A Comparative Theoretical Study of Picric Acid and Its Cocrystals
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis
2.2. X-ray Crystallography
2.3. Computational Method
3. Results and Discussion
3.1. Crystal Structure
| Parameter | Data |
|---|---|
| Empirical formula | C6H3N3O7·C7H8O |
| Formula weight | 349.26g/mol |
| Temperature | 293(2) K |
| Crystal system | Monoclinic |
| Space group | P21/c |
| Cell parameters | a = 8.494(18) Å |
| b = 17.41(4) Å | |
| c = 10.18(2) Å | |
| β = 92.76°(4) | |
| Volume | 1504(5) Å3 |
| Z | 4 |
| Calculated density | 1.543 g/cm3 |
| Absorption coefficient | 0.129 mm−1 |
| F(000) | 720 |
| Crystal size | 0.10 × 0.10 × 0.10 mm |
| Theta range for data collection | 2.32–28.25° |
| Max. and min. transmission | 0.9879 and 0.9872 |
| Data/restraints/parameters | 3427/0/226 |
| Goodness-of-fit on F2 | 1.005 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0754, wR2 = 0.1615 |
| R indices (all data) | R1 = 0.1652, wR2 = 0.1993 |
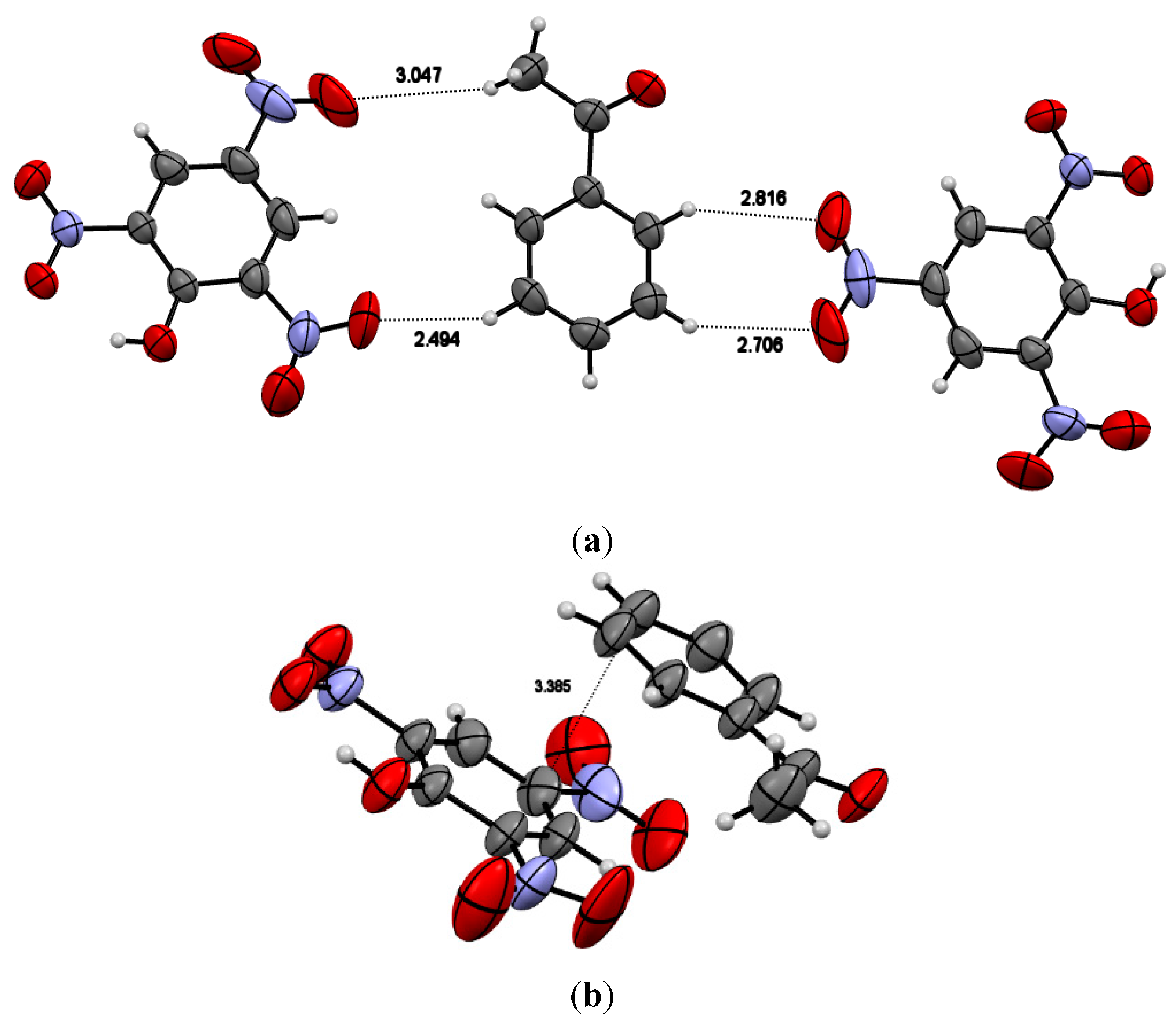
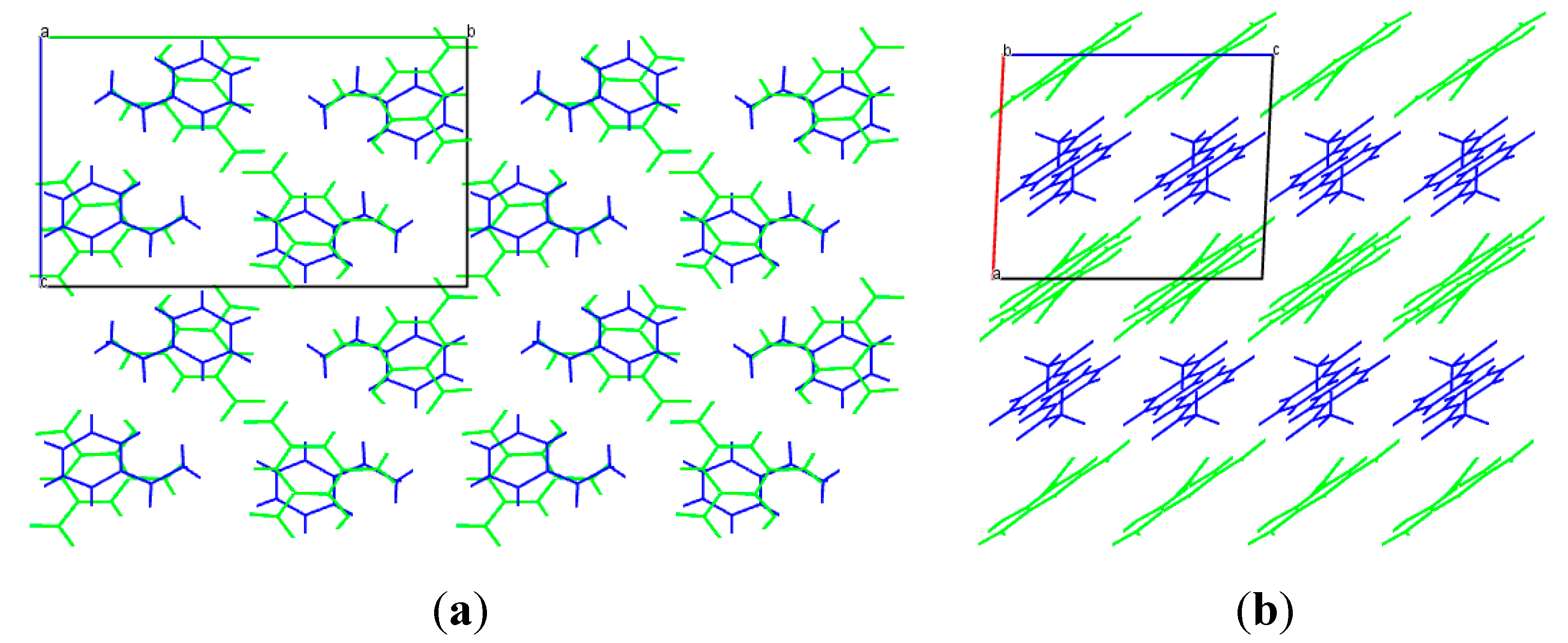
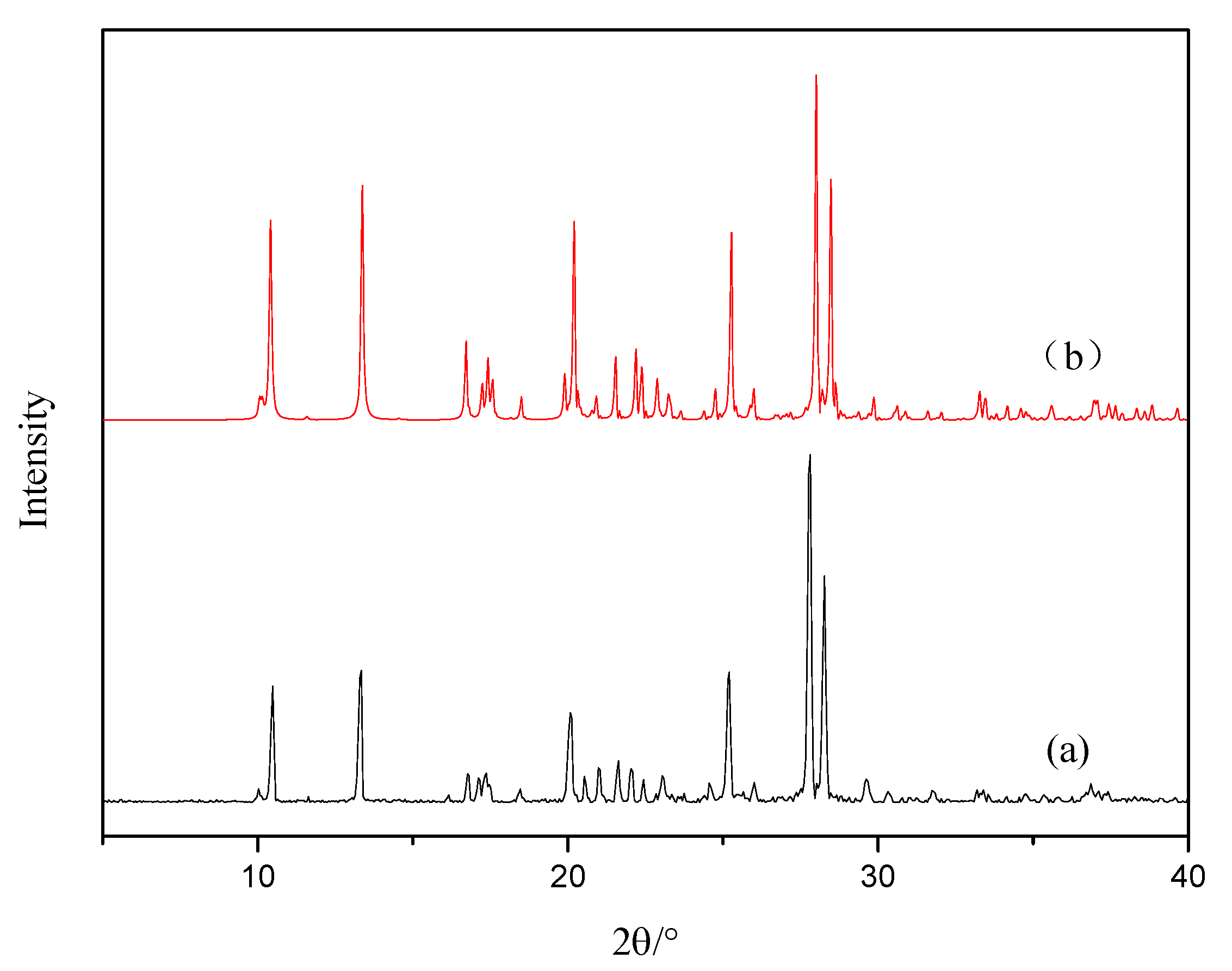
3.2. PXRD Analysis
3.3. Hirshfeld Surface Analysis
| Interaction | Picric Acid | Cocrystal 1 | Cocrystal 2 | Cocrystal 3 |
|---|---|---|---|---|
| O…O | 26.5 | 13.9 | 14.5 | 21.9 |
| O…H | 33.7 | 49.6 | 54.9 | 41.1 |
| O…C | 26.1 | 11.1 | 1.6 | 16.0 |
| O…N | 10.7 | 3.9 | 4.0 | 8.8 |
| H…H | 0.8 | 4.4 | 7.0 | 2.4 |
| H…C | 0.2 | 7.5 | 2.7 | 4.3 |
| C…C | 0 | 3.9 | 10.0 | 2.9 |
3.4. Binding Energy and Mulliken Charge
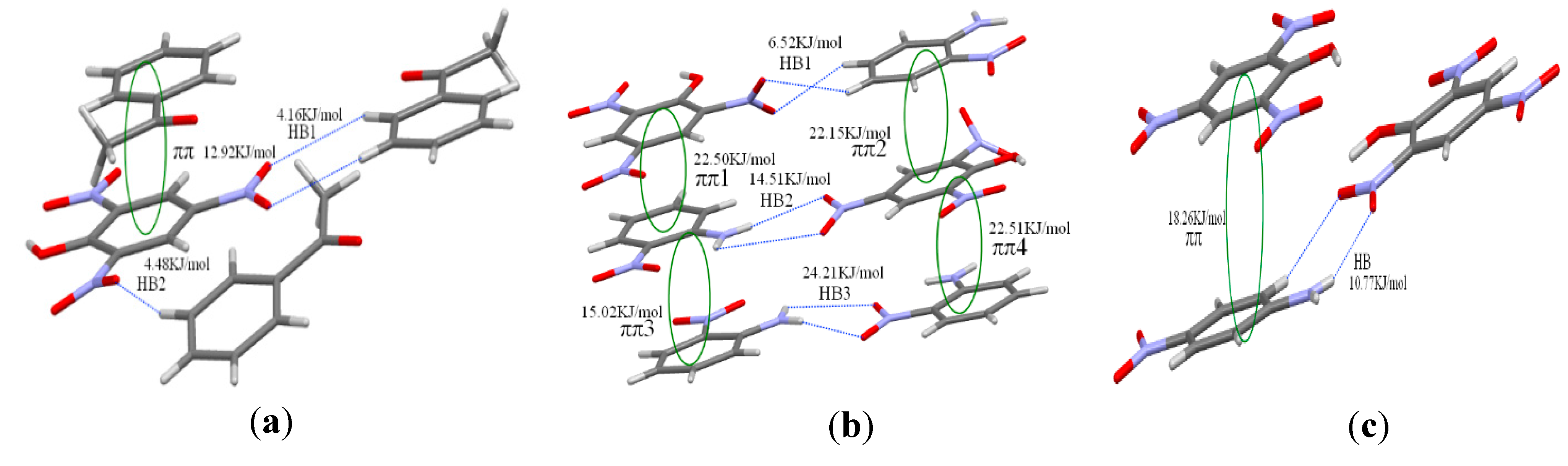
| Crystal | Interaction | Molecule | O1 | O2 | N | H1 | H2 |
|---|---|---|---|---|---|---|---|
| Cocrystal1 | HB1 | picric acid | –0.098 | –0.093 | 0.019 | 0.185 | 0.167 |
| complex | –0.003 | –0.004 | –0.174 | 0.183 | 0.170 | ||
| HB2 | picric acid | –0.093 | –0.092 | – | 0.139 | 0.169 | |
| complex | –0.066 | –0.069 | – | 0.162 | 0.221 | ||
| Cocrystal2 | HB1 | picric acid | –0.092 | –0.050 | –0.052 | 0.169 | 0.168 |
| complex | 0.006 | 0.039 | –0.248 | 0.178 | 0.128 | ||
| HB2 | picric acid | –0.098 | –0.093 | 0.019 | 0.178 | 0.136 | |
| complex | –0.022 | –0.019 | –0.159 | 0.258 | 0.157 | ||
| HB3 | picric acid | –0.148 | –0.194 | 0.059 | 0.136 | 0.178 | |
| complex | –0.080 | –0.066 | –0.154 | 0.185 | 0.272 | ||
| Cocrystal3 | HB1 | picric acid | –0.080 | –0.058 | –0.015 | 0.179 | 0.143 |
| complex | –0.033 | 0.023 | –0.255 | 0.244 | 0.129 |
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Lin, H.; Zhu, S.G.; Li, H.Z.; Peng, X.H. Synthesis, characterization, AIM and NBO analysis of HMX/DMI cocrystal explosive. J. Mol. Struct. 2013, 1048, 339–348. [Google Scholar] [CrossRef]
- Li, S.H.; Wang, Y.; Qi, C.; Zhao, X.X.; Zhang, J.C.; Zhang, S.W.; Pang, S.P. 3D energetic metal-organic frameworks: Synthesis and properties of high energy materials. Angew. Chem. Int. Ed. 2013, 52, 14031–14035. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Qi, C.; Li, S.H.; Zhang, H.J.; Sun, C.H.; Yu, Y.Z.; Pang, S.P. 1,1’-Azobis-1,2,3-triazole: A high-nitrogen compound with stable N8 structure and photochromism. J. Am. Chem. Soc. 2010, 132, 12172–12173. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.X.; Yang, H.W.; Cheng, G.B. Synthesis and characterization of a stable, catenated N11 energetic salt. Angew. Chem. Int. Ed. 2013, 52, 4875–4877. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Kim, K.J. Effect of supersaturation on the morphology of coated surface in coating by solution crystallization. Ind. Eng. Chem. Res. 2011, 50, 3475–3482. [Google Scholar] [CrossRef]
- Landenberger, K.B.; Matzger, A.J. Cocrystals of 1,3,5,7-tetranitro-1,3,5,7-tetrazacyclooctane(HMX). Cryst. Growth Des. 2012, 12, 3603–3609. [Google Scholar] [CrossRef]
- Yang, Z.W.; Li, H.Z.; Zhou, X.Q.; Zhang, C.Y. Characterization and properties of a novel energetic-energetic cocrystal explosive composed of HNIW and BTF. Cryst. Growth Des. 2012, 12, 5155–5158. [Google Scholar] [CrossRef]
- Landenberger, K.B.; Bolton, O.; Matzger, A.J. Two isostructural explosive cocrystals with significantly different thermodynamic stabilities. Angew. Chem. Int. Ed. 2013, 52, 6468–6471. [Google Scholar] [CrossRef] [PubMed]
- Landenberger, K.B.; Matzger, A.J. Cocrystal engineering of a prototype energetic material: Supramolecular chemistry of 2,4,6-trinitrotoluene. Cryst. Growth Des. 2012, 10, 5341–5347. [Google Scholar] [CrossRef]
- Bolton, O.; Matzger, A.J. Improved stability and smart-material functionality realized in an energetic cocrystal. Angew. Chem. Int. Ed. 2011, 50, 8960–8963. [Google Scholar] [CrossRef] [PubMed]
- Bolton, O.; Simke, L.R.; Matzger, A.J. High power explosive with good sensitivity: A 2:1 cocrystal of CL-20:HMX. Cryst. Growth Des. 2012, 12, 4311–4314. [Google Scholar] [CrossRef]
- Zhang, H.B.; Guo, C.Y.; Wang, X.C.; Xu, J.J.; He, X.; Liu, Y.; Liu, X.F.; Huang, H.; Sun, J. Five energetic cocrystals of BTF by intermolecular hydrogen bond and π-stacking interactions. Cryst. Growth Des. 2013, 2, 679–687. [Google Scholar] [CrossRef]
- Dobrowolska, W.S.; Bator, G.; Sobczyk, L. The (2:1) complex of picric acid with tetramethylpyrazine: The structure, IR spectra and tunnel splitting of methyl groups. J. Mol. Struct. 2010, 975, 298–302. [Google Scholar] [CrossRef]
- Bertolasi, V.; Gilli, P.; Gilli, G. Hydrogen bonding and Electron Donor-Acceptor (EDA) interactions controlling the crystal packing of picric acid and its adducts with nitrogen bases. Their rationalization in terms of the pKa equalization and electron-pair saturation concepts. Cryst. Growth Des. 2011, 11, 2724–2735. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.X.; Gong, X.D. 2,4-Diazido-5-iodo-pyrimidine crystal under high pressure: A comparison of DFT and DFT-D studies. Comput. Theor. Chem. 2012, 1000, 60–69. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.C.; Wang, G.X.; Gong, X.D. Comparative theoretical studies of high pressure effect on polymorph I of 2,2’,4,4’,6,6’-hexanitroazobenzene crystal. Struct. Chem. 2012, 23, 1631–1642. [Google Scholar] [CrossRef]
- Saminathan, K.; Sivakumar, K. A 2:1 complex of 2-nitro-aniline and picric acid. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, 354–356. [Google Scholar] [CrossRef]
- Thakuria, H.; Borah, B.M.; Pramanik, A.; Das, G. Solid state synthesis and hierarchical supramolecular self-assembly of organic salt cocrystals. J. Chem. Crystallogr. 2007, 37, 807–816. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Foundations of crystallography. J. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C. A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Xue, X.G.; Cao, Y.F.; Zhou, Y.; Li, H.Z.; Zhou, J.H.; Gao, T. Intermolecular friction symbol derived from crystal information. Cryst. Eng. Comm. 2013, 15, 6837–6844. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. Cryst. Eng. Comm. 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. Cryst. Eng. Comm. 2012, 4, 378–392. [Google Scholar] [CrossRef]
- Lorenzo, I.; Graña, A.M. Stacking and hydrogen bond interactions between adenine and gallic acid. J. Mol. Model. 2013, 19, 5293–5299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y. Review of the establishment of nitro group charge method and its applications. J. Hazard. Mater. 2009, 161, 21–28. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-Y.; Zhang, L.; Zhu, S.-G.; Cheng, G.-B. A Comparative Theoretical Study of Picric Acid and Its Cocrystals. Crystals 2015, 5, 346-354. https://doi.org/10.3390/cryst5030346
Chen P-Y, Zhang L, Zhu S-G, Cheng G-B. A Comparative Theoretical Study of Picric Acid and Its Cocrystals. Crystals. 2015; 5(3):346-354. https://doi.org/10.3390/cryst5030346
Chicago/Turabian StyleChen, Peng-Yuan, Lin Zhang, Shun-Guan Zhu, and Guang-Bin Cheng. 2015. "A Comparative Theoretical Study of Picric Acid and Its Cocrystals" Crystals 5, no. 3: 346-354. https://doi.org/10.3390/cryst5030346
APA StyleChen, P.-Y., Zhang, L., Zhu, S.-G., & Cheng, G.-B. (2015). A Comparative Theoretical Study of Picric Acid and Its Cocrystals. Crystals, 5(3), 346-354. https://doi.org/10.3390/cryst5030346





