Recent Advances in the Raman Investigation of Structural and Optical Properties of Graphene and Other Two-Dimensional Materials
Abstract
:1. Introduction
2. Structure of Graphene, Graphene Oxide, and Reduced Graphene Oxide
3. State of the Art
4. Materials and Methods
5. Results and Discussion
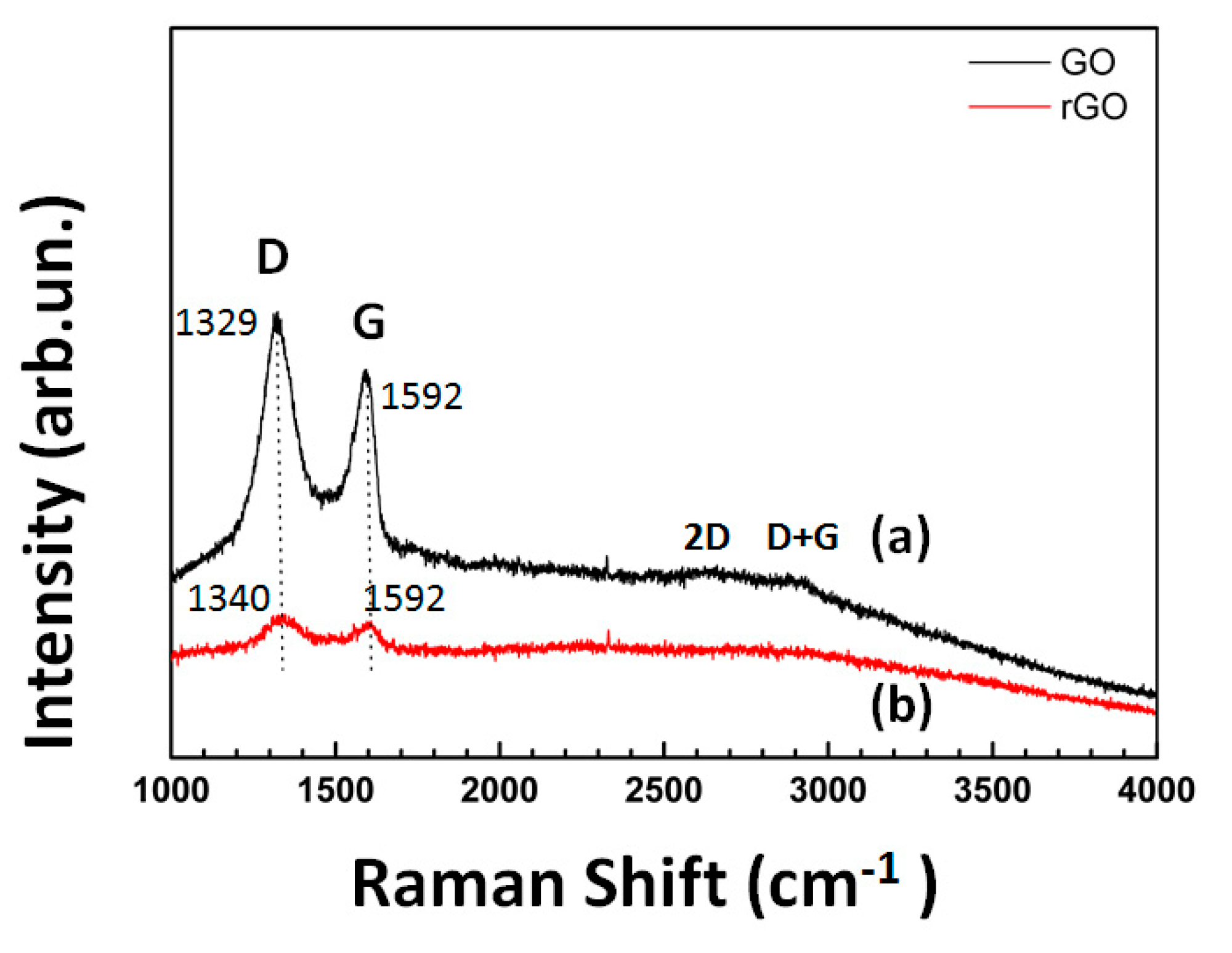
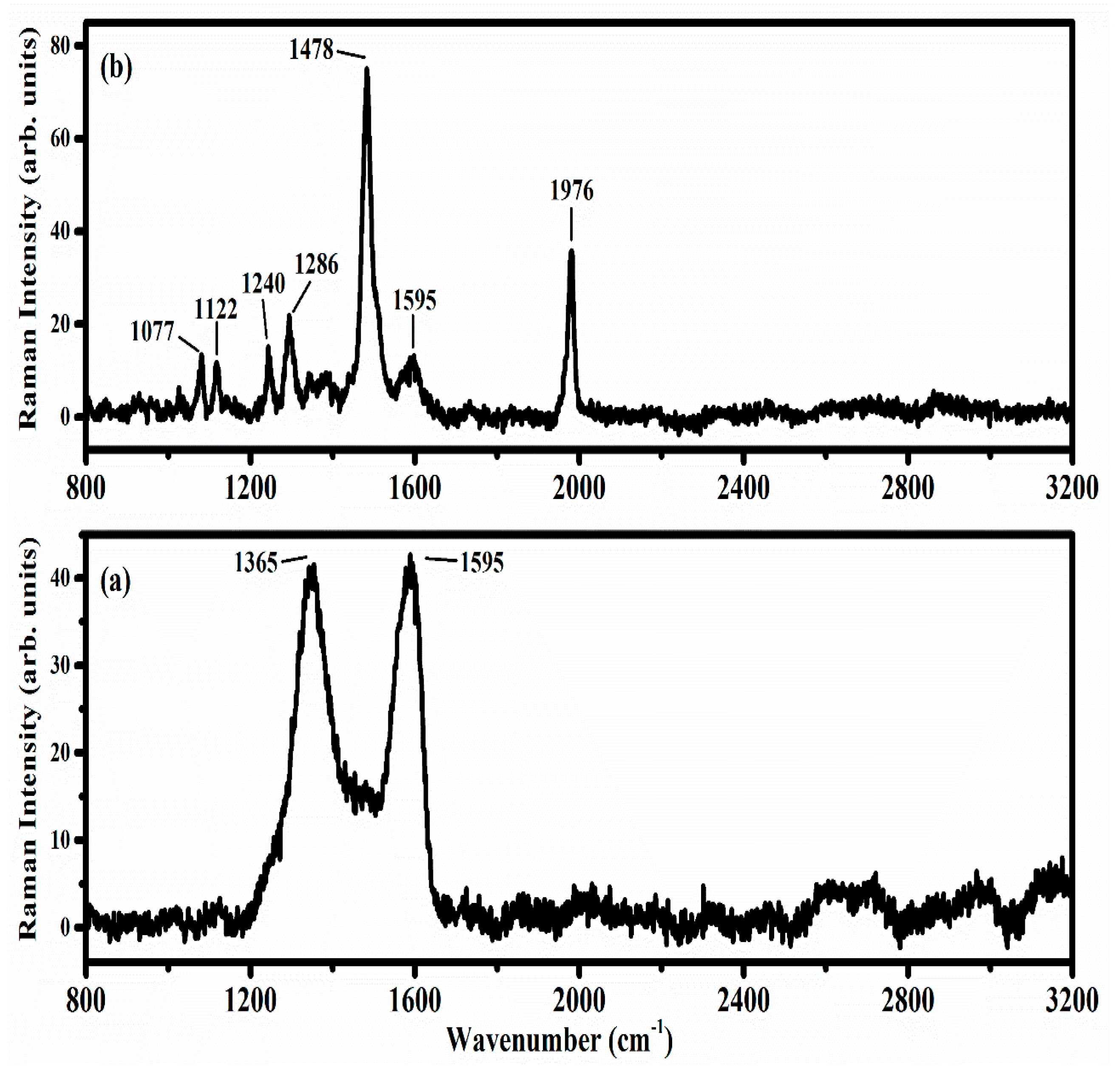
5.1. Thin Films of Graphene Oxide and Reduced Graphene Oxide Deposited by Electrophoresis
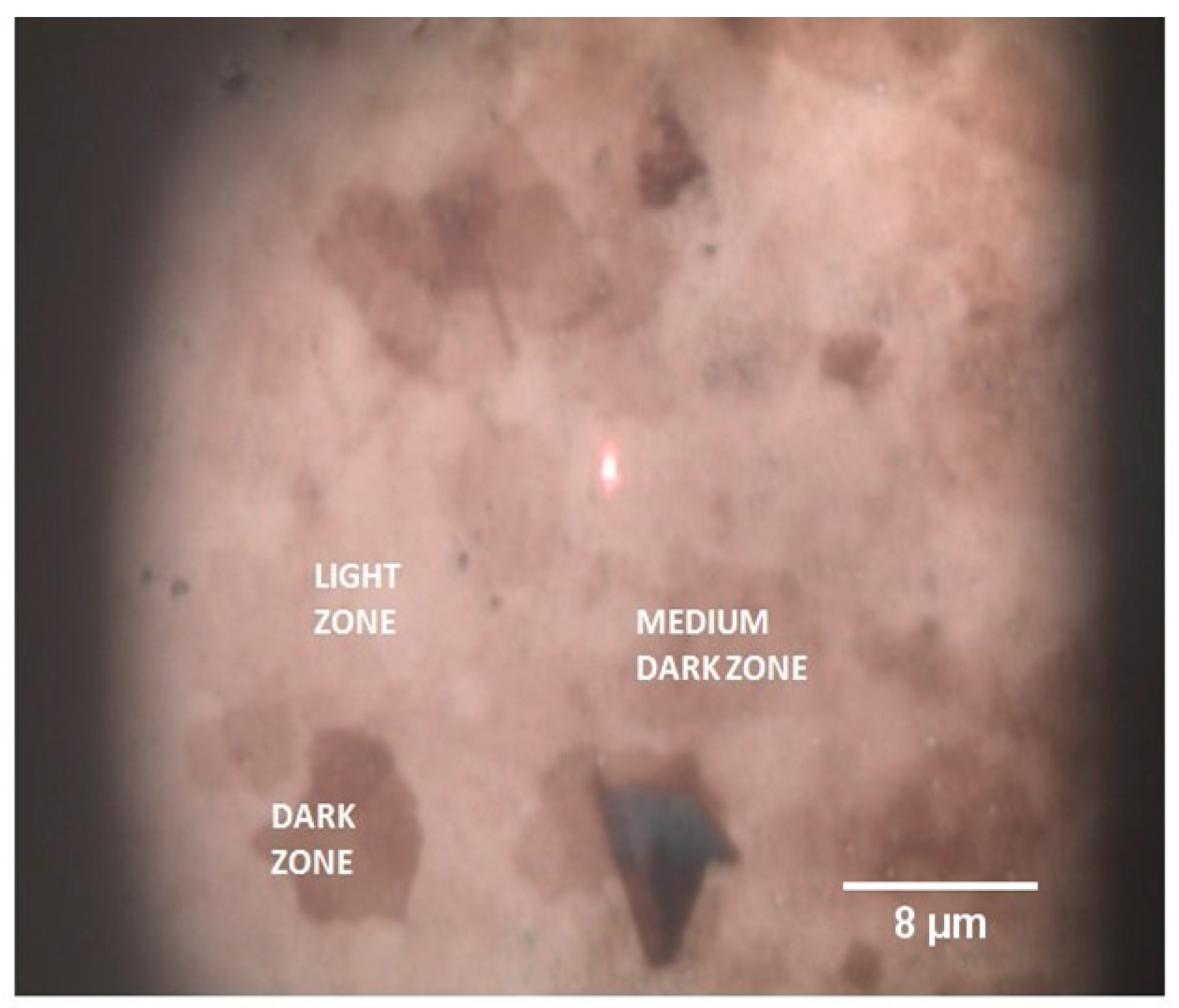
5.2. Graphene Oxide on Magnetron-Sputtered Silver Thin Films for Surface-Enhanced Raman Spectroscopy Applications
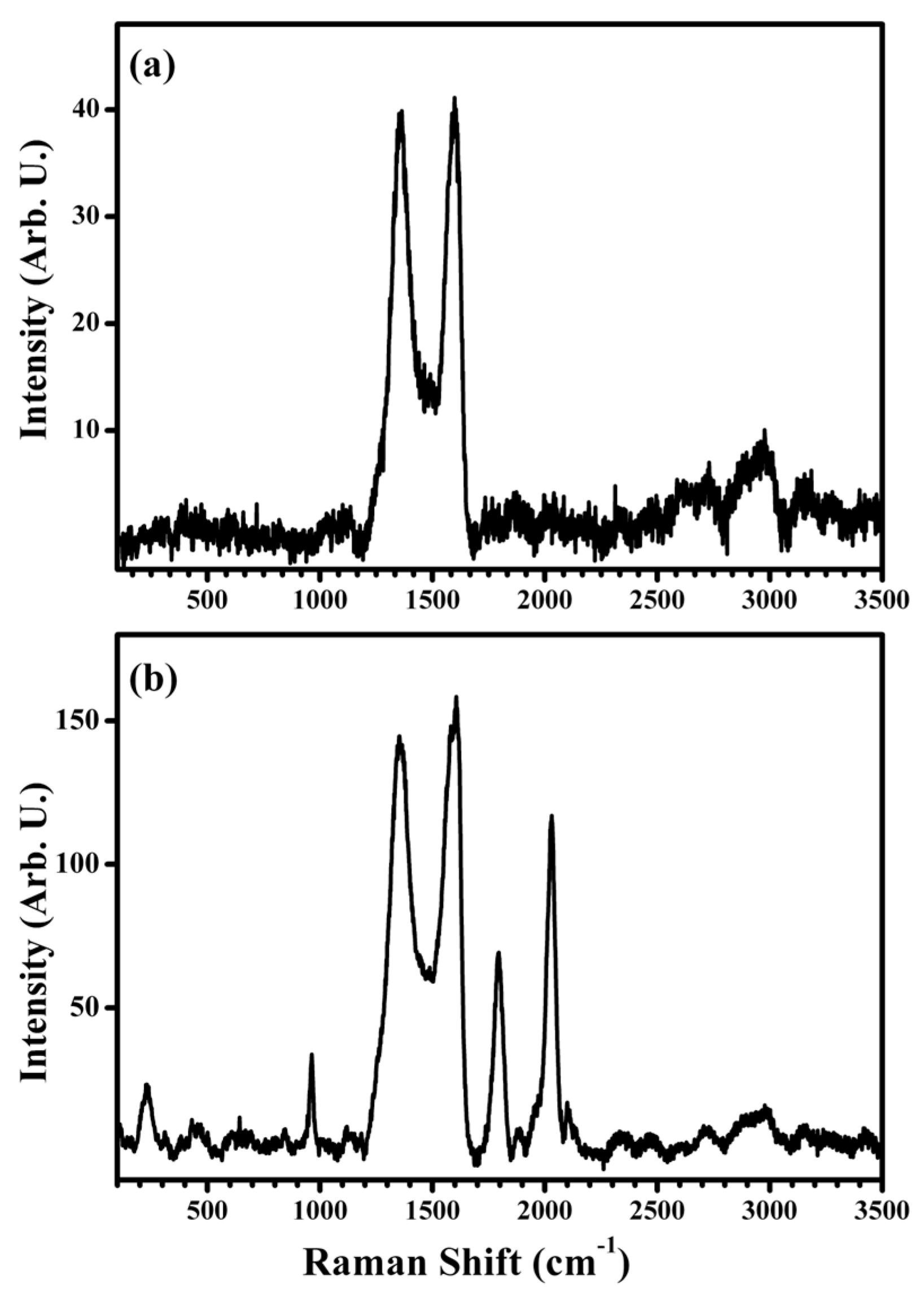
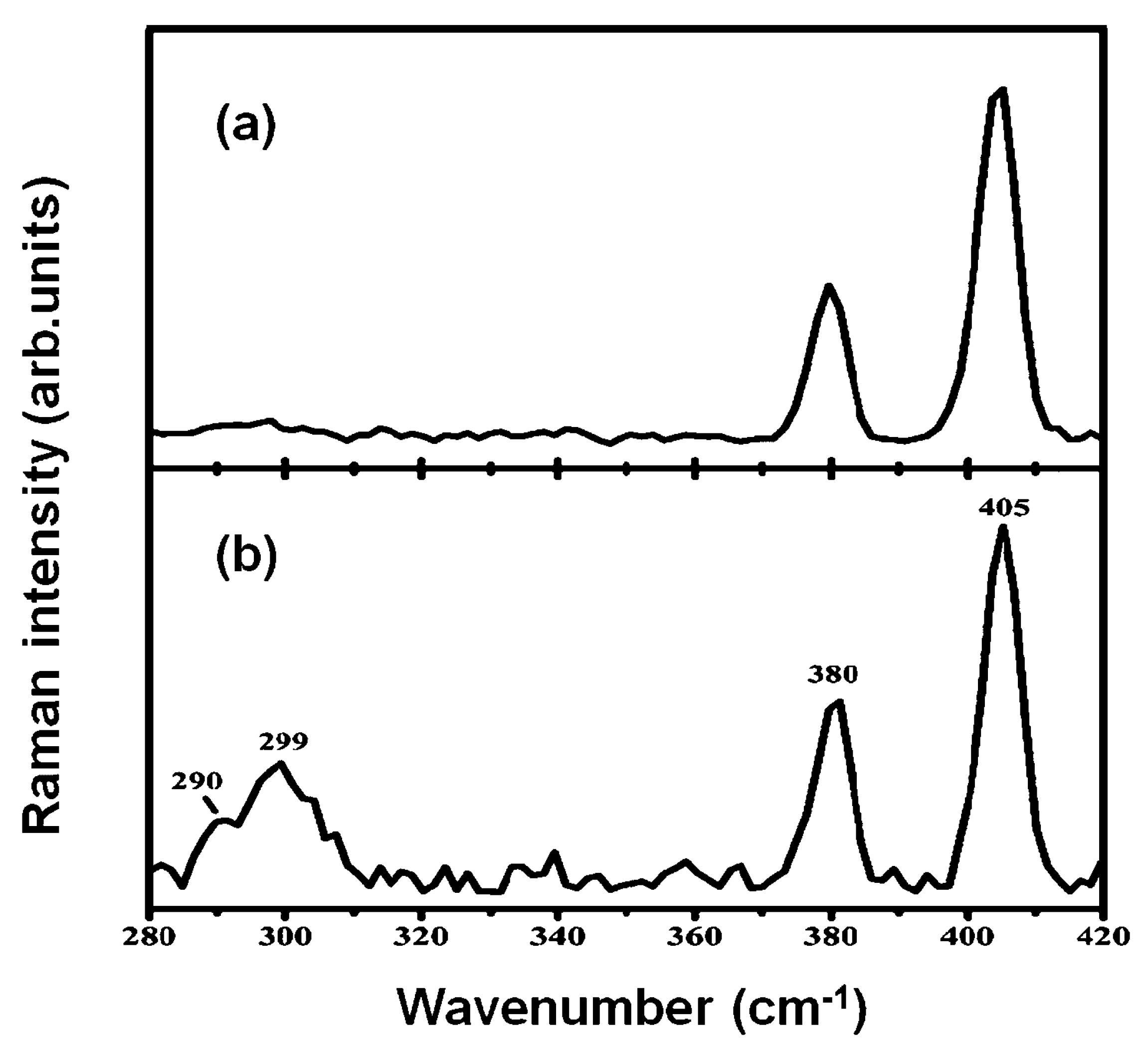
5.3. Micro-Raman Spectroscopy Measurements of Spin-Coated MoS2 Films
5.4. Micro-Raman Spectroscopy Measurements of Graphene Nanoplatelets
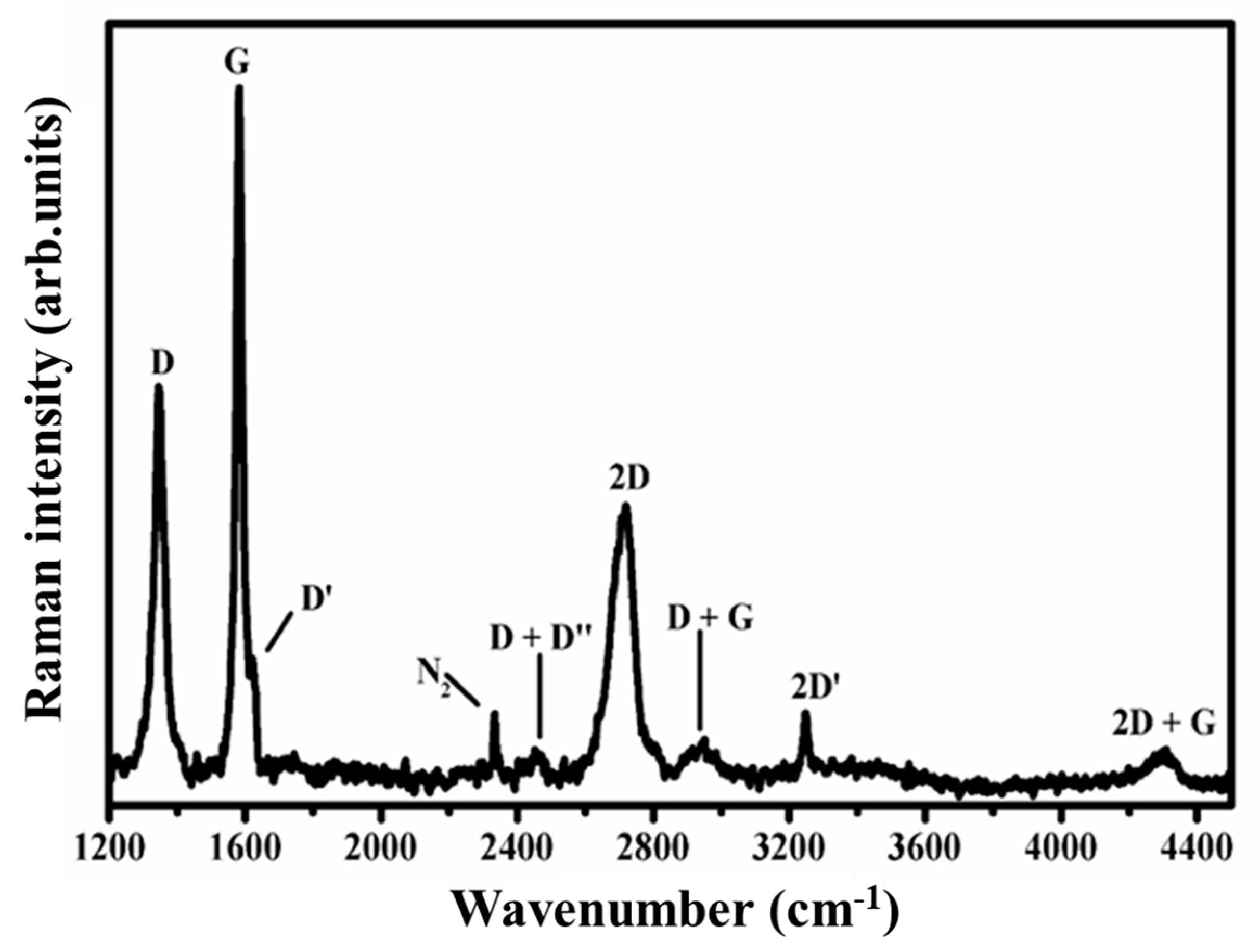
5.5. Micro-Raman Spectroscopy Measurements of CVD-Grown Monolayer Graphene
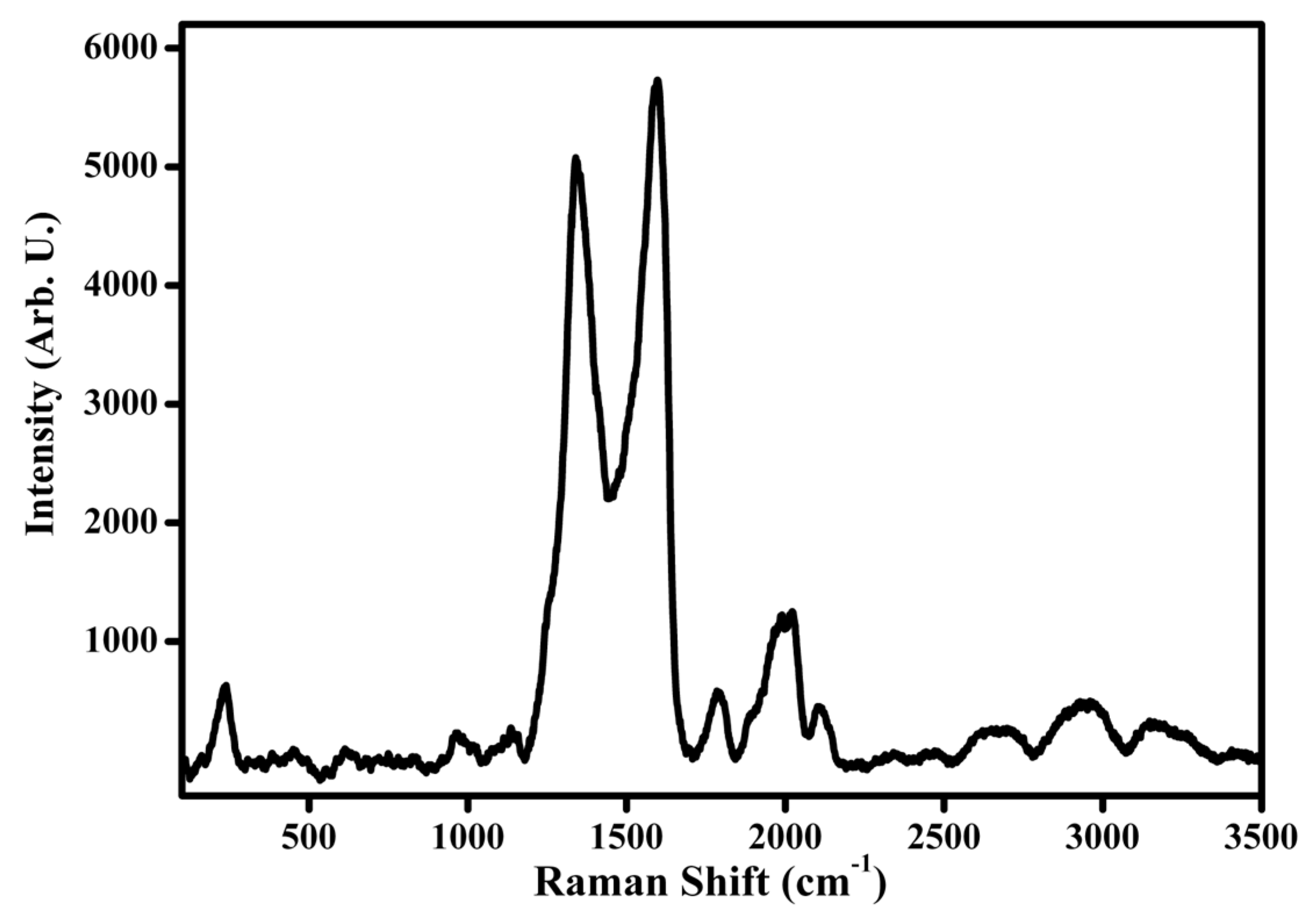
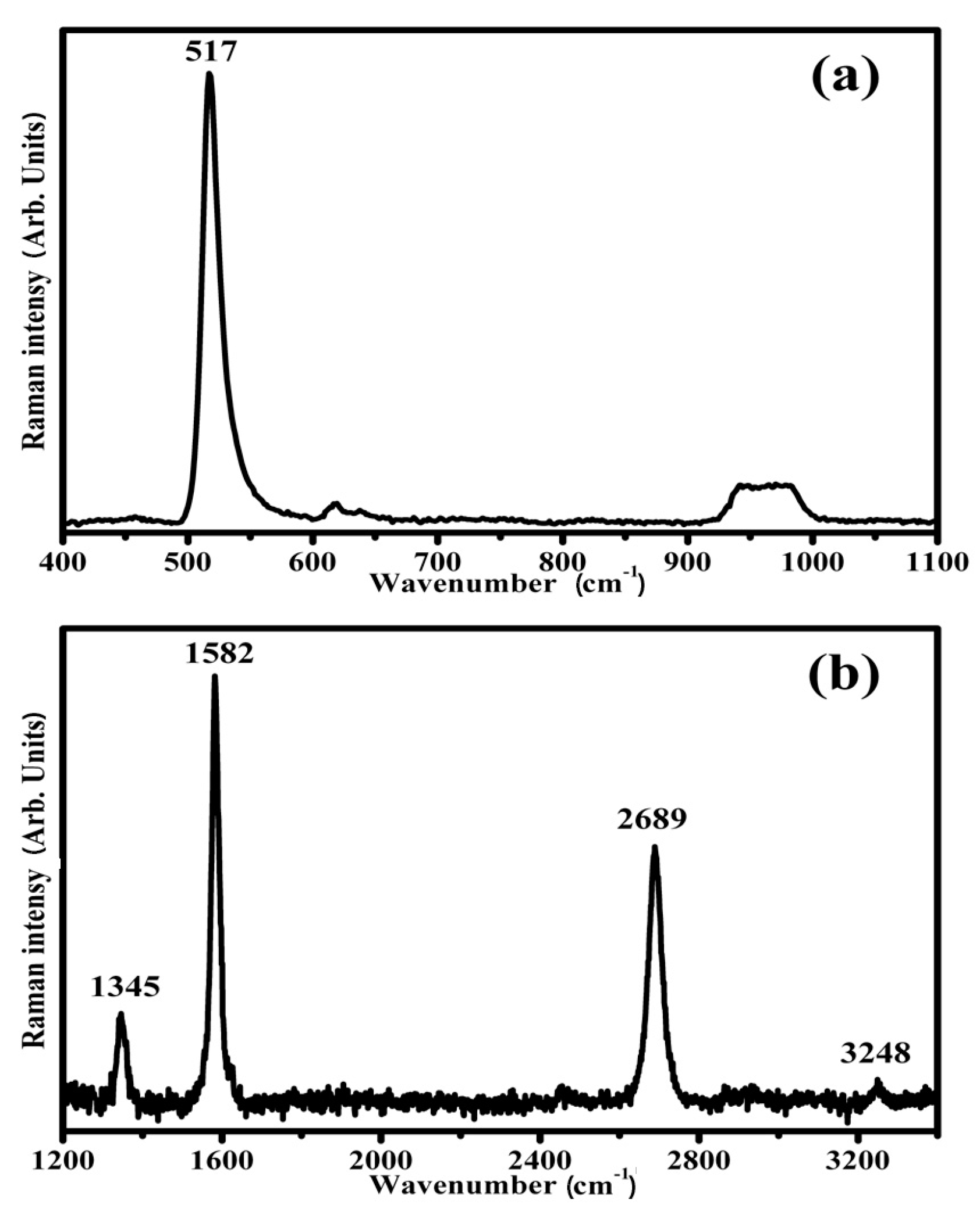
5.6. Micro-Raman Spectroscopy Measurements of Ag/GO/Au Sandwich Structure
6. Conclusions, Applications, and Outlook
6.1. Conclusions
- GO Thin Films on Ti/Glass Substrates: We used micro-Raman spectroscopy to study the properties of GO layers that were deposited on titanium substrates through electrophoretic deposition. This technique proved invaluable for gaining insights into the structural and optical characteristics of the GO layers.
- GO Thin Films on Magnetron-Sputtered Silver: A significant portion of the review is dedicated to the study of GO thin films fabricated by dip-coating onto magnetron-sputtered silver substrates. Micro-Raman analysis reveals that these films exhibit SERS activity.
- Optical Properties of MoS2: This review also includes an exploration of the optical properties of solution-based MoS2, with micro-Raman spectroscopy employed for its characterization.
- Investigation of GNPs: This review includes a section on the investigation of GNPs.
- Monolayer Graphene on SiO2/Si Substrates: The optical properties of monolayer graphene samples grown via CVD on SiO2/Si substrates are discussed.
- Silver/GO/Gold Sandwich Structure: The review reports on the micro-Raman investigation of a silver/GO/gold sandwich structure. Notably, sharp Raman modes have been observed, sometimes even exhibiting greater intensity than typical GO bands, particularly under low laser power conditions.
6.2. Applications of Graphene and Other Two-Dimensional Materials
6.3. Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, H.; Zhang, X. Sodium Intercalation in Nitrogen-Doped Graphene-Based Anode: A First-Principles Study. Crystals 2023, 13, 1011. [Google Scholar] [CrossRef]
- Sultana, S.M.N.; Helal, E.; Gutiérrez, G.; David, E.; Moghimian, N.; Demarquette, N.R. Effect of Few-Layer Graphene on the Properties of Mixed Polyolefin Waste Stream. Crystals 2023, 13, 358. [Google Scholar] [CrossRef]
- Sartanavičius, A.; Žemgulytė, J.; Ragulis, P.; Ratautas, K.; Trusovas, R. Laser-Induced Graphene in Polyimide for Antenna Applications. Crystals 2023, 13, 1003. [Google Scholar] [CrossRef]
- Zhu, C.; Lin, Z.; Zhang, D.; Shi, J.; Peng, S.; Jin, Z. Predicting the Level of Background Current Noise in Graphene Biosensor through a Non-Covalent Functionalization Process. Crystals 2023, 13, 359. [Google Scholar] [CrossRef]
- Le, H.C.; Pham, N.T.; Vu, D.C.; Pham, D.L.; Nguyen, S.H.; Nguyen, T.T.; Nguyen, C.D. Nitrogen-Doped Graphene Quantum Dot–Tin Dioxide Nanocomposite Ultrathin Films as Efficient Electron Transport Layers for Planar Perovskite Solar Cells. Crystals 2023, 13, 961. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, B.; Rente, B.; Alford, N.; Petrov, P.K. Deposition of Nanocrystalline Multilayer Graphene Using Pulsed Laser Deposition. Crystals 2023, 13, 881. [Google Scholar] [CrossRef]
- Hui, Y.; Zu, H.; Song, R.; Fu, H.; Luo, K.; Tian, C.; Wu, B.; Huang, G.-L.; Kou, Z.; Cheng, X.; et al. Graphene-Assembled Film-Based Reconfigurable Filtering Antenna with Enhanced Corrosion-Resistance. Crystals 2023, 13, 747. [Google Scholar] [CrossRef]
- Jibin, K.P.; Augustine, S.; Velayudhan, P.; George, J.S.; Krishnageham Sidharthan, S.; Poulose, S.V.; Thomas, S. Unleashing the Power of Graphene-Based Nanomaterials for Chromium(VI) Ion Elimination from Water. Crystals 2023, 13, 1047. [Google Scholar] [CrossRef]
- Bhuyan, M.S.A.; Uddin, M.N.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.C.; Banks, C.E. A decade of graphene research: Production, applications and outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Berry, V. Impermeability of graphene and its applications. Carbon N. Y. 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal′ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Biswas, K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene, the new nanocarbon. J. Mater. Chem. 2009, 19, 2457–2469. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Lui, C.H.; Liu, L.; Mak, K.F.; Flynn, G.W.; Heinz, T.F. Ultraflat graphene. Nature 2009, 462, 339–341. [Google Scholar] [CrossRef]
- Bai, J.; Zhong, X.; Jiang, S.; Huang, Y.; Duan, X. Graphene nanomesh. Nat. Nanotechnol. 2010, 5, 190–194. [Google Scholar] [CrossRef]
- Obraztsova, E.D.; Rybin, M.G.; Obraztsov, P.A. 7—Optical properties of graphene. In Woodhead Publishing Series in Electronic and Optical Materials; Skakalova, V., Kaiser, A.B.B.T.-G., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 133–142. ISBN 978-0-08-102848-3. [Google Scholar] [CrossRef]
- Jafari, B.; Gholizadeh, E.; Golmohammadi, S.; Ebadzadeh, M.; Soofi, H.; Aghili, S. An Innovative method for adjustable broadband THz to Mid-IR optical modulator using Graphene Gratings surface plasmon Fabry–Perot resonances with low insertion loss, high speed and modulation depth. Opt. Commun. 2023, 530, 129200. [Google Scholar] [CrossRef]
- Rogalski, A. Graphene-based materials in the infrared and terahertz detector families: A tutorial. Adv. Opt. Photonics 2019, 11, 314–379. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; Khan, A. ullah Graphene synthesis, characterization and its applications: A review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Chen, S. Large area CVD growth of graphene. Synth. Met. 2015, 210, 95–108. [Google Scholar] [CrossRef]
- Shafi, M.; Duan, P.; Liu, W.; Zhang, W.; Zhang, C.; Hu, X.; Zha, Z.; Liu, R.; Liu, C.; Jiang, S.; et al. SERS Sensing Using Graphene-Covered Silver Nanoparticles and Metamaterials for the Detection of Thiram in Soil. Langmuir 2022, 38, 16183–16193. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, T.; Sun, Q.; Tang, Y.; Hu, B.; Levy, U.; Yu, W. Graphene–Silver Hybrid Metamaterial for Tunable and High Absorption at Mid-Infrared Waveband. IEEE Photonics Technol. Lett. 2018, 30, 475–478. [Google Scholar] [CrossRef]
- Shaban, N.; Prajapati, Y.K.; Mohammadkhani, R. Performance enhancement of waveguide-coupled and metamaterial surface plasmon resonance sensors based on silver-bismuth ferrite and graphene. J. Mater. Sci. Mater. Electron. 2023, 34, 309. [Google Scholar] [CrossRef]
- Kaushik, V.; Kagdada, H.L.; Singh, D.K.; Pathak, S. Enhancement of SERS effect in Graphene-Silver hybrids. Appl. Surf. Sci. 2022, 574, 151724. [Google Scholar] [CrossRef]
- Yu, X.; Cai, H.; Zhang, W.; Li, X.; Pan, N.; Luo, Y.; Wang, X.; Hou, J.G. Tuning Chemical Enhancement of SERS by Controlling the Chemical Reduction of Graphene Oxide Nanosheets. ACS Nano 2011, 5, 952–958. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef]
- Huq, T.; Ong, H.C.; Chew, B.T.; Leong, K.Y.; Kazi, S.N. Review on aqueous graphene nanoplatelet Nanofluids: Preparation, Stability, thermophysical Properties, and applications in heat exchangers and solar thermal collectors. Appl. Therm. Eng. 2022, 210, 118342. [Google Scholar] [CrossRef]
- Ajala, O.J.; Tijani, J.O.; Bankole, M.T.; Abdulkareem, A.S. A critical review on graphene oxide nanostructured material: Properties, Synthesis, characterization and application in water and wastewater treatment. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100673. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Yang, Q.-D.; Li, J.; Cheng, Y.; Li, H.-W.; Guan, Z.; Yu, B.; Tsang, S.-W. Graphene oxide as an efficient hole-transporting material for high-performance perovskite solar cells with enhanced stability. J. Mater. Chem. A 2017, 5, 9852–9858. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.-H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon N. Y. 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Yee, K.; Ghayesh, M.H. A review on the mechanics of graphene nanoplatelets reinforced structures. Int. J. Eng. Sci. 2023, 186, 103831. [Google Scholar] [CrossRef]
- Moosa, A.; Ramazani, S.A.A.; Ibrahim, M. Mechanical and Electrical Properties of Graphene Nanoplates and Carbon-Nanotubes Hybrid Epoxy Nanocomposites. Am. J. Mater. Sci. 2016, 6, 157–165. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, K.; Verma, V.; Bhatti, H.S. Microwave assisted synthesis and characterization of graphene nanoplatelets. Appl. Nanosci. 2016, 6, 97–103. [Google Scholar] [CrossRef]
- Krittayavathananon, A.; Li, X.; Batchelor-McAuley, C.; Sawangphruk, M.; Compton, R.G. Comparing the effect of different surfactants on the aggregation and electrical contact properties of graphene nanoplatelets. Appl. Mater. Today 2018, 12, 163–167. [Google Scholar] [CrossRef]
- Moriche, R.; Jiménez-Suárez, A.; Sánchez, M.; Prolongo, S.G.; Ureña, A. High sensitive damage sensors based on the use of functionalized graphene nanoplatelets coated fabrics as reinforcement in multiscale composite materials. Compos. Part B Eng. 2018, 149, 31–37. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Han, S.A.; Bhatia, R.; Kim, S.-W. Synthesis, properties and potential applications of two-dimensional transition metal dichalcogenides. Nano Converg. 2015, 2, 17. [Google Scholar] [CrossRef]
- Babu, G.; Masurkar, N.; Al Salem, H.; Arava, L.M.R. Transition Metal Dichalcogenide Atomic Layers for Lithium Polysulfides Electrocatalysis. J. Am. Chem. Soc. 2017, 139, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chao, D.; Liu, E.; Jaroniec, M.; Zhao, N.; Qiao, S.-Z. Transition metal dichalcogenides for alkali metal ion batteries: Engineering strategies at the atomic level. Energy Environ. Sci. 2020, 13, 1096–1131. [Google Scholar] [CrossRef]
- Politano, G.G.; Versace, C.; Vena, C.; Castriota, M.; Ciuchi, F.; Fasanella, A.; Desiderio, G.; Cazzanelli, E. Physical investigation of electrophoretically deposited graphene oxide and reduced graphene oxide thin films. J. Appl. Phys. 2016, 120, 195307. [Google Scholar] [CrossRef]
- Politano, G.G.; Cazzanelli, E.; Versace, C.; Vena, C.; De Santo, M.P.; Castriota, M.; Ciuchi, F.; Bartolino, R. Graphene oxide on magnetron sputtered silver thin films for SERS and metamaterial applications. Appl. Surf. Sci. 2018, 427, 927–933. [Google Scholar] [CrossRef]
- Politano, G.G.; Cazzanelli, E.; Versace, C.; Castriota, M.; Desiderio, G.; Davoli, M.; Vena, C.; Bartolino, R. Micro-Raman investigation of Ag/graphene oxide/Au sandwich structure. Mater. Res. Express 2019, 6, 075605. [Google Scholar] [CrossRef]
- Politano, G.G.; Nucera, A.; Castriota, M.; Desiderio, G.; Vena, C.; Versace, C. Spectroscopic and morphological study of graphene nanoplatelets thin films on Si/SiO2 substrates. Mater. Res. Express 2019, 6, 106432. [Google Scholar] [CrossRef]
- Politano, G.G.; Castriota, M.; De Santo, M.P.; Pipita, M.M.; Desiderio, G.; Vena, C.; Versace, C. Variable angle spectroscopic ellipsometry characterization of spin-coated MoS2 films. Vacuum 2021, 189, 110232. [Google Scholar] [CrossRef]
- Castriota, M.; Politano, G.G.; Vena, C.; De Santo, M.P.; Desiderio, G.; Davoli, M.; Cazzanelli, E.; Versace, C. Variable Angle Spectroscopic Ellipsometry investigation of CVD-grown monolayer graphene. Appl. Surf. Sci. 2019, 467–468, 213–220. [Google Scholar] [CrossRef]
- Bruna, M.; Ott, A.K.; Ijäs, M.; Yoon, D.; Sassi, U.; Ferrari, A.C. Doping Dependence of the Raman Spectrum of Defected Graphene. ACS Nano 2014, 8, 7432–7441. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611. [Google Scholar] [CrossRef]
- Katsnelson, M.I. Graphene: Carbon in two dimensions. Mater Today 2007, 10, 20. [Google Scholar] [CrossRef]
- Fang, W.; Song, Y.; Hsu, A.L.; Kong, J. A review of large-area bilayer graphene synthesis by chemical vapor deposition. Nanoscale 2015, 7, 20335. [Google Scholar] [CrossRef]
- Yu, X.Z.; Hwang, C.G.; Jozwiak, C.M.; Köhl, A.; Schmid, A.K.; Lanzara, A. New synthesis method for the growth of epitaxial graphene. J. Electron Spectros. Relat. Phenomena 2011, 184, 100–106. [Google Scholar] [CrossRef]
- Sinclair, R.C.; Suter, J.L.; Coveney, P. V Micromechanical exfoliation of graphene on the atomistic scale. Phys. Chem. Chem. Phys. 2019, 21, 5716–5722. [Google Scholar] [CrossRef]
- Mishra, N.; Boeckl, J.; Motta, N.; Iacopi, F. Graphene growth on silicon carbide: A review. Phys. Status Solidi 2016, 213, 2277–2289. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Alternative methods and nature-based reagents for the reduction of graphene oxide: A review. Carbon N. Y. 2015, 94, 224–242. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon N. Y. 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Katsnelson, M.I. Modeling of Graphite Oxide. J. Am. Chem. Soc. 2008, 130, 10697–10701. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Matsuo, Y. Formation process and structure of graphite oxide. Carbon N. Y. 1994, 32, 469–475. [Google Scholar] [CrossRef]
- Smirnov, V.A.; Arbuzov, A.A.; Shul’ga, Y.M.; Baskakov, S.A.; Martynenko, V.M.; Muradyan, V.E.; Kresova, E.I. Photoreduction of graphite oxide. High Energy Chem. 2011, 45, 57–61. [Google Scholar] [CrossRef]
- Petridis, L.V.; Kokkinos, N.C.; Mitropoulos, A.C.; Kyzas, G.Z. Chapter 8—Graphene aerogels for oil absorption. In Advanced Low-Cost Separation Techniques in Interface Science; Kyzas, G.Z., Mitropoulos, A.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 30, pp. 173–197. ISBN 1573-4285. [Google Scholar] [CrossRef]
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through pKa Measurements. J. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. Graphene Oxide Dispersions in Organic Solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient Synthesis of Graphene Oxide Based on Improved Hummers Method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, K.E.; Lee, H.; Koh, S.J.; Ko, J.-H.; Kim, Y.-H.; Kim, S.O.; Park, J.Y. The Effect of Thickness and Chemical Reduction of Graphene Oxide on Nanoscale Friction. J. Phys. Chem. B 2018, 122, 543–547. [Google Scholar] [CrossRef]
- Zhao, C.; Xing, L.; Xiang, J.; Cui, L.; Jiao, J.; Sai, H.; Li, Z.; Li, F. Formation of uniform reduced graphene oxide films on modified PET substrates using drop-casting method. Particuology 2014, 17, 66–73. [Google Scholar] [CrossRef]
- Sun, H.B.; Yang, J.; Zhou, Y.Z.; Zhao, N.; Li, D. Preparation of reduced graphene oxide films by dip coating technique and their electrical conductivity. Mater. Technol. 2014, 29, 14–20. [Google Scholar] [CrossRef]
- Nasiłowska, B.; Bogdanowicz, Z.; Hińcza, K.; Mierczyk, Z.; Góźdź, S.; Djas, M.; Kowiorski, K.; Bombalska, A.; Kowalik, A. Graphene Oxide Aerosol Deposition and its Influence on Cancer Cells. Preliminary Results. Materials 2020, 13, 4464. [Google Scholar] [CrossRef]
- Komarov, I.A.; Danilova, E.A.; Denisenko, E.I.; Peretiyagin, V.G.; Rabchinskii, M.K. Spin-coating deposition of graphene oxide from mixed water-organic suspensions. Fuller. Nanotub. Carbon Nanostruct. 2022, 30, 146–151. [Google Scholar] [CrossRef]
- Marcin Behunová, D.; Gallios, G.; Girman, V.; Kolev, H.; Kaňuchová, M.; Dolinská, S.; Václavíková, M. Electrophoretic Deposition of Graphene Oxide on Stainless Steel Substrate. Nanomaterials 2021, 11, 1779. [Google Scholar] [CrossRef]
- Li, X.; Shen, Q.; Zhang, Y.; Wang, L.; Nie, C. Wear behavior of electrodeposited nickel/graphene composite coating. Diam. Relat. Mater. 2021, 119, 108589. [Google Scholar] [CrossRef]
- Tseluikin, V.; Dzhumieva, A.; Tikhonov, D.; Yakovlev, A.; Strilets, A.; Tribis, A.; Lopukhova, M. Pulsed Electrodeposition and Properties of Nickel-Based Composite Coatings Modified with Graphene Oxide. Coatings 2022, 12, 656. [Google Scholar] [CrossRef]
- Ray, S.C. Applications of Graphene and Graphene-Oxide based Nanomaterials. In Applications of Graphene and Graphene-Oxide Based Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–84. [Google Scholar]
- Tkachev, S.V.; Buslaeva, E.Y.; Naumkin, A.V.; Kotova, S.L.; Laure, I.V.; Gubin, S.P. Reduced graphene oxide. Inorg. Mater. 2012, 48, 796–802. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite-and graphene oxide. Carbon N. Y. 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.I.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J.M.D. Vitamin C Is an Ideal Substitute for Hydrazine in the Reduction of Graphene Oxide Suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Jung, I.; Dikin, D.A.; Piner, R.D.; Ruoff, R.S. Tunable Electrical Conductivity of Individual Graphene Oxide Sheets Reduced at “Low” Temperatures. Nano Lett. 2008, 8, 4283–4287. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon N. Y. 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Sadhukhan, S.; Ghosh, T.K.; Rana, D.; Roy, I.; Bhattacharyya, A.; Sarkar, G.; Chakraborty, M.; Chattopadhyay, D. Studies on synthesis of reduced graphene oxide (RGO) via green route and its electrical property. Mater. Res. Bull. 2016, 79, 41–51. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Pisana, S.; Chakraborty, B.; Piscanec, S.; Saha, S.K.; Waghmare, U.V.; Novoselov, K.S.; Krishnamurthy, H.R.; Geim, A.K.; Ferrari, A.C.; et al. Monitoring dopants by Raman scattering in an electrochemically top-gated graphene transistor. Nat. Nanotechnol. 2008, 3, 210–215. [Google Scholar] [CrossRef]
- Sharma, N.; Tomar, S.; Shkir, M.; Kant Choubey, R.; Singh, A. Study of Optical and Electrical Properties of Graphene Oxide. Mater. Today Proc. 2021, 36, 730–735. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- De Silva, K.K.H.; Viswanath, P.; Rao, V.K.; Suzuki, S.; Yoshimura, M. New Insight into the Characterization of Graphene Oxide and Reduced Graphene Oxide Monolayer Flakes on Si-Based Substrates by Optical Microscopy and Raman Spectroscopy. J. Phys. Chem. C 2021, 125, 7791–7798. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Verde, M.; Peiteado, M.; Caballero, A.C.; Villegas, M.; Ferrari, B. Electrophoretic Deposition of Transparent ZnO Thin Films from Highly Stabilized Colloidal Suspensions. J. Colloid Interface Sci. 2012, 373, 27–33. [Google Scholar] [CrossRef]
- Yan, Y.; Peng, S.; Jin, Z.; Zhang, D.; Shi, J. Recovering the Intrinsic Electrical Property of a Graphene Field-Effect Transistor by Interface Cleaning Technology. ACS Appl. Electron. Mater. 2023, 5, 3113–3119. [Google Scholar] [CrossRef]
- Kim, S.; Kim, B.; Park, S.; Chang, W.S.; Kang, H.; Kim, S.; Lee, H.; Kim, S. Hysteretic behavior of all CVD h-BN/graphene/h-BN heterostructure field-effect transistors by interfacial charge trap. Surf. Interfaces 2023, 36, 102615. [Google Scholar] [CrossRef]
- Chaudhary, M.; Xin, C.; Hu, Z.; Zhang, D.; Radtke, G.; Xu, X.; Billot, L.; Tripon-Canseliet, C.; Chen, Z. Nitrogen-Doped Carbon Quantum Dots on Graphene for Field-Effect Transistor Optoelectronic Memories. Adv. Electron. Mater. 2023, 9, 2300159. [Google Scholar] [CrossRef]
- Khan, M.F.; Elahi, E.; Hassan, N.U.; Rehman, M.A.; Khalil, H.M.W.; Khan, M.A.; Rehman, S.; Hao, A.; Noh, H.; Khan, K.; et al. Bipolar Photoresponse of a Graphene Field-Effect Transistor Induced by Photochemical Reactions. ACS Appl. Electron. Mater. 2023. [Google Scholar] [CrossRef]
- Vignesh, G.; Devendran, P.; Nallamuthu, N.; Sudhahar, S.; Kumar, P.S.; Kumar, M.K. Effects of nitrogen, sulphur, and temperature treatments on the spectral, structural, and electrochemical characteristics of graphene oxide for energy storage applications. Carbon Trends 2023, 11, 100262. [Google Scholar] [CrossRef]
- Zhang, D.; Du, J.; Zhang, W.; Tong, B.; Sun, Y.; Zhao, T.-Y.; Ma, L.-P.; Sun, D.-M.; Cheng, H.-M.; Ren, W. Carrier Transport Regulation of Pixel Graphene Transparent Electrodes for Active-Matrix Organic Light-Emitting Diode Display. Small 2023, 2302920. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Smith, A.D.; Forsberg, F.; Wagner, S.; Schröder, S.; Akbari, S.S.A.; Fischer, A.C.; Villanueva, L.G.; Östling, M.; Lemme, M.C.; et al. Manufacture and characterization of graphene membranes with suspended silicon proof masses for MEMS and NEMS applications. Microsyst. Nanoeng. 2020, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Han, N.; Zhang, J.; Wang, J.; Chen, X.; Zhao, J.; Gan, X. Emergent second-harmonic generation in van der Waals heterostructure of bilayer MoS2 and monolayer graphene. Sci. Adv. 2023, 9, eadf4571. [Google Scholar] [CrossRef]
- Thakkar, S.; De Luca, L.; Gaspa, S.; Mariani, A.; Garroni, S.; Iacomini, A.; Stagi, L.; Innocenzi, P.; Malfatti, L. Comparative Evaluation of Graphene Nanostructures in GERS Platforms for Pesticide Detection. ACS Omega 2022, 7, 5670–5678. [Google Scholar] [CrossRef]
- Szczęśniak, D.; Kais, S. Gap states and valley-spin filtering in transition metal dichalcogenide monolayers. Phys. Rev. B 2020, 101, 115423. [Google Scholar] [CrossRef]
- Pak, J.; Lee, I.; Cho, K.; Kim, J.-K.; Jeong, H.; Hwang, W.-T.; Ahn, G.H.; Kang, K.; Yu, W.J.; Javey, A.; et al. Intrinsic Optoelectronic Characteristics of MoS2 Phototransistors via a Fully Transparent van der Waals Heterostructure. ACS Nano 2019, 13, 9638–9646. [Google Scholar] [CrossRef]
- Szczęśniak, D.; Hoehn, R.D.; Kais, S. Canonical Schottky barrier heights of transition metal dichalcogenide monolayers in contact with a metal. Phys. Rev. B 2018, 97, 195315. [Google Scholar] [CrossRef]
- Lan, Y.-W.; Torres, C.M., Jr.; Tsai, S.-H.; Zhu, X.; Shi, Y.; Li, M.-Y.; Li, L.-J.; Yeh, W.-K.; Wang, K.L. Atomic-Monolayer MoS2 Band-to-Band Tunneling Field-Effect Transistor. Small 2016, 12, 5676–5683. [Google Scholar] [CrossRef] [PubMed]
- Ferralis, N. Probing Mechanical Properties of Graphene with Raman Spectroscopy. J. Mater. Sci. 2010, 45, 5135–5149. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Politano, G.G.; Versace, C. Recent Advances in the Raman Investigation of Structural and Optical Properties of Graphene and Other Two-Dimensional Materials. Crystals 2023, 13, 1357. https://doi.org/10.3390/cryst13091357
Politano GG, Versace C. Recent Advances in the Raman Investigation of Structural and Optical Properties of Graphene and Other Two-Dimensional Materials. Crystals. 2023; 13(9):1357. https://doi.org/10.3390/cryst13091357
Chicago/Turabian StylePolitano, Grazia Giuseppina, and Carlo Versace. 2023. "Recent Advances in the Raman Investigation of Structural and Optical Properties of Graphene and Other Two-Dimensional Materials" Crystals 13, no. 9: 1357. https://doi.org/10.3390/cryst13091357




