Synthesis, Crystal Structure and Thermal Stability of 1D Linear Silver(I) Coordination Polymers with 1,1,2,2-Tetra(pyrazol-1-yl)ethane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Coordination Polymers
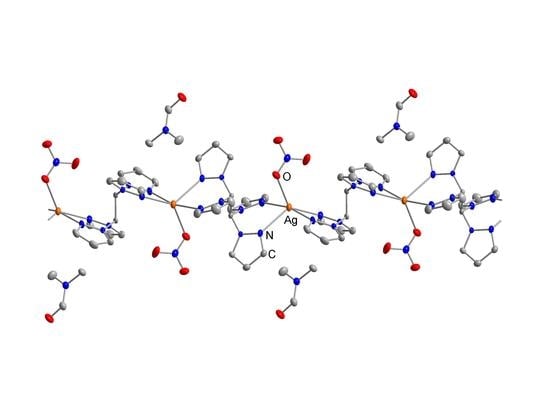
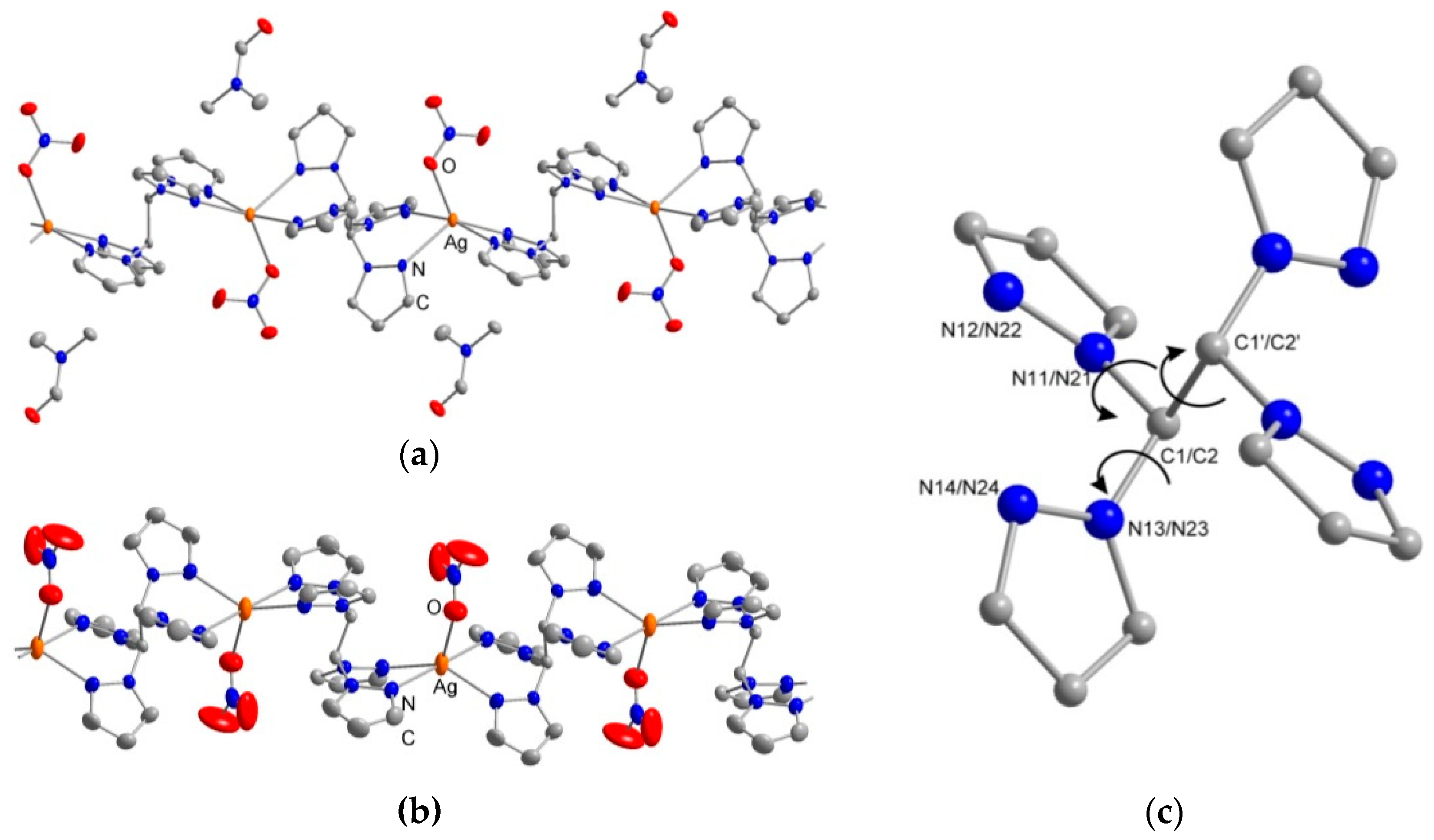
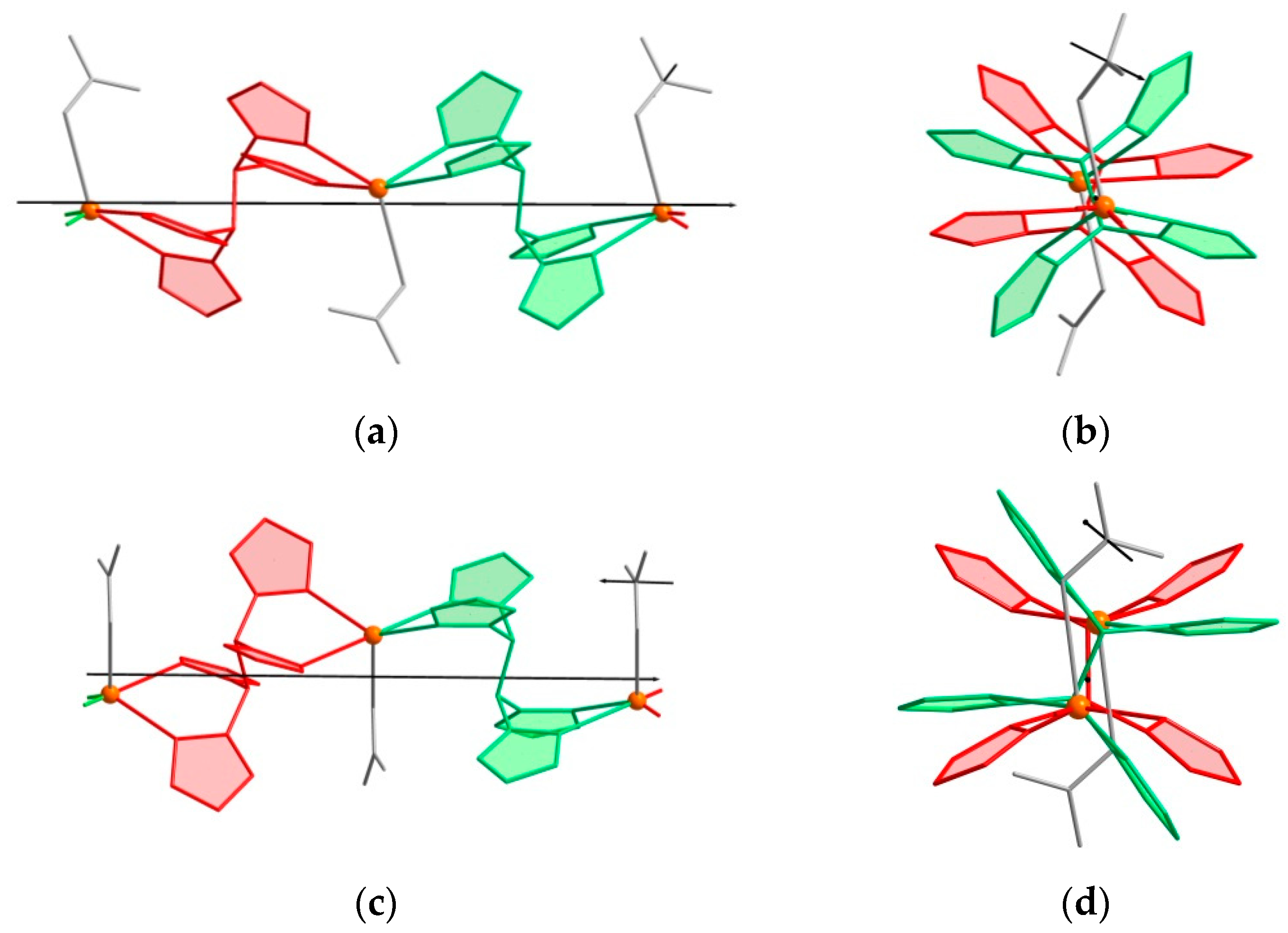
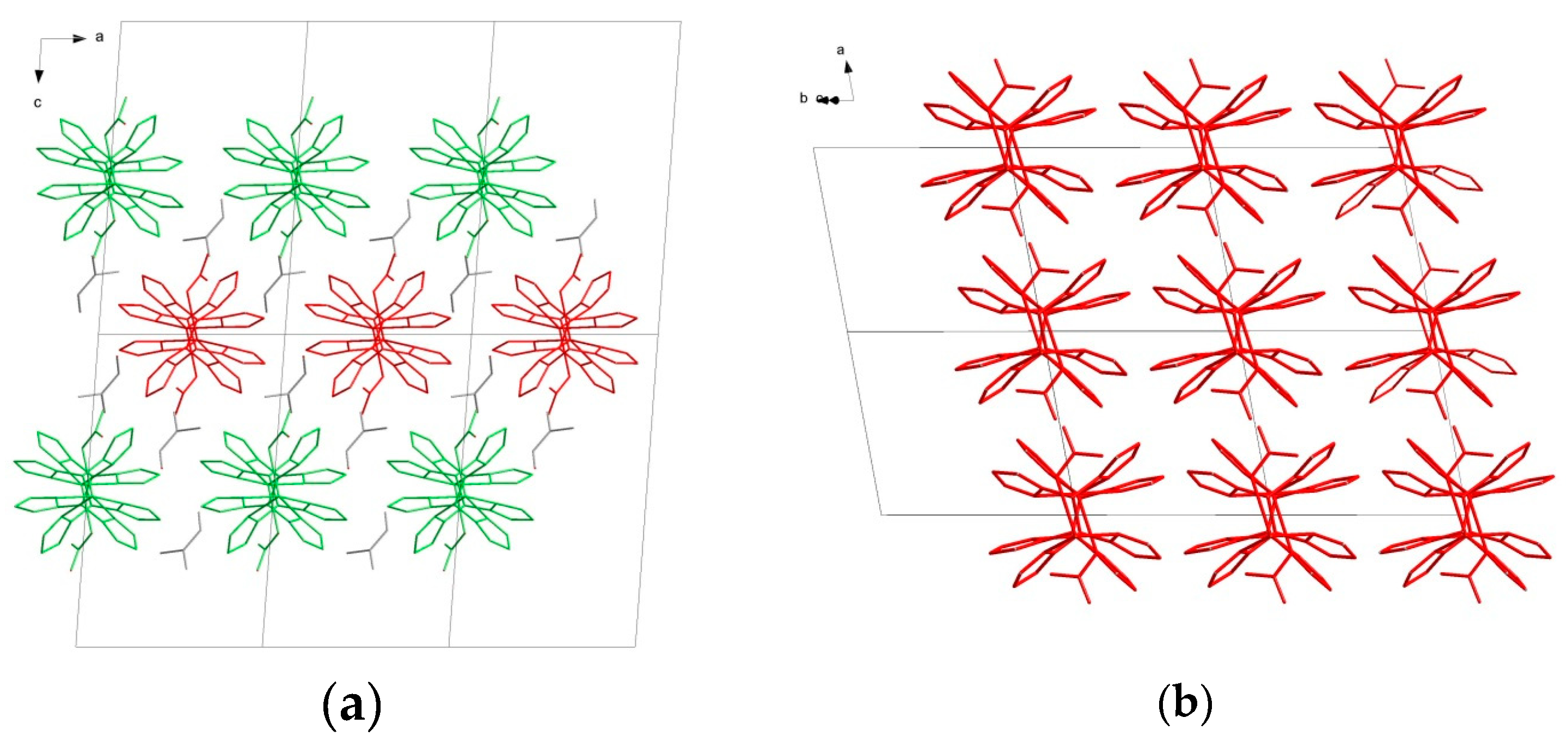
2.2. Crystal Structures
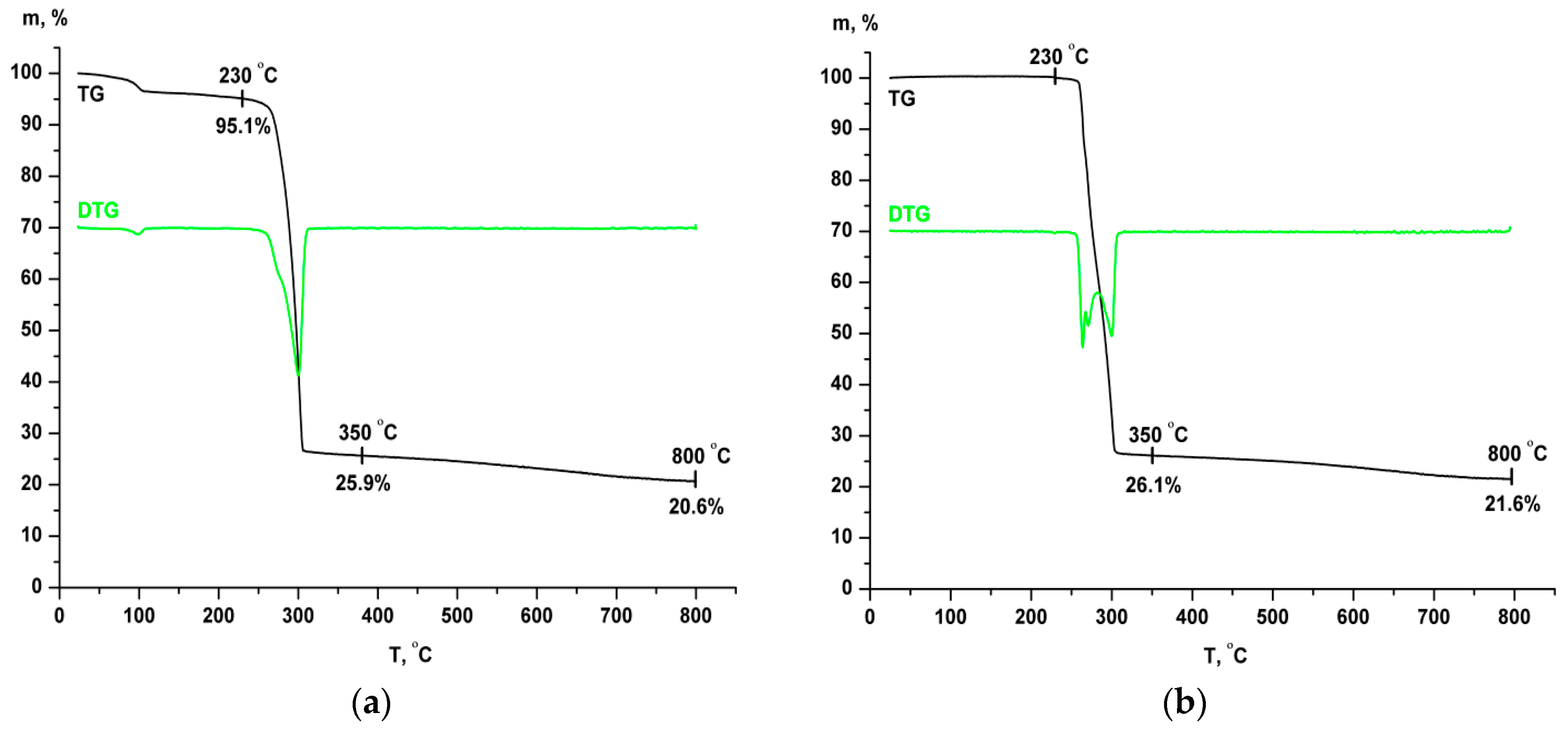
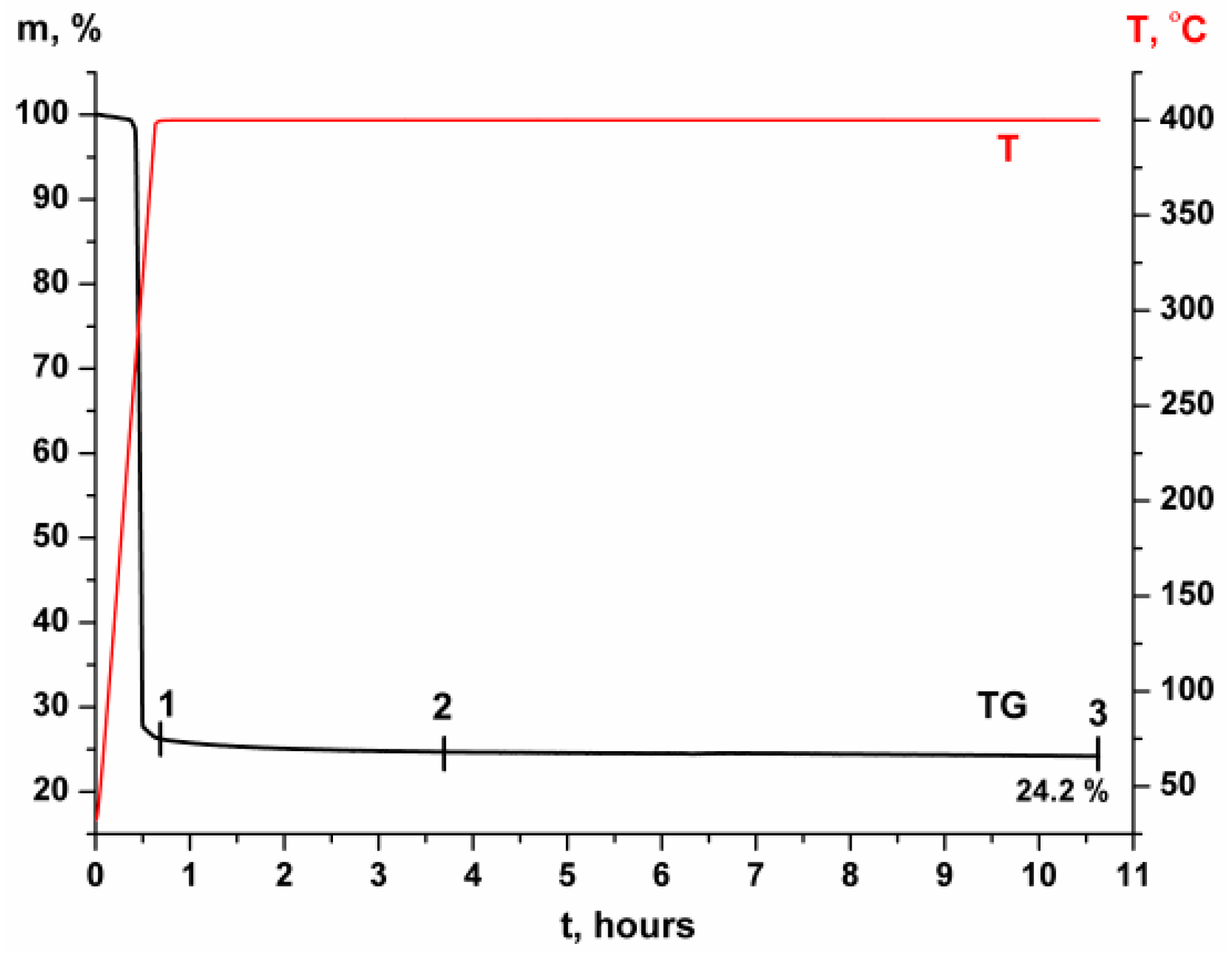
2.3. Thermal and XRD Analyses
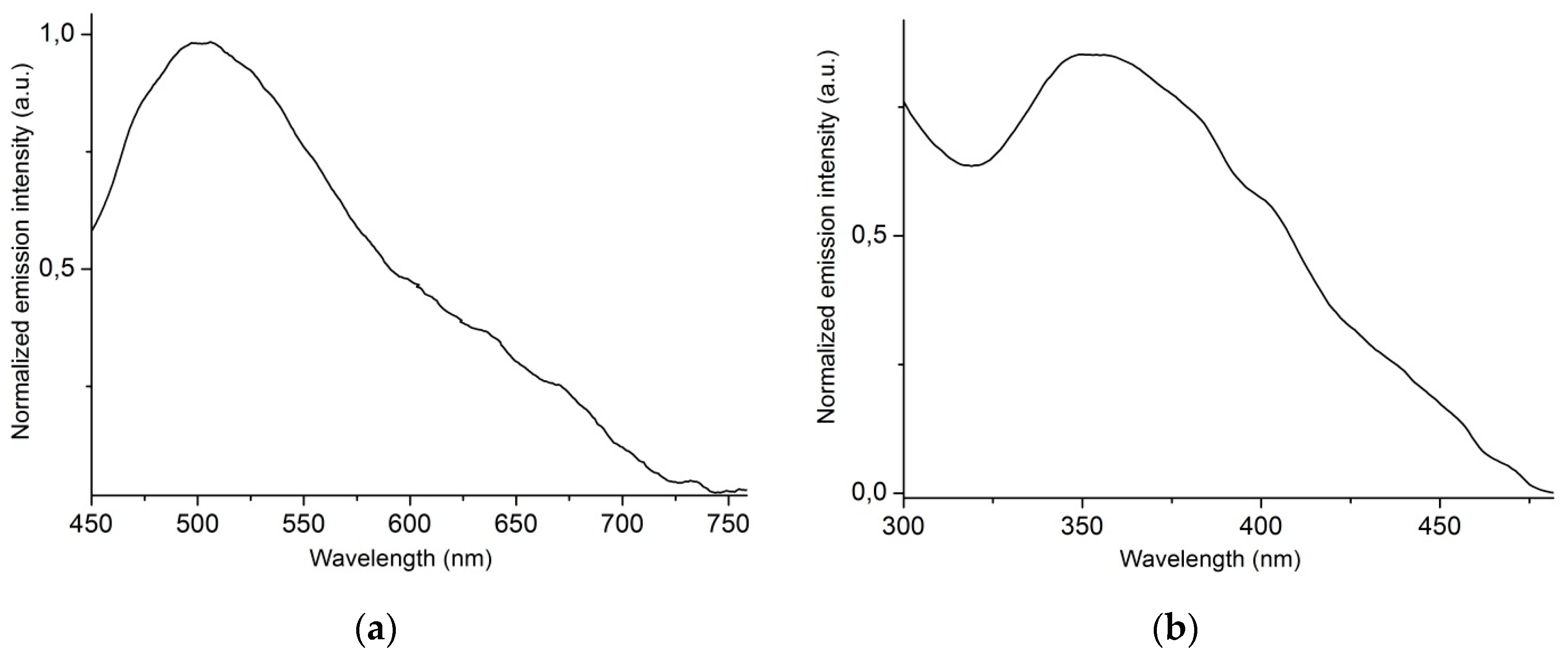
2.4. Solid-State Luminescence Studies
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of Compounds
3.2.1. Synthesis of {[Ag(µ2‑Pz4)(NO3)]·DMF}n (1)
3.2.2. Synthesis of [{Ag(µ2‑Pz4)(NO3)}n] (2)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Beheshti, A.; Zafarian, H.R.; Khorramdin, R.; Fattahi Monavvar, M.; Abrahams, C.T. Novel silver(I) pyrazole-based coordination polymers: Synthetic and structural studies. Polyhedron 2012, 48, 245–252. [Google Scholar] [CrossRef]
- Khlobystov, A.N.; Blake, A.J.; Champness, N.R.; Lemenovskii, D.A.; Majouga, A.G.; Zyk, N.V.; Schröder, M. Supramolecular design of one-dimensional coordination polymers based on silver(I) complexes of aromatic nitrogen-donor ligands. Coord. Chem. Rev. 2001, 222, 155–192. [Google Scholar] [CrossRef]
- Bassanetti, I.; Atzeri, C.; Tinonin, D.A.; Marchiò, L. Silver(I) and Thioether-bis(pyrazolyl)methane Ligands: The Correlation between Ligand Functionalization and Coordination Polymer Architecture. Cryst. Growth Des. 2016, 16, 3543–3552. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Malinina, E.A.; Sivaev, I.B.; Bregadze, V.I.; Kuznetsov, N.T. Silver and Copper Complexes with closo-Polyhedral Borane, Carborane and Metallacarborane Anions: Synthesis and X-ray Structure. Crystals 2016, 6, 60. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Wieczorek, S.W.; Lis, A.; Guedes da Silva, M.F.C.; Florek, M.; Król, J.; Staroniewicz, Z.; Smoleński, P.; Pombeiro, A.J.L. 1,3,5-Triaza-7-phosphaadamantane-7-oxide (PTA═O): New Diamondoid Building Block for Design of Three-Dimensional Metal–Organic Frameworks. Cryst. Growth Des. 2011, 11, 2711–2716. [Google Scholar] [CrossRef]
- Jaros, S.W.; Guedes da Silva, M.F.C.; Florek, M.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Silver(I) 1,3,5-Triaza-7-phosphaadamantane Coordination Polymers Driven by Substituted Glutarate and Malonate Building Blocks: Self-Assembly Synthesis, Structural Features, and Antimicrobial Properties. Inorg. Chem. 2016, 55, 5886–5894. [Google Scholar] [CrossRef] [PubMed]
- Fromm, K.M. Silver coordination compounds with antimicrobial properties. Appl. Organomet. Chem. 2013, 27, 683–687. [Google Scholar] [CrossRef]
- Jaros, S.W.; Guedes da Silva, M.F.C.; Król, J.; Conceição Oliveira, M.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Bioactive Silver–Organic Networks Assembled from 1,3,5-Triaza-7-phosphaadamantane and Flexible Cyclohexanecarboxylate Blocks. Inorg. Chem. 2016, 55, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Jaros, S.W.; Smolenski, P.; da Silva, M.F.C.; Florek, M.; Krol, J.; Staroniewicz, Z.; Pombeiro, A.J.L.; Kirillov, A.M. New silver BioMOFs driven by 1,3,5-triaza-7-phosphaadamantane-7-sulfide (PTA=S): Synthesis, topological analysis and antimicrobial activity. CrystEngComm 2013, 15, 8060–8064. [Google Scholar] [CrossRef]
- Smolenski, P.; Jaros, S.W.; Pettinari, C.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Vitali, L.A.; Petrelli, D.; Kochel, A.; Kirillov, A.M. New water-soluble polypyridine silver(i) derivatives of 1,3,5-triaza-7-phosphaadamantane (PTA) with significant antimicrobial and antiproliferative activities. Dalt. Trans. 2013, 42, 6572–6581. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.-B.; Li, Z.-H.; Xu, C.-Y.; Ji, B.-M. Syntheses, characterization, and luminescence properties of three novel Ag(I) coordination polymers based on polycarboxylic acid ligands and 1,3-di-(1,2,4-triazole-4-yl)benzene. CrystEngComm 2016, 18, 4636–4642. [Google Scholar] [CrossRef]
- Beheshti, A.; Zafarian, H.R.; Abrahams, C.T.; Bruno, G.; Rudbari, H.A. Investigating the effect of anion substitutions on the structure of silver-based coordination polymers. Inorg. Chim. Acta 2015, 438, 196–202. [Google Scholar] [CrossRef]
- Gardinier, J.R.; Tatlock, H.M.; Hewage, J.S.; Lindeman, S.V. Cyclic versus Polymeric Supramolecular Architectures in Metal Complexes of Dinucleating Ligands: Silver(I) Trifluoromethanesulfonate Complexes of the Isomers of Bis(di(1H-pyrazolyl)methyl)-1,1′-biphenyl. Cryst. Growth Des. 2013, 13, 3864–3877. [Google Scholar] [CrossRef]
- Reger, D.L.; Foley, E.A.; Smith, M.D. Synthesis of a tritopic, third-generation bis(1-pyrazolyl)methane ligand and its silver(I) complex: Unexpected structure with high coordination numbers. Inorg. Chem. Commun. 2010, 13, 568–572. [Google Scholar] [CrossRef]
- Morin, T.J.; Merkel, A.; Lindeman, S.V.; Gardinier, J.R. Breaking the Cycle: Impact of Sterically-Tailored Tetra(pyrazolyl)lutidines on the Self-Assembly of Silver(I) Complexes. Inorg. Chem. 2010, 49, 7992–8002. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zang, H.; Sun, C.; Xu, G.; Wang, X.; Shao, K.; Lan, Y.; Su, Z. Anion-directed genuine meso-helical supramolecular isomers of two 1D Ag(I) complexes based on arene-linked bis(pyrazolyl)methane ligands. CrystEngComm 2010, 12, 3458. [Google Scholar] [CrossRef]
- Reger, D.L.; Watson, R.P.; Smith, M.D. Silver(I) complexes of fixed, polytopic bis(pyrazolyl)methane ligands: Influence of ligand geometry on the formation of discrete metallacycles and coordination polymers. Inorg. Chem. 2006, 45, 10077–10087. [Google Scholar] [CrossRef] [PubMed]
- Reger, D.L.; Watson, R.P.; Gardinier, J.R.; Smith, M.D. Impact of Variations in Design of Flexible Bitopic Bis(pyrazolyl)methane Ligands and Counterions on the Structures of Silver(I) Complexes: Dominance of Cyclic Dimeric Architecture. Inorg. Chem. 2004, 43, 6609–6619. [Google Scholar] [CrossRef] [PubMed]
- Reger, D.L.; Gardinier, J.R.; Christian Grattan, T.; Smith, M.R.; Smith, M.D. Synthesis of the silver(I) complex of CH2[CH(pz4Et)2]2 containing the unprecedented [Ag(NO3)4]3− anion: A general method for the preparation of 4-(alkyl)pyrazoles. New J. Chem. 2003, 27, 1670–1677. [Google Scholar] [CrossRef]
- Reger, D.L.; Semeniuc, R.F.; Silaghi-Dumitrescu, I.; Smith, M.D. Influences of Changes in Multitopic Tris(pyrazolyl)methane Ligand Topology on Silver(I) Supramolecular Structures. Inorg. Chem. 2003, 42, 3751–3764. [Google Scholar] [CrossRef] [PubMed]
- Potapov, A.S.; Nudnova, E.A.; Khlebnikov, A.I.; Ogorodnikov, V.D.; Petrenko, T.V. Synthesis, crystal structure and electrocatalytic activity of discrete and polymeric copper(II) complexes with bitopic bis(pyrazol-1-yl) methane ligands. Inorg. Chem. Commun. 2015, 53, 72–75. [Google Scholar] [CrossRef]
- Semitut, E.Y.; Komarov, V.Y.; Filatov, E.Y.; Kuznetsova, A.S.; Khlebnikov, A.I.; Potapov, A.S. Synthesis and structural characterization of copper(II) coordination polymers with 1,1,2,2-tetra(pyrazol-1-yl)ethane. Inorg. Chem. Commun. 2016, 64, 23–26. [Google Scholar] [CrossRef]
- Wang, J.-X.; Zhu, Z.-R.; Bai, F.-Y.; Wang, X.-Y.; Zhang, X.-X.; Xing, Y.-H. Molecular design and the optimum synthetic route of the compounds with multi-pyrazole and its derivatives and the potential application in antibacterial agents. Polyhedron 2015, 99, 59–70. [Google Scholar] [CrossRef]
- Dehury, N.; Maity, N.; Tripathy, S.K.; Basset, J.-M.; Patra, S. Dinuclear Tetrapyrazolyl Palladium Complexes Exhibiting Facile Tandem Transfer Hydrogenation/Suzuki Coupling Reaction of Fluoroarylketone. ACS Catal. 2016, 6, 5535–5540. [Google Scholar] [CrossRef]
- Heath, R.; Muller-Bunz, H.; Albrecht, M. Silver(I) NHC mediated C-C bond activation of alkyl nitriles and catalytic efficiency in oxazoline synthesis. Chem. Commun. 2015, 51, 8699–8701. [Google Scholar] [CrossRef] [PubMed]
- Nudnova, E.A.; Potapov, A.S.; Khlebnikov, A.I.; Ogorodnikov, V.D. Synthesis of ditopic ligands containing bis(1H-pyrazol-1-yl)-methane fragments. Russ. J. Org. Chem. 2007, 43, 1698–1702. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B. Applications in Coordination, Organometallic, and Bioinorganic Chemistry; Wiley-Interscience: Hoboken, NJ, USA, 2009. [Google Scholar]
- Kleywegt, G.J.; Wiesmeijer, W.G.R.; Van Driel, G.J.; Driessen, W.L.; Reedijk, J.; Noordik, J.H. Unidentate versus symmetrically and unsymmetrically bidentate nitrate co-ordination in pyrazole-containing chelates. The crystal and molecular structures of (nitrato-O)[tris (3, 5-dimethylpyrazol-1-ylmethyl) amine] copper (II) nitrate,(nitrato-O, O′)[tris (3, 5-dimethylpyrazol-1-ylmethyl) amine] nickel (II) nitrate, and (nitrato-O)(nitrato-O, O′)[tris (3, 5-dimethylpyrazol-1-ylmethyl) amine] cadmium (II). J. Chem. Soc. Dalt. Trans. 1985, 2177–2184. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2’-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalt. Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, J.; Xi, X.; Yang, P.; Shi, Q. A Novel Silver(I) Complex with 4,5-Diazafluoren-9-one and the Interaction Study Between the Complex and DNA. J. Chem. Crystallogr. 2011, 41, 209–213. [Google Scholar] [CrossRef]
- Marandi, F.; Hosseini, N.; Krautscheid, H.; Lässig, D.; Lincke, J.; Rafiee, M.; Asl, Y.A. Synthesis and structural characterization of new dinuclear silver(I) complexes: Different coordination modes of substituted 1,2,4-triazine ligands. J. Mol. Struct. 2011, 1006, 324–329. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, Y.-L. (Nitrato-κO)bis[5-(pyridin-2-yl)pyrazine-2-carbonitrile-κ2N4,N5]silver(I). Acta Crystallogr. Sect. E 2011, 67, m1863. [Google Scholar] [CrossRef] [PubMed]
- Potapov, A.S.; Nudnova, E.A.; Khlebnikov, A.I.; Ogorodnikov, V.D.; Petrenko, T.V. Synthesis of new polydentate pyrazolyl-ethene ligands by interaction of 1H-pyrazole and 1,1,2,2-tetrabromoethane in a superbasic medium. J. Heterocycl. Chem. 2011, 48, 645–651. [Google Scholar] [CrossRef]
- Bruker. APEX2 (Version 2.0), SAINT (Version 8.18c), and SADABS (Version 2.11); Bruker Advanced X-ray Solutions, Bruker AXS Inc.: Madison, WI, USA, 2000–2012. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- PCPDFWIN. v. 1.30, JCPDS-ICDD: Swarthmore, PA, USA, 1997.
- Krumm, S. An Interactive Windows Program for Profile Fitting and Size/Strain Analysis. Mater. Sci. Forum 1996, 228–231, 183–190. [Google Scholar] [CrossRef]

| Parameter | Compound 1 | Compound 2 |
|---|---|---|
| ν3, cm−1 | 1390 1294 | 1393 1309 |
| Δν3, cm−1 | 96 | 84 |
| ν4, cm−1 | 770 | 755 |
| l2-l1, Å 1 | 0.699 | 0.932 |
| A1-A2, ° 1 | 33.49 | 47.72 |
| l3-l2, Å 1 | 0.006 | −0.085 |
| Parameter | Compound 1 | Compound 2 |
|---|---|---|
| Empirical formula | C17H21AgN10O4 | C14H14AgN9O3 |
| Formula weight | 537.31 | 464.21 |
| Crystal system | Monoclinic | Triclinic |
| Space group | P21/n | P-1 |
| Unit cell dimensions a, Å | 9.394(2) | 7.8650(9) |
| b, Å | 14.299(4) | 8.8031(10) |
| c, Å | 15.793(4) | 13.2418(15) |
| α, ° | 90 | 74.380(4) |
| β, ° | 94.173(7) | 76.990(3) |
| γ, ° | 90 | 83.647(4) |
| Volume, Å3 | 2115.7(9) | 859.07(17) |
| Z | 4 | 2 |
| Density (calcd.), g·cm−3 | 1.687 | 1.795 |
| F(000) | 1088 | 464 |
| Abs. coefficient, mm-1 | 1.001 | 1.211 |
| Crystal size, mm3 | 0.8 × 0.15 × 0.15 | 0.32 × 0.08 × 0.08 |
| 2θmax, ° | 51.38 | 53.58 |
| Index range | −11 ≤ h ≤11 −17 ≤ k ≤17 −19 ≤ l ≤11 | −9 ≤ h ≤ 9 −10 ≤ k ≤ 8 −16 ≤ l ≤ 16 |
| Reflections collected | 14199 | 7730 |
| Independent reflections | 3967 [R(int) = 0.0215] | 3239 [R(int) = 0.0549] |
| Completness to 2θ = 51.38, % | 98.7 | 99.4 |
| Reflections, I ≥ 2σ(I) | 3786 | 2328 |
| Parameters | 291 | 250 |
| Final R indices [I > 2σ(I)] | R1 = 0.0665 wR2 = 0.1958 | R1 = 0.0681 wR2 = 0.1577 |
| R indices (all data) | R1 = 0.0679 wR2 = 0.1962 | R1 = 0.0924 wR2 = 0.1771 |
| GoF | 1.277 | 1.037 |
| Residual electron density (min/max, e/Å3) | −0.931/2.534 | −2.227/1.646 |
| Parameter | {[Ag(µ2‑Pz4)(NO3)]DMF}n (1) | [{Ag(µ2‑Pz4)(NO3)}n] (2) |
|---|---|---|
| α (°) | 128.0 | 141.5 |
| β (°) | 166.8 | 178.0 |
| τ5 = (β – α)/60 | 0.64 | 0.61 |
| Parameter | {[Ag(µ2‑Pz4)(NO3)]DMF}n (1) | [{Ag(µ2‑Pz4)(NO3)}n] (2) |
|---|---|---|
| Ag–N12 | 2.41 | 2.30 |
| Ag–N14 | 2.40 | 2.63 |
| Ag–N22 | 2.54 | 2.49 |
| Ag–N24 | 2.40 | 2.31 |
| Ag–O1 | 2.58 | 2.60 |
| Parameter | Nmn–C1–C1’–Nmn | C1–C1’–Nmn–Nmn |
|---|---|---|
| {[Ag(µ2‑Pz4)(NO3)]DMF}n (1) | ±55.5; ±54.8 | ±57.9; ±53.8; ±50.4; ±54.8 |
| [{Ag(µ2‑Pz4)(NO3)}n] (2) | ±54.8; ±54.0 | ±57.6; ±58.1; ±50.5; ±58.2 |
| [Cu2(µ2‑Pz4)(H2O)2(NO3)4] [21] | −58.9; 56.1; −57.3; 55.4 | 60.8; −70.4; 69.1; −61.7 70.4; −61.5; 63.2; −67.0 |
| [Cu2(µ2‑Pz4)(H2O)6(NO3)4] [22] | 52.6; −61.7 | 59.5; −74.9; 67.7; −61.0. |
| [Cu(Pz4)(NO3)2]n [22] | ±56.0 | ±72.2; ±61.5; |
| [{Cu(Pz4)(H2O)(NO3)2}2]n [22] | ±55.3 | ±70.0; ±72.5 |
| Thermolysis Conditions (T, Annealing Time) | Crystallite Size Range for Different Atmospheres (nm) | |
|---|---|---|
| He | H2/He (7.2%) | |
| 400 °C, 0 h | 12–23 | 11–20 |
| 400 °C, 3 h | - | 9–20 |
| 400 °C, 10 h | 17–30 | 10–20 |
| 600 °C, 0 h | - | 10–21 |
| 800 °C, 0 h | 26–53 | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semitut, E.; Komarov, V.; Sukhikh, T.; Filatov, E.; Potapov, A. Synthesis, Crystal Structure and Thermal Stability of 1D Linear Silver(I) Coordination Polymers with 1,1,2,2-Tetra(pyrazol-1-yl)ethane. Crystals 2016, 6, 138. https://doi.org/10.3390/cryst6110138
Semitut E, Komarov V, Sukhikh T, Filatov E, Potapov A. Synthesis, Crystal Structure and Thermal Stability of 1D Linear Silver(I) Coordination Polymers with 1,1,2,2-Tetra(pyrazol-1-yl)ethane. Crystals. 2016; 6(11):138. https://doi.org/10.3390/cryst6110138
Chicago/Turabian StyleSemitut, Evgeny, Vladislav Komarov, Taisiya Sukhikh, Evgeny Filatov, and Andrei Potapov. 2016. "Synthesis, Crystal Structure and Thermal Stability of 1D Linear Silver(I) Coordination Polymers with 1,1,2,2-Tetra(pyrazol-1-yl)ethane" Crystals 6, no. 11: 138. https://doi.org/10.3390/cryst6110138