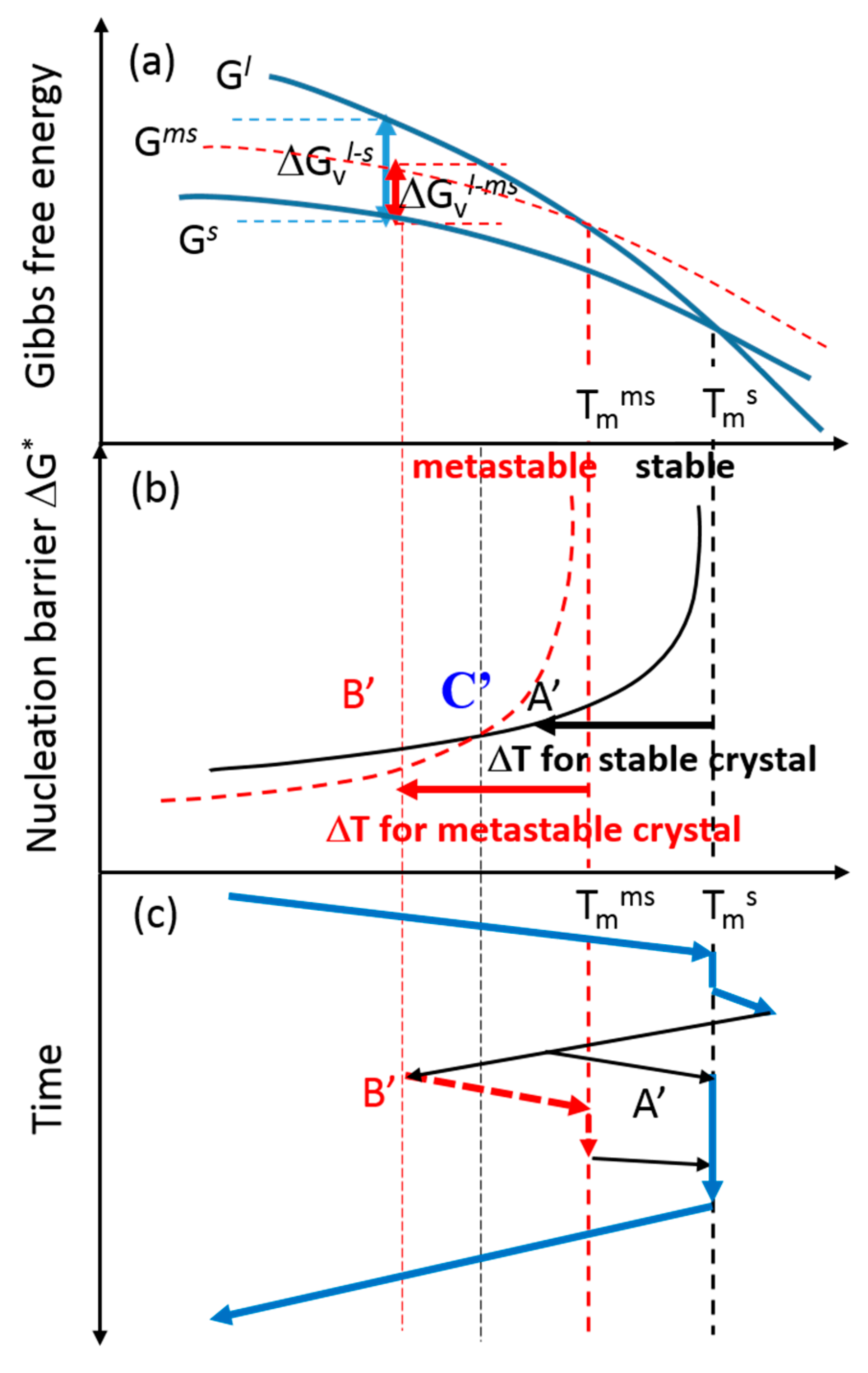
1.1. Classical Nucleation Theory: Estimation of Crystal-Liquid Interfacial Free Energy
1.2. Formation of a Metastable Crystal from a Metastable Liquid
2. Results and Discussion
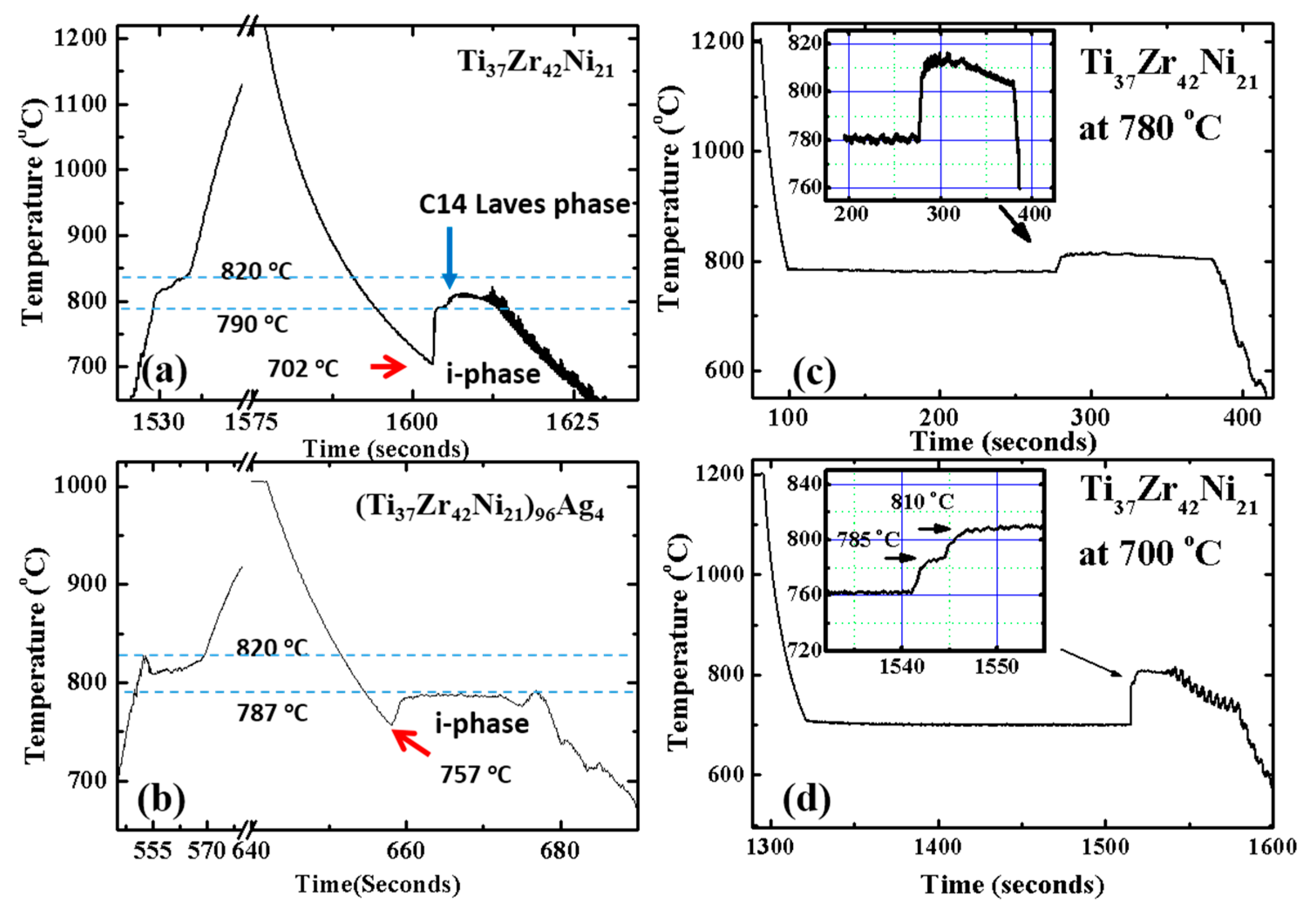
2.1. Metastable Icosahedral Quasicrystal Formation from Supercooled Ti-Zr-Ni Alloy Liquids
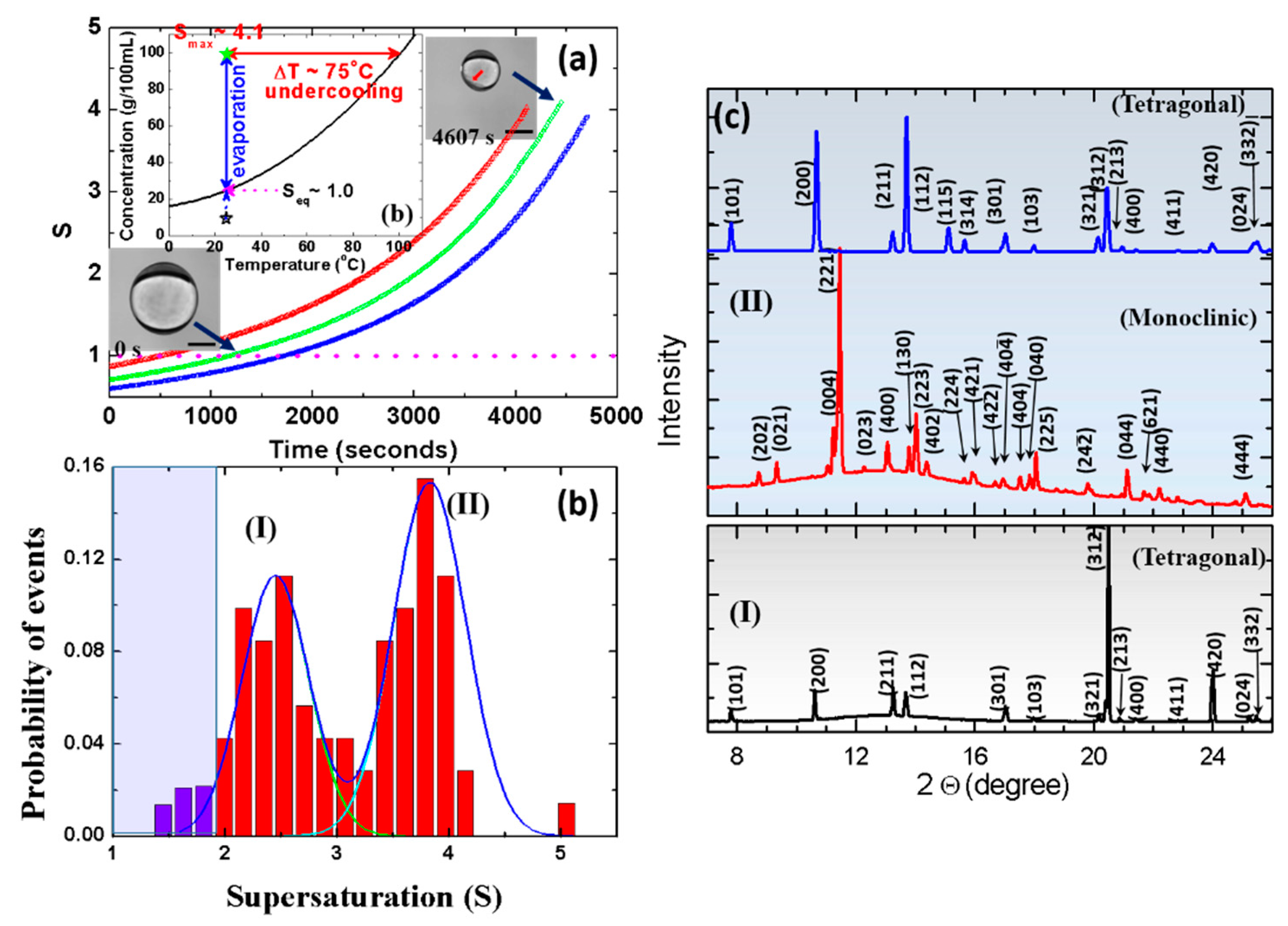
2.2. Metastable Monoclinic KH2PO4 (KDP) Crystal Formation From A Supersaturated KDP Solution
2.3. Formation of Metastable Ice VII Crystal And High Density Amorphous From Supercompressed Water
3. Summary
Acknowledgments
Conflicts of Interest
References
- Ostwald, W. Studien über die bildung und umwandlung fester körper. Z. Phys. Chem. 1897, 22, 289–330. [Google Scholar] [CrossRef]
- Van Santen, R.A. The ostwald step rule. J. Phys. Chem. 1984, 88, 5768–5769. [Google Scholar] [CrossRef]
- Vekilov, V.G. Phase diagrams and kinetics of phase transitions in protein solutions. J. Phys.: Condens. Matter 2012, 24, 193101. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Wi, H.S.; Jo, W.; Cho, Y.C.; Lee, H.H.; Jeong, S.-Y.; Kim, Y.-I.; Lee, G.W. Multiple pathways of crystal nucleation in an extremely supersaturated aqueous potassium dihydrogen phosphate (KDP) solution droplet. Proc. Natl. Acad. Sci. USA 2016, 113, 13618–13623. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Völkel, A.; Cölfen, H. Stable prenucleation calcium carbonate clusters. Science 2008, 322, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.; Dey, A.; Bomans, P.H.H.; Coadou, C.L.; Fratzl, P.; Sommerdijk, N.A.J.M.; Faivre, D. Nucleation and growth of magnetite from solution. Nat. Mater. 2013, 12, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Kelton, K.F.; Lee, G.W.; Gangopadhyay, A.K.; Croat, T.K.; Rathz, T.J.; Hyers, R.W.; Rogers, J.R.; Robinson, M.B.; Robinson, D.S. First X-ray scattering studies on electrostatically levitated metallic liquids: Demonstrated influence of local icosahedral order on the nucleation barrier. Phys. Rev. Lett. 2003, 90, 195504. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.W.; Cho, Y.C.; Lee, B.; Kelton, K.F. Interfacial free energy and medium range order: Proof of an inverse of Frank’s hypothesis. Phys. Rev. B 2017, 95, 054202. [Google Scholar] [CrossRef]
- Lee, G.W.; Evans, W.J.; Yoo, C.S. Crystallization of water in a dynamic diamond-anvil cell: Evidence for ice VII-like local order in supercompressed water. Phys. Rev. B 2006, 74, 134112. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Yoo, C.-S. High density amorphous ice at room temperature. Proc. Natl. Acad. Sci. USA 2011, 108, 7685–7688. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.-Y.; Kim, Y.-M.; Kim, J.-G.; Kim, Y.-J. Multiphase transformation and Ostwald’s rule of stages during crystallization of a metal phosphate. Nat. Phys. 2009, 5, 68–73. [Google Scholar] [CrossRef]
- Cahn, J.W. On spinodal decomposition. Acta Metall. 1961, 9, 795. [Google Scholar] [CrossRef]
- Turnbull, D.; Cech, R.E. Formation of crystal nuclei in liquid metals. J. Appl. Phys. 1950, 21, 804. [Google Scholar] [CrossRef]
- Waseda, Y. The Structure of Non-Crystalline Materials; McGraw-Hill Inc.: Pennsylvania Plaza, NY, USA, 1980. [Google Scholar]
- Frank, F.C. Supercooling of liquids. Proc. R. Soc. London A 1952, 215, 43. [Google Scholar] [CrossRef]
- Spaepen, F. Solid State Physics; Ehrenreich, H., Turnbull, D., Eds.; Academic Press: Boston, MA, USA, 1994; Volume 47, p. 1. [Google Scholar]
- Spaepen, F.; Meyer, R. The surface tension in a structural model for the solid-liquid interface. Scr. Metall. 1976, 10, 257. [Google Scholar] [CrossRef]
- Spaepen, F. A structural model for the solid-liquid interface in monatomic systems. Acta Metall. 1975, 23, 729. [Google Scholar] [CrossRef]
- Murty, B.S.; Ping, D.H.; Hono, K. Nanoquasicrystallization of binary Zr–Pd metallic glasses. Appl. Phys. Lett. 2000, 77, 1102. [Google Scholar] [CrossRef]
- Köster, U.; Meinhardt, J.; Roos, S.; Liebertz, H. Formation of quasicrystals in bulk glass forming Zr-Cu-Ni-Al alloys. Appl. Phys. Lett. 1996, 69, 179. [Google Scholar] [CrossRef]
- Lee, G.W.; Gangopadhyay, A.K.; Croat, T.K.; Rathz, T.J.; Hyers, R.W.; Rogers, J.R.; Kelton, K.F. Link between liquid structure and the nucleation barrier for icosahedral quasicrystal, polytetrahedral, and simple crystalline phases in Ti-Zr-Ni alloys; verification of Frank’s hypothesis. Phys. Rev. B 2005, 72, 174107. [Google Scholar] [CrossRef]
- Notthoff, C.; Feuerbacher, B.; Franz, H.; Herlach, D.M.; Holland-Moritz, D. Direct determination of metastable phase diagram by synchrotron radiation experiments on undercooled metallic melt. Phys. Rev. Lett. 2011, 86, 1038–1041. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.W.; Gangopadhyay, A.K.; Kelton, K.F.; Hyers, R.W.; Rathz, T.J.; Rogers, J.R.; Robinson, D.S. Difference in icosahedral short-range order in early and late transition metal liquids. Phys. Rev. Lett. 2004, 93, 037802. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.W.; Jeon, S.; Park, C.; Kang, D.-H. Crystal–liquid interfacial free energy and thermophysical properties of pure liquid Ti using electrostatic levitation: Hypercooling limit, specific heat, total hemispherical emissivity, density, and interfacial free energy. J. Chem. Thermodyn. 2013, 63, 1. [Google Scholar] [CrossRef]
- Lee, G.W.; Jeon, S.; Kang, D.H. Crystal–liquid interfacial free energy of supercooled liquid Fe using a containerless technique. Cryst. Growth Des. 2013, 13, 1786–1792. [Google Scholar] [CrossRef]
- Jeon, S.; Kang, D.H.; Kang, S.H.; Kang, S.E.; Okada, J.T.; Ishikawa, T.; Lee, S.; Lee, G.W. Non-contact measurement of thermophysical properties of Fe, Fe-C, and Fe-C-Mn alloys in solid, supercooled, and stable liquid phases. ISIJ Int. 2016, 56, 719–722. [Google Scholar] [CrossRef]
- Kang, D.-H.; Jeon, S.; Yoo, H.; Ishikawa, T.; Okada, J.T.; Paradis, P.-F.; Lee, G.W. Nanosized nucleus-supercooled liquid interfacial free energy and thermophysical properties of early and late transition liquid metals. Cryst. Growth Des. 2014, 14, 1103. [Google Scholar] [CrossRef]
- Schenk, T.; Holland-Moritz, D.; Simonet, V.; Bellissent, R.; Herlach, D.M. Icosahedral short-range order in deeply undercooled metallic melts. Phys. Rev. Lett. 2002, 89, 075507. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.W.; Gangopadhyay, A.K.; Hyers, R.W.; Rathz, T.J.; Rogers, J.R.; Robinson, D.S.; Goldman, A.I.; Kelton, K.F. Local structure of equilibrium and supercooled Ti-Zr-Ni liquids. Phys. Rev. B 2008, 77, 184102. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, G.W.; Gangopadhyay, A.K.; Hyers, R.W.; Rogers, J.R.; Goldman, A.I.; Kelton, K.F. Structural studies of a Ti-Zr-Ni quasicrystal-forming liquid. J. Phys.: Conden. Matt. 2007, 19, 455212. [Google Scholar] [CrossRef]
- Kim, T.H.; Gangopadhyay, A.K.; Xing, L.Q.; Lee, G.W.; Shen, Y.T.; Kelton, K.F.; Goldman, A.I.; Hyers, R.W.; Rogers, J.R. Role of Ti in the formation of Zr-Ti-Cu-Ni-Al glasses. Appl. Phys. Lett. 2005, 87, 251924. [Google Scholar] [CrossRef]
- Herlach, D.M.; Gao, J.; Volkmann, T. Nucleation and phase-selection in undercooled melts. Mater. Sci. Eng. A 2004, 375–377, 9–15. [Google Scholar] [CrossRef]
- Lee, G.W.; Gangopadhyay, A.K.; Kelton, K.F. Effect of microalloying on the formation and stability of the Ti-Zr-Ni icosahedral quasicrystal. J. Alloy Compd. 2012, 537, 171–174. [Google Scholar] [CrossRef]
- Lee, S.; Jo, W.; Cho, Y.C.; Lee, H.H.; Lee, G.W. Solution electrostatic levitator for measuring surface properties and bulk structures of an extremely supersaturated solution drop above metastable zone width limit. Rev. Sci. Instrum. 2017, 88, 055101. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, C.G.; Kohl, I.; Loerting, T.; Mayer, E.; Hallbrucker, A. Pure ices IV and XII from high-density amorphous ice. Can. J. Phys. 2003, 81, 25. [Google Scholar] [CrossRef]
- Koh, C.A. Towards a fundamental understanding of natural gas hydrates. Chem. Soc. Rev. 2002, 31, 157. [Google Scholar] [CrossRef] [PubMed]
- Cavalleri, M.; Ogasawara, H.; Pettersson, L.G.M.; Nilsson, A. The interpretation of X-ray absorption spectra of water and ice. Chem. Phys. Lett. 2002, 364, 363. [Google Scholar] [CrossRef]
- Loerting, T.; Winkel, K.; Seidl, M.; Bauer, M.; Mitterdorfer, C.; Handle, P.H.; Salzmann, C.G.; Mayer, E.; Finney, J.L.; Bowron, D.T. How many amorphous ices are there? Phys. Chem. Chem. Phys. 2011, 13, 8783. [Google Scholar] [CrossRef] [PubMed]
- Mishima, O.; Calvert, L.D.; Whalley, E. ‘Melting ice’ I at 77 K and 10 kbar: A new method of making amorphous solids. Nature 1984, 310, 393. [Google Scholar] [CrossRef]
- Loerting, T.; Salzman, C.; Kohl, I.; Meyer, E.; Hallbrucker, A. A second distinct structural “state” of high-density amorphous ice at 77 K and 1 bar. Phys. Chem. Chem. Phys. 2001, 3, 5355. [Google Scholar] [CrossRef]
- Lee, G.W.; Evans, W.J.; Yoo, C.S. Dynamic pressure-induced dendritic and shock crystal growth of ice VI. Proc. Natl. Acad. Sci. USA 2007, 104, 9178–9181. [Google Scholar] [CrossRef] [PubMed]
- Kelton, K.F. Solid State Physics; Ehrenreich, H., Turnbull, D., Eds.; Academic Press: Boston, MA, USA, 1991; p. 75. [Google Scholar]
- Turnbull, D. Kinetics of solidification of supercooled liquid mercury droplets. J. Chem. Phys. 1952, 20, 411. [Google Scholar] [CrossRef]
- Thompson, C.V.; Spaepen, F. On the approximation of the free energy change on crystallization. Acta Metall. 1979, 27, 1855. [Google Scholar] [CrossRef]
- Battezzati, L.; Garonne, E. On the approximations of the free energy of undercooled glass-forming metallic melts. Z. Metallk. 1984, 75, 305. [Google Scholar]
- Paradis, P.-F.; Ishikawa, T.; Lee, G.W.; Holland-Moritz, D.; Brillo, J.; Rhim, W.-K.; Okada, J.T. Materials properties measurements and particle beam interactions studies using electrostatic levitation. Mater. Sci. Eng. R 2014, 76, 1. [Google Scholar] [CrossRef]
- Lee, G.W.; Gangopadhyay, A.K.; Kelton, K.F. Phase diagram studies of Ti-Zr-Ni alloys containing less than 40 at.% Ni and estimated critical cooling rate for icosahedral quasicrystal formation from the liquid. Acta Mater. 2011, 59, 4964–4973. [Google Scholar] [CrossRef]
- Kang, D.H.; Zhang, H.; Yoo, H.; Lee, H.H.; Lee, S.; Lee, G.W.; Lou, H.; Wang, X.; Cao, Q.; Zhang, D.; et al. Interfacial free energy controlling glass-forming ability of Cu-Zr alloys. Sci. Rep. 2014, 4, 5167. [Google Scholar] [CrossRef] [PubMed]
- Steinhardt, P.J. Solid-state physics: How does your quasicrystal grow? Nature 2008, 452, 43. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.S.; Glotzer, S.C. How do quasicrystals grow? Phys. Rev. Lett. 2007, 99, 235503. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Xu, Y.; Hui, X.; Lu, Z.P.; Li, F.; Chen, G.L.; Lu, J.; Liu, C.T. Metallic liquids and glasses: Atomic order and global packing. Phys. Rev. Lett. 2010, 105, 155501. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, S.; Yang, F.; Wang, J.; Lang, J. Effect of alcoholic additives on the nucleation of KDP and DKDP crystals. J. Cryst. Growth 1997, 179, 226–230. [Google Scholar]
- Wojciechowski, K.; Kibalczyc, W. Light scattering study of KH2PO4 and BaSO4 nucleation process. J. Cryst. Growth 1986, 76, 379–382. [Google Scholar] [CrossRef]
- Shanmungham, M.; Gnanam, F.D.; Ramasamy, P. Nucleation studies in supersaturated potassium dihydrogen orthophosphate solution and the effect of soluble impurities. J. Mater. Sci. 1984, 19, 2837–2844. [Google Scholar] [CrossRef]
- Joshi, M.S.; Antony, A.V. Nucleation in supersaturated potassium dihydrogen orthophosphate solutions. J. Cryst. Growth 1979, 46, 7–9. [Google Scholar] [CrossRef]
- Zaitseva, N.; Carman, L. Rapid growth of KDP-type crystals. Prog. Cryst. Growth Charact. Mater. 2001, 43, 1–118. [Google Scholar] [CrossRef]
- Parikh, K.D.; Parekh, B.B.; Dave, D.J.; Joshi, M.J. Nucleation kinetics of L-arginine, L-lysine and L-alanine doped potassium dihydrogen phosphate crystals. JCPT 2013, 3, 92–96. [Google Scholar] [CrossRef]
- Chernov, A.A. Secondary nucleation induced by the cracking of a growing crystal: KH2PO4 (KDP) and K(H,D)2PO4 (DKDP). J. Cryst. Growth 1990, 102, 793–800. [Google Scholar] [CrossRef]
- Söhnel, O. Electrolyte crystal-aqueous solution interfacial tensions from crystallization data. J. Cryst. Growth 1982, 57, 101–108. [Google Scholar] [CrossRef]
- Evans, W.J.; Yoo, C.S.; Lee, G.W.; Cynn, H.; Lipp, M.J.; Visbeck, K. Dynamic diamond anvil cell (dDAC): A novel device for studying the dynamic-pressure properties of materials. Rev. Sci. Instrum. 2007, 78, 073904. [Google Scholar] [CrossRef] [PubMed]
- Saitta, A.M.; Datchi, F. Structure and phase diagram of high-density water: The role of interstitial molecules. Phys. Rev. E 2003, 67, 020201(R). [Google Scholar] [CrossRef] [PubMed]

| Compositions & Used Parameters (Tl, ρ, Cp, ΔHf) | ΔT/Tm | Interfacial Energy (σ) (±0.0002) | α (=σ/ΔHf) | W*/kBT at Tr | r*(I) (nm) | Coherence Length (nm) on as Cast i-Phase | ||
|---|---|---|---|---|---|---|---|---|
| ΔGl-s(1) | ΔGl-s(2) | ΔGl-s(3) | ||||||
| Ti37Zr42Ni21 (Tl = 1060 K, ρ = 5.95 g/cm3, Cp = 44.24 J/mol-K, ΔHf = 8.1 kJ/mol) | 0.1 | 0.061 | 0.049 | 0.050 | 0.324 | 665.17 | 1.735 | 25 |
| (Ti37Zr42Ni21)96Ag4 (Tl = 1060 K, ρ = 5.98 g/cm3, Cp = 44.24 J/mol-K, ΔHf = 7.93 kJ/mol) | 0.029 | 0.027 | 0.022 | 0.022 | 0.146 | 58.84 | 2.736 | 43 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.W. Formation of Metastable Crystals from Supercooled, Supersaturated, and Supercompressed Liquids: Role of Crystal-Liquid Interfacial Free Energy. Crystals 2017, 7, 326. https://doi.org/10.3390/cryst7110326
Lee GW. Formation of Metastable Crystals from Supercooled, Supersaturated, and Supercompressed Liquids: Role of Crystal-Liquid Interfacial Free Energy. Crystals. 2017; 7(11):326. https://doi.org/10.3390/cryst7110326
Chicago/Turabian StyleLee, Geun Woo. 2017. "Formation of Metastable Crystals from Supercooled, Supersaturated, and Supercompressed Liquids: Role of Crystal-Liquid Interfacial Free Energy" Crystals 7, no. 11: 326. https://doi.org/10.3390/cryst7110326




