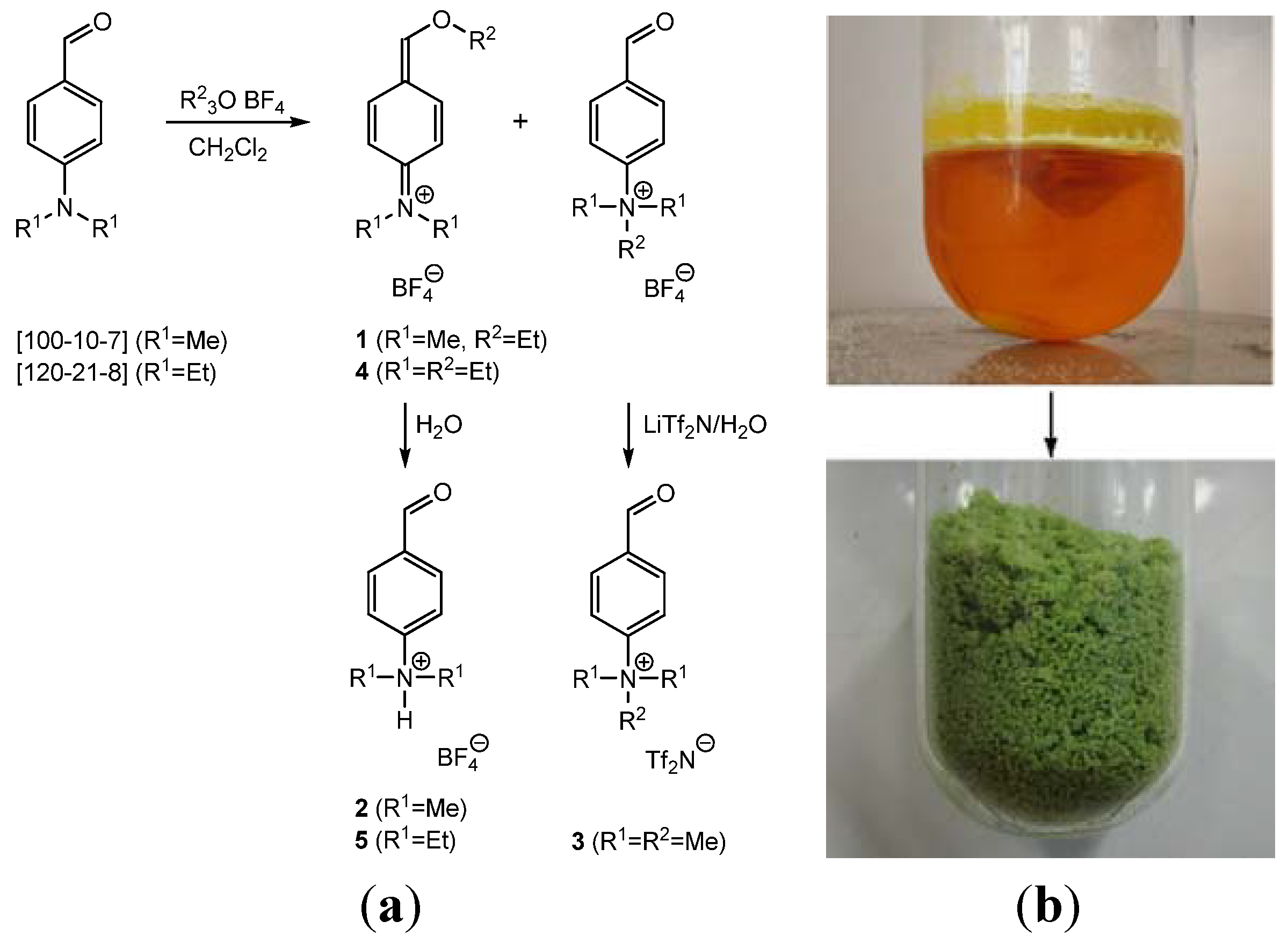
Iminium Salts by Meerwein Alkylation of Ehrlich’s Aldehyde
Abstract
:1. Introduction
2. Results and Discussion
| Compound | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Chemical formula | C11H16NO·BF4 | C9H12NO·BF4 | C10H14NO·C2F6NO4S2 | C13H20NO·BF4 | C11H16NO·BF4 |
| Mr | 265.06 | 237.01 | 444.37 | 293.11 | 265.06 |
| Crystal system | Monoclinic | Monoclinic | Triclinic | Triclinic | Triclinic |
| Space group | C2/c | P21/n | P  | P  | P  |
| a/Å | 18.9425 (5) | 8.4727 (4) | 7.7374 (4) | 8.1170 (12) | 7.2055 (5) |
| b/Å | 7.4564 (3) | 13.5659 (6) | 10.3506 (7) | 9.2368 (16) | 9.0529 (6) |
| c/Å | 21.134 (1) | 10.4411 (5) | 12.6004 (8) | 10.6937 (17) | 10.2060 (7) |
| α/° | 90 | 90 | 72.577 (6) | 67.069 (15) | 94.378 (6) |
| β/° | 118.979 (7) | 109.730 (6) | 73.231 (5) | 77.760 (13) | 90.403 (6) |
| γ/° | 90 | 90 | 72.301 (9) | 89.080 (13) | 97.839 (5) |
| V/Å3 | 2611.3 (2) | 1129.64 (9) | 895.34 (10) | 719.6 (2) | 657.50 (8) |
| Z | 8 | 4 | 2 | 2 | 2 |
| Dx/g·cm−3 | 1.35 | 1.39 | 1.65 | 1.35 | 1.34 |
| μ/mm−1 | 1.08 | 0.13 | 0.39 | 1.03 | 0.12 |
| Crystal size/mm3 | 0.32 × 0.24 × 0.24 | 0.32 × 0.32 × 0.02 | 0.28 × 0.16 × 0.12 | 0.20 × 0.20 × 0.12 | 0.32 × 0.2 × 0.08 |
| F(000)/e | 1104 | 488 | 452 | 308 | 276 |
| Θ range/° | 4.8–67.6 | 2.6–25.2 | 2.8–25.4 | 4.6–67.5 | 3.2–25.3 |
| h, k, l range | –22 ≤ h ≤ 16 | –10 ≤ h ≤ 10 | –7 ≤ h ≤ 9 | –9 ≤ h ≤ 9 | –8 ≤ h ≤ 7 |
| –8 ≤ k ≤ 8 | –16 ≤ k ≤ 16 | –11 ≤ k ≤ 12 | –11 ≤ k ≤ 10 | –10 ≤ k ≤ 10 | |
| –19 ≤ l ≤ 25 | –11 ≤ l ≤ 12 | –14 ≤ l ≤ 15 | –10 ≤ l ≤ 12 | –12 ≤ l ≤ 9 | |
| Measured reflections | 7103 | 10599 | 5493 | 4283 | 4097 |
| Independent reflections (Rint) | 2337 (0.022) | 2020 (0.033) | 3248 (0.019) | 2421 (0.027) | 2369 (0.025) |
| Observed reflections [I ≥ 2σ(I)] | 1902 | 1641 | 2529 | 1928 | 1893 |
| Restraints/parameters | 21/185 | 0/153 | 0/251 | 0/188 | 132/261 |
| R1/wR2 [I ≥ 2σ(I)] | 0.059/0.172 | 0.039/0.11 | 0.029/0.067 | 0.043/0.108 | 0.039/0.084 |
| R1/wR2 (all data) | 0.069/0.181 | 0.048/0.116 | 0.039/0.071 | 0.055/0.119 | 0.053/0.093 |
| ∆ρmax/min/e Å−3 | 0.50/–0.33 | 0.32/–0.28 | 0.35/–0.34 | 0.18/–0.20 | 0.17/–0.17 |
2.1. N-(4-(Ethoxymethylene)cyclohexa-2,5-dienylidene)-N,N-dimethylammonium Tetrafluoroborate (1)

2.2. 4-(Dimethylamino)benzaldehyde hydrotetrafluoroborate (2)
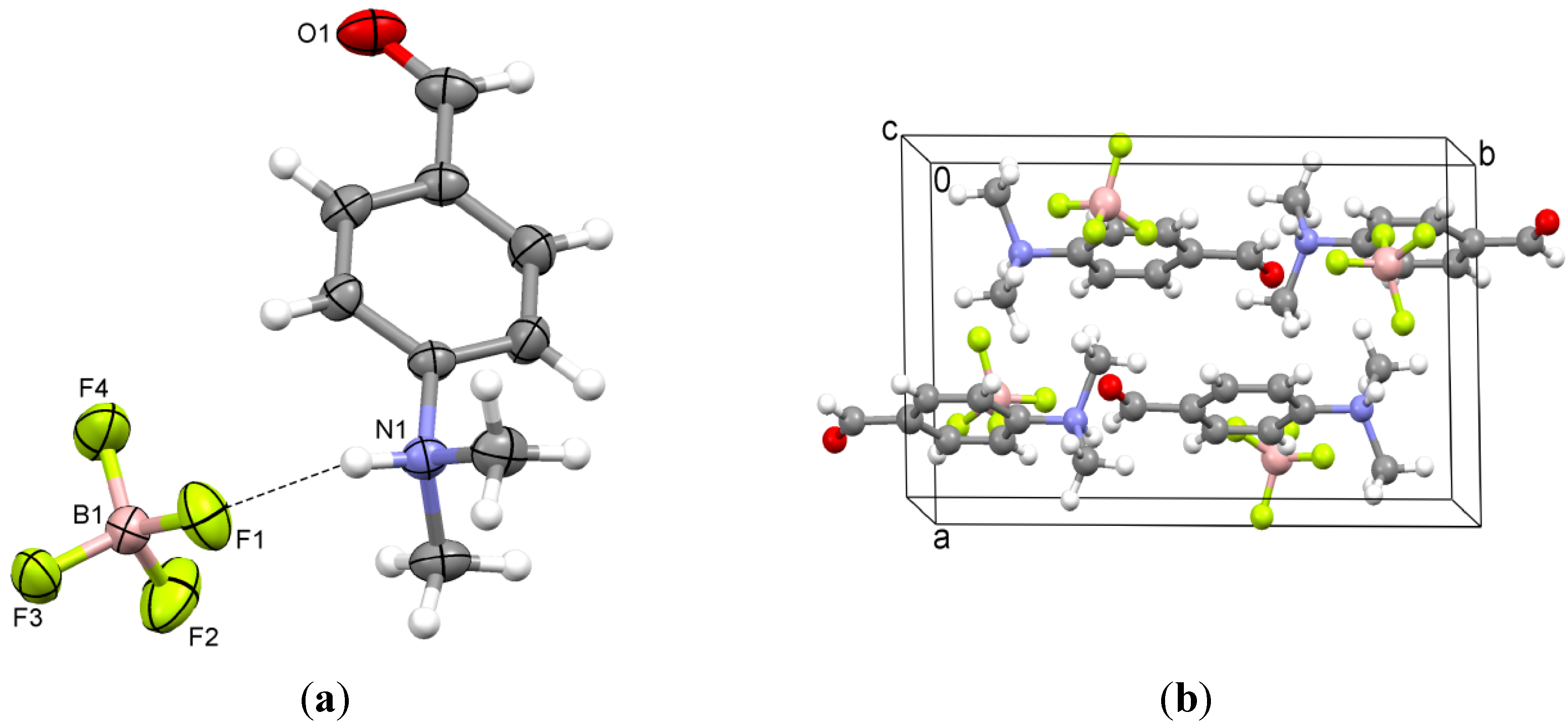
2.3. 4-(Trimethylammonio)benzaldehyde bis(trifluoromethylsulfonyl)imide (3)
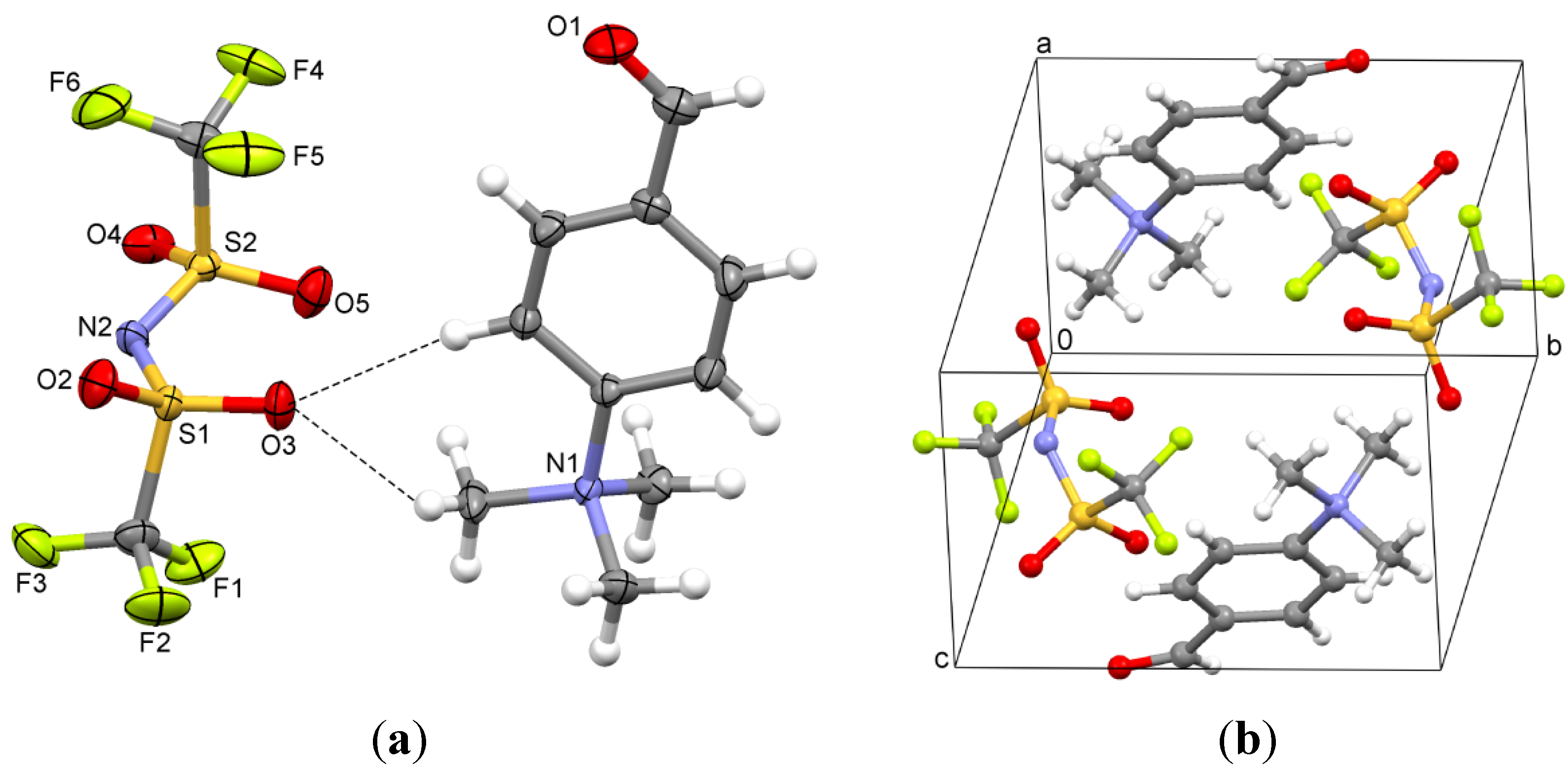
2.4. N-(4-(Ethoxymethylene)cyclohexa-2,5-dienylidene)-N,N-diethylammonium Tetrafluoroborate (4)

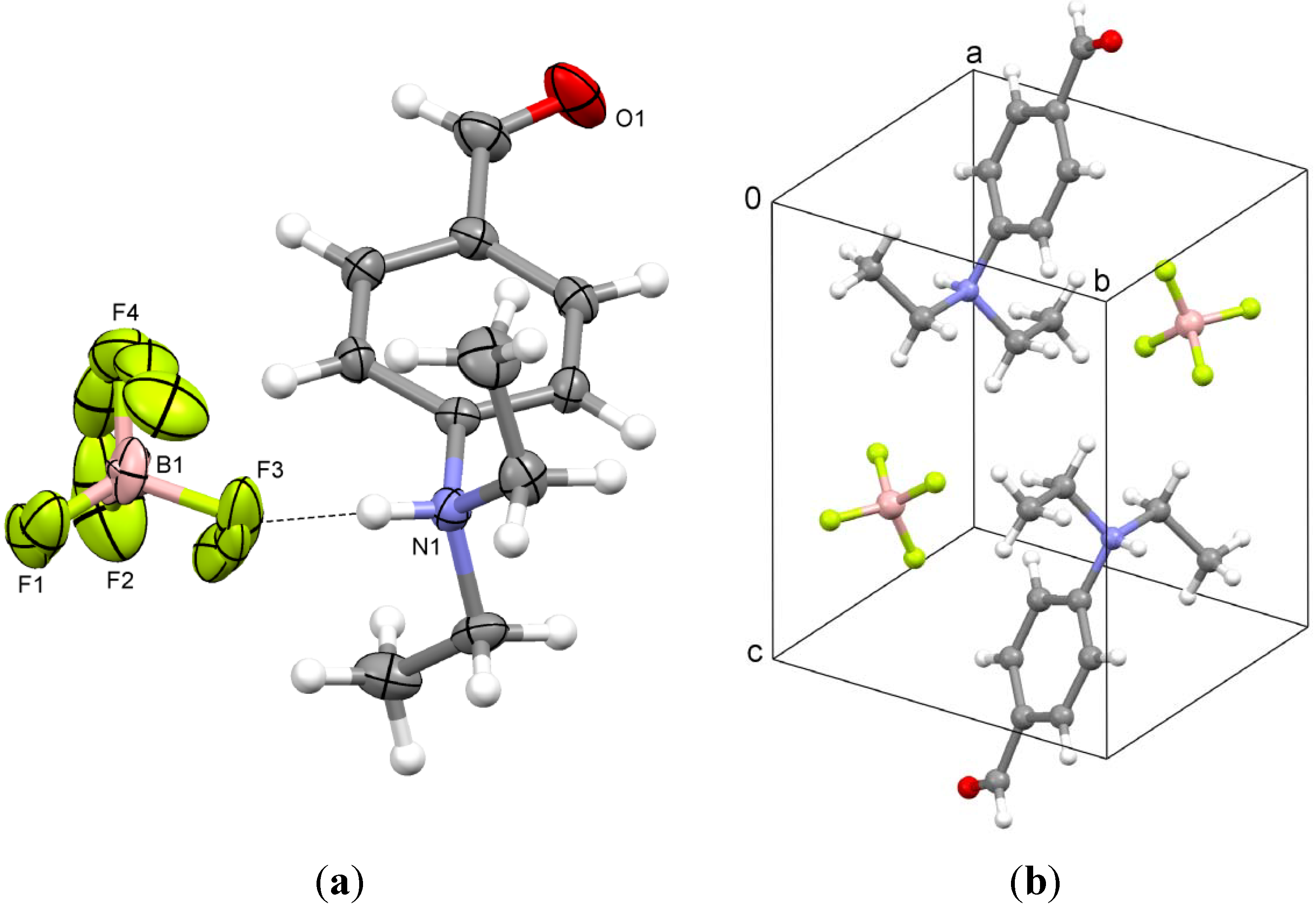
2.5. 4-(Diethylamino)benzaldehyde Hydrotetrafluoroborate (5)
3. Experimental Section
3.1. N-(4-(Ethoxymethylene)cyclohexa-2,5-dienylidene)-N,N-dimethylammonium Tetrafluoroborate (1) and 4-(Dimethylamino)benzaldehyde Hydrotetrafluoroborate (2)
3.2. 4-(Trimethylammonio)benzaldehyde Bis(trifluoromethylsulfonyl)imide (3)
3.3. N-(4-(Ethoxymethylene)cyclohexa-2,5-dienylidene)-N,N-diethylammonium Tetrafluoroborate (4)
3.4. 4-(Diethylamino)benzaldehyde Hydrotetrafluoroborate (5)
4. Conclusions
Acknowledgments
References
- Fahim, H.A.; Galaby, M. Quaternary ammonium salts. The formation and decomposition of ethyldimethylanilinium salts. The synthesis of N-ethyl-N-methylanilines. J. Chem. Soc. 1950, 1950, 3529–3532. [Google Scholar] [CrossRef]
- Yuan, H.; Zhou, Z.; Xiao, J.; Liang, L.; Dai, L. Preparation of quarternary ammonium salt-tagged ferrocenylphosphine-imine ligands and their application to palladium-catalyzed asymmetric allylic substitution. Tetrahedron Asymmetry 2010, 21, 1874–1884. [Google Scholar] [CrossRef]
- Royer, J.; Bonin, M.; Micouin, L. Chiral heterocycles by iminium ion cyclization. Chem. Rev. 2004, 104, 2311–2352. [Google Scholar] [CrossRef]
- El Kaim, L.; Grimaud, L. Beyond the Ugi reaction: Less conventional interactions between isocyanides and iminium species. Tetrahedron 2009, 65, 2153–2171. [Google Scholar] [CrossRef]
- Brown, E.R. Quinonediimines, monoimines, and related compounds. In Chemistry of Quinonoid Compounds; Patai, S., Rappoport, Z., Eds.; Wiley: Chichester, UK, 1988; pp. 1231–1292. [Google Scholar]
- Meerwein, H.; Florian, W.; Schön, N.; Stopp, G. Acid amide acetals, urea acetals, and lactam acetals (in German). Ann. Chem. 1961, 641, 1–39. [Google Scholar] [CrossRef]
- Emde, H.; Götz, A.; Hofmann, K.; Simchen, G. Reaktionen der Trialkylsilyl-trifluormethansulfonate, I. Synthese von Trialkylsilyl-Enolethern (in German). Ann. Chem. 1981, 1981, 1643–1657. [Google Scholar]
- Elslager, E.F.; Haley, N.F.; McLean, J.R.; Perricone, S.C.; Potoczak, D.; Veloso, H.; Worth, D.F.; Wheelock, R.H. Inhibitors of platelet aggregation. 2. 9-{[(Dialkylamino)alkyl]thio]-3-(dimethylamino)acridines and related acridine derivatives. J. Med. Chem. 1971, 14, 782–788. [Google Scholar] [CrossRef]
- Bruus-Jensen, K.; Poethko, T.; Schottelius, M.; Hauser, A.; Schwaiger, M.; Wester, H.-J. Chemoselective hydrazone formation between HYNIC-functionalized peptides and 18F-fluorinated aldehydes. Nucl. Med. Biol. 2006, 33, 173–183. [Google Scholar] [CrossRef]
- Poethko, T.; Schottelius, M.; Thumshirn, G.; Hersel, U.; Herz, M.; Henriksen, G.; Kessler, H.; Schwaiger, M.; Wester, H.-J. Two-step methodology for high-yield routine radiohalogenation of peptides: 18F-labeled RGD and octreotide analogs. J. Nucl. Med. 2004, 45, 892–902. [Google Scholar]
- Shamsuri, A.A.; Abdullah, D.K. Protonation and complexation approaches for production of protic eutectic ionic liquids. J. Phys. Sci. 2010, 21, 15–28. [Google Scholar]
- Dattagupta, J.K.; Saha, N.N. The crystal structure of p-dimethylaminobenzaldehyde hydrobromide. Acta Crystallogr. 1973, B29, 1228–1233. [Google Scholar]
- Thakuria, H.; Borah, B.M.; Pramanik, A.; Das, G. Solid state synthesis and hierarchical supramolecular self-assembly of organic salt cocrystals. J. Chem. Cryst. 2007, 37, 807–816. [Google Scholar] [CrossRef]
- Laus, G.; Hummel, M.; Többens, D.M.; Gelbrich, T.; Kahlenberg, V.; Wurst, K.; Griesser, U.J.; Schottenberger, H. The 1:1 and 1:2 salts of 1,4-diazabicyclo[2.2.2]octane and bis(trifluoromethyl-sulfonyl)amine: Thermal behaviour and polymorphism. CrystEngComm 2011, 13, 5439–5446. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Froschauer, C.; Weber, H.K.; Kahlenberg, V.; Laus, G.; Schottenberger, H. Iminium Salts by Meerwein Alkylation of Ehrlich’s Aldehyde. Crystals 2013, 3, 248-256. https://doi.org/10.3390/cryst3010248
Froschauer C, Weber HK, Kahlenberg V, Laus G, Schottenberger H. Iminium Salts by Meerwein Alkylation of Ehrlich’s Aldehyde. Crystals. 2013; 3(1):248-256. https://doi.org/10.3390/cryst3010248
Chicago/Turabian StyleFroschauer, Carmen, Hedda K. Weber, Volker Kahlenberg, Gerhard Laus, and Herwig Schottenberger. 2013. "Iminium Salts by Meerwein Alkylation of Ehrlich’s Aldehyde" Crystals 3, no. 1: 248-256. https://doi.org/10.3390/cryst3010248
APA StyleFroschauer, C., Weber, H. K., Kahlenberg, V., Laus, G., & Schottenberger, H. (2013). Iminium Salts by Meerwein Alkylation of Ehrlich’s Aldehyde. Crystals, 3(1), 248-256. https://doi.org/10.3390/cryst3010248





