Unleashing the Power of Graphene-Based Nanomaterials for Chromium(VI) Ion Elimination from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Graphene Oxide
2.2.2. Synthesis of Amine Modified Silica
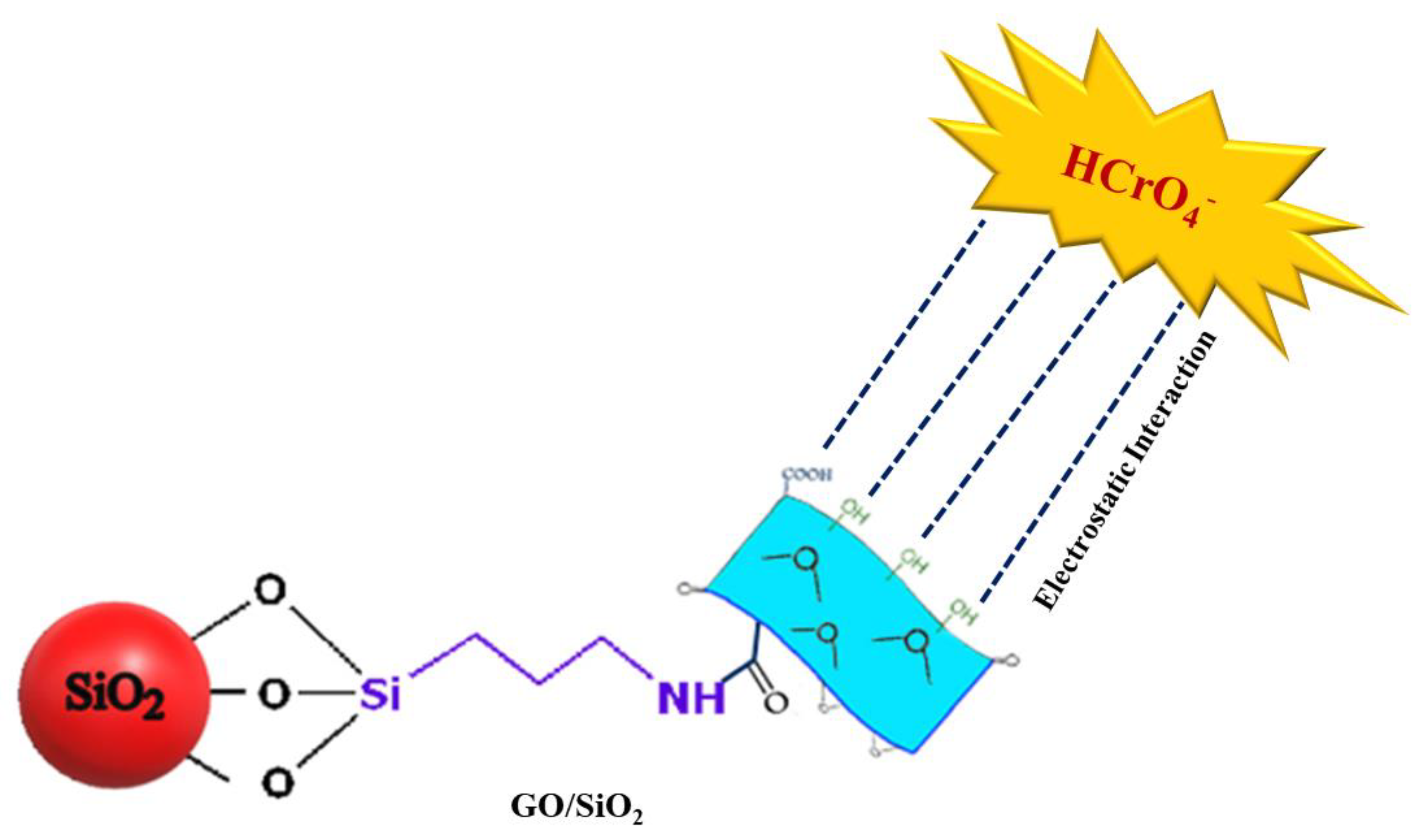
2.2.3. Synthesis of Self-Assembled Graphene-Oxide-Coated Silica Nanoparticle
2.2.4. Preparation of Stock Solution
2.2.5. Optimization of pH
2.2.6. Batch Adsorption Test on GO and GO-Coated Silica Nanoparticles
2.3. Characterizations
3. Results and Discussion
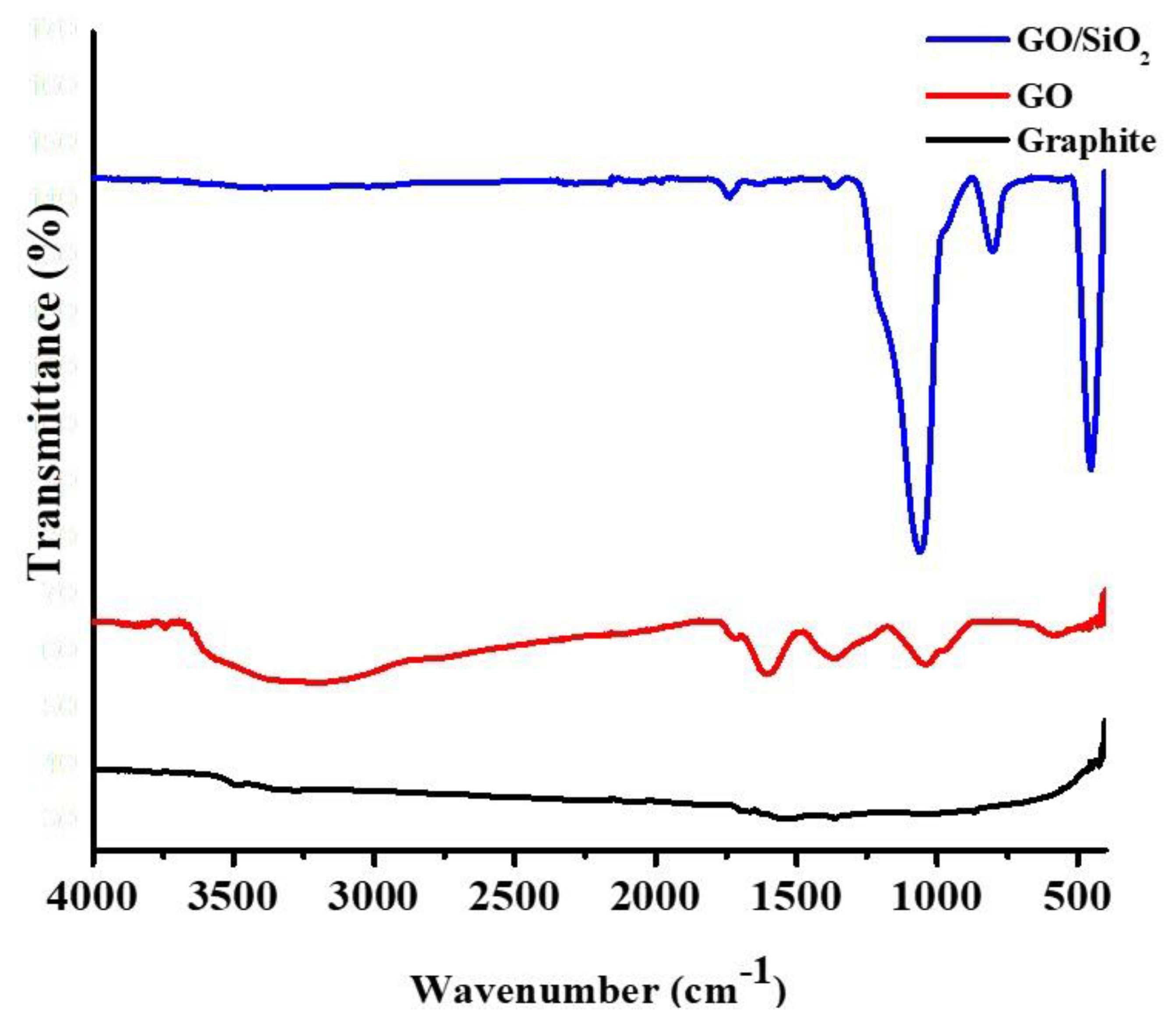
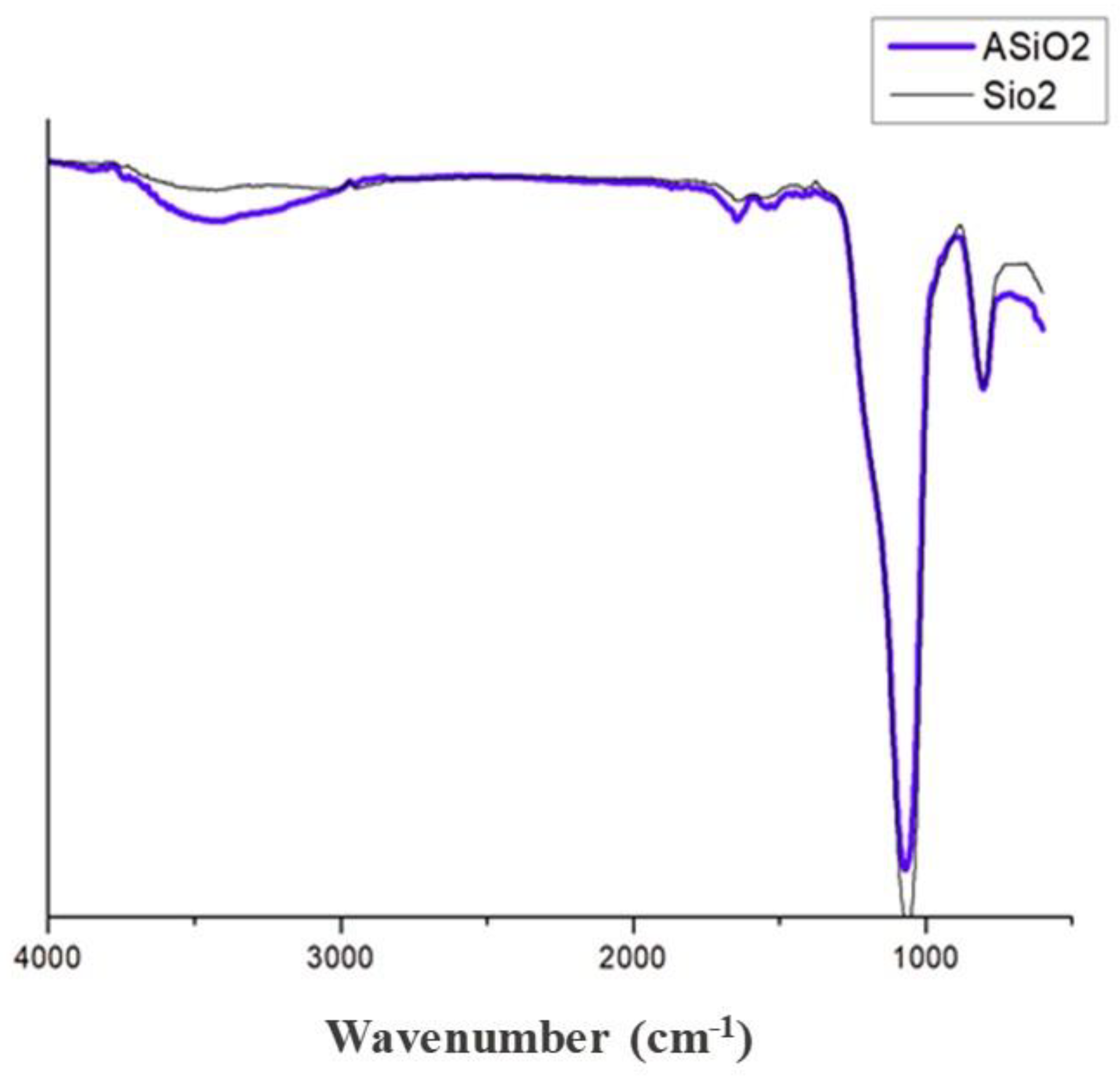
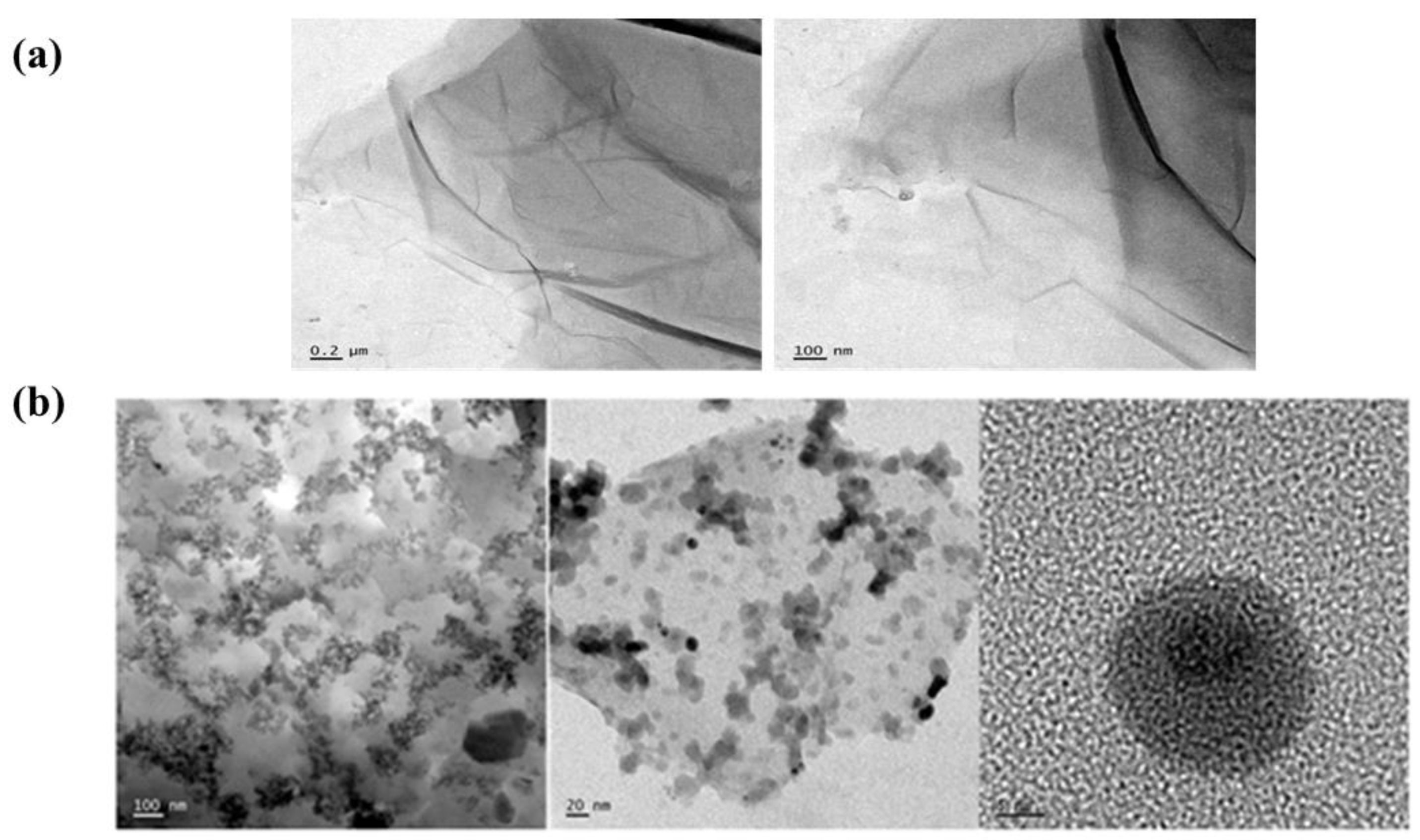
3.1. Characterization of GO and GO/SiO2
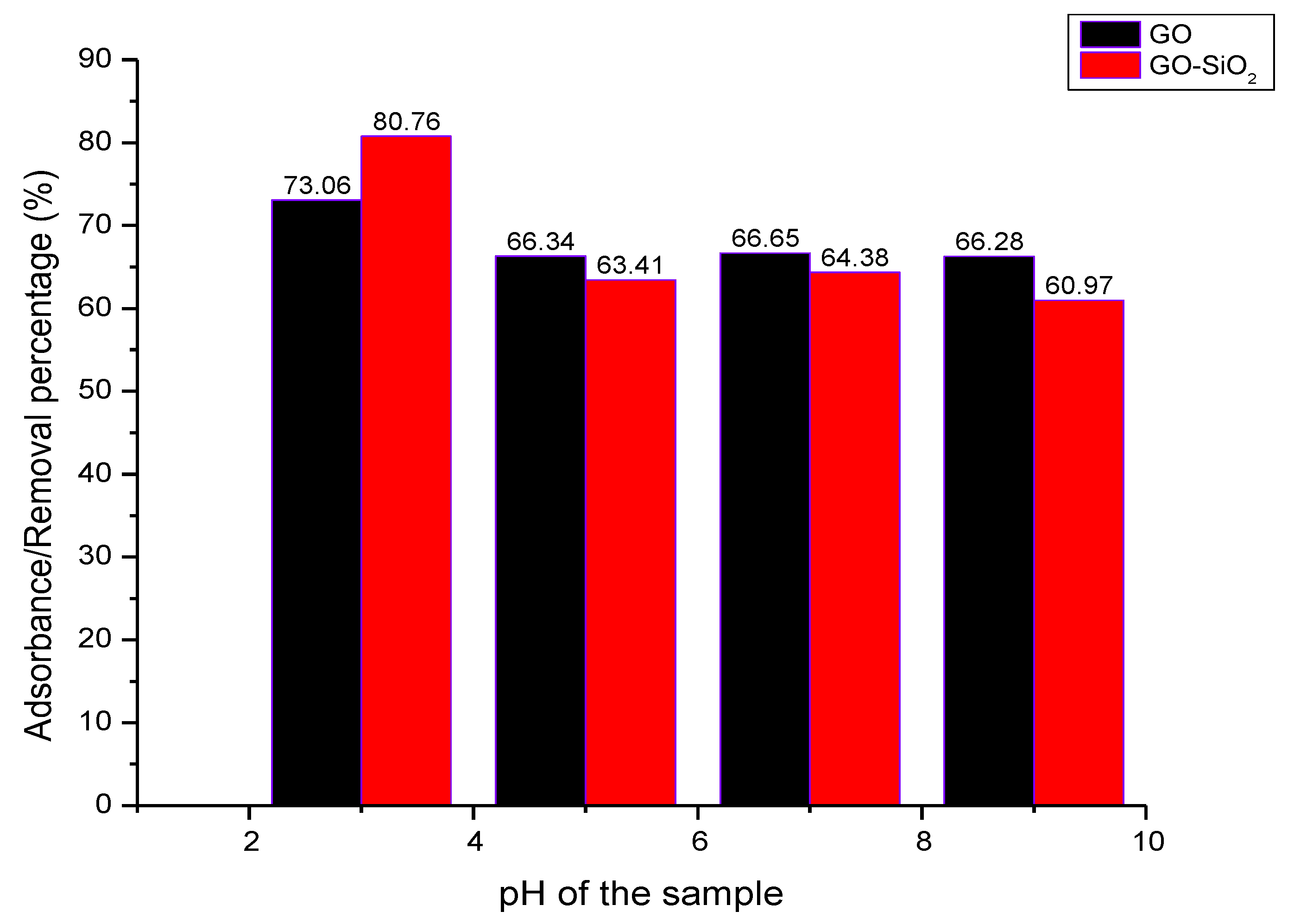
3.2. Effect of pH on Adsorption of Cr(VI) Ions
3.3. Evaluation of the Adsorption Efficiency of GO and Si/GO at Different Concentrations of Solutions
3.4. Comparison of Removal Efficiency of Cr(VI) Ions Using Different Adsorbents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Järup, L. Medical bulletin and undefined 2003: Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef]
- Brevik, E.C.; Burgess, L.C. Soils and Human Health; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Sperling, M. Chromium. Encycl. Anal. Sci. Second Ed. 2004, 113. [Google Scholar] [CrossRef]
- Rodríguez, M.C.; Barsanti, L.; Passarelli, V.; Evangelista, V.; Conforti, V.; Gualtieri, P. Effects of chromium on photosynthetic and photoreceptive apparatus of the alga Chlamydomonas reinhardtii. Environ. Res. 2007, 105, 234. [Google Scholar] [CrossRef]
- Saha, R.; Nandi, R.; Saha, B. Chemistry, and undefined 2011: Sources and toxicity of hexavalent chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.; Ren, B.; Luo, J.; Yuan, J.; Ding, X.; Bian, H.; Yao, X. Trends and Health Risks of Dissolved Heavy Metal Pollution in Global River and Lake Water from 1970 to 2017. Rev. Environ. Contam. Toxicol. 2020, 251, 1–24. [Google Scholar]
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium pollution in European water, sources, health risk, and remediation strategies: An overview. Int. J. Environ. Res. Public Health 2020, 17, 5438. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Lisiak-Zielińska, M.; Borowiak, K.; Budka, A.; Kanclerz, J.; Janicka, E.; Kaczor, A.; Żyromski, A.; Biniak-Pieróg, M.; Podawca, K.; Mleczek, M.; et al. How polluted are cities in central Europe?—Heavy metal contamination in Taraxacum officinale and soils collected from different land use areas of three representative cities. Chemosphere 2021, 266, 129113. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2021, 92, 407. [Google Scholar] [CrossRef]
- Singh, K.; Renu, N.A.; Agarwal, M. Methodologies for removal of heavy metal ions from wastewater: An overview. Interdiscip. Environ. Rev. 2017, 18, 124. [Google Scholar] [CrossRef]
- Zhao, D.; Gao, X.; Chen, S.; Xie, F.; Feng, S.; Alsaedi, A.; Hayat, T.; Chen, C. Interaction between U(VI) with sulfhydryl groups functionalized graphene oxides investigated by batch and spectroscopic techniques. J. Colloid Interface Sci. 2018, 524, 129. [Google Scholar] [CrossRef] [PubMed]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373. [Google Scholar] [CrossRef]
- Arora, R. Adsorption of heavy metals—A review. Mater. Today Proc. 2019, 18, 4745. [Google Scholar] [CrossRef]
- Prachi; Gautam, P.; Madathil, D.; Nair, A.N.B. Nanotechnology in waste water treatment: A review. Int. J. ChemTech Res. 2013, 5, 2303. [Google Scholar]
- Gangadhar, G.; Maheshwari, U.; Gupta, S. Application of nanomaterials for the removal of pollutants from effluent streams. Nanosci. Nanotechnol.-Asia 2012, 2, 140–150. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Sadegh, H.; Ali, G.A.; Gupta, V.K.; Makhlouf, A.S.H.; Shahryari-Ghoshekandi, R.; Nadagouda, M.N.; Sillanpää, M.; Megiel, E. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanostructure Chem. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Wang, X.; Ma, Y.; Yang, Y.; Zhuang, L.; Zhang, S.; Jehan, R.; Chen, J.; Wang, X. Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: A review. Environ. Pollut. 2019, 252, 62. [Google Scholar] [CrossRef]
- Li, L.; Zhao, L.; Ma, J.; Tian, Y.; Tian, Y. Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions. Green Process. Synth. 2020, 9, 294. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Kakan, S.S.; Rajesh, N. A novel amine impregnated graphene oxide adsorbent for the removal of hexavalent chromium. Chem. Eng. J. 2013, 230, 328. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. The application of graphene-based materials for the removal of heavy metals and radionuclides from water and wastewater. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1042. [Google Scholar] [CrossRef]
- Deng, J.H.; Zhang, X.R.; Zeng, G.M.; Gong, J.L.; Niu, Q.Y.; Liang, J. Simultaneous removal of Cd (II) and ionic dyes from aqueous solution using magnetic graphene oxide nanocomposite as an adsorbent. Chem. Eng. J. 2013, 226, 189–200. [Google Scholar] [CrossRef]
- Peer, F.E.; Bahramifar, N.; Younesi, H. Removal of Cd (II), Pb (II) and Cu (II) ions from aqueous solution by polyamidoamine dendrimer grafted magnetic graphene oxide nanosheets. J. Taiwan Inst. Chem. Eng. 2018, 87, 225–240. [Google Scholar] [CrossRef]
- Shahzad, A.; Miran, W.; Rasool, K.; Nawaz, M.; Jang, J.; Lim, S.R.; Lee, D.S. Heavy metals removal by EDTA-functionalized chitosan graphene oxide nanocomposites. RSC Adv. 2017, 7, 9764. [Google Scholar] [CrossRef]
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Manyangadze, M.; Chikuruwo, N.M.H.; Narsaiah, T.B.; Chakra, C.S.; Charis, G.; Danha, G.; Mamvura, T.A. Adsorption of lead ions from wastewater using nano silica spheres synthesized on calcium carbonate templates. Heliyon 2020, 6, e05309. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Sheet, I.; Kabbani, A.; Holail, H. Removal of heavy metals using nanostructured graphite oxide, silica nanoparticles and silica/graphite oxide composite. Energy Procedia 2014, 50, 130. [Google Scholar] [CrossRef]
- Mondal, N.K.; Chakraborty, S. Adsorption of Cr(VI) from aqueous solution on graphene oxide (GO) prepared from graphite: Equilibrium, kinetic and thermodynamic studies. Appl. Water Sci. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Lee, D.W.; De Los Santos V, L.; Seo, J.W.; Felix, L.L.; Bustamante D, A.; Cole, J.M.; Barnes, C.H.W. The structure of graphite oxide: Investigation of its surface chemical groups. J. Phys. Chem. B 2010, 114, 5723–5728. [Google Scholar]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Langmuir, H.C. Silica-graphene oxide hybrid composite particles and their electroresponsive characteristics. ACS Publ. 2012, 28, 7055. [Google Scholar] [CrossRef] [PubMed]
- Björkman, Å. Thermische Klärschlammbehandlung. Schweiz. Z. Hydrol. 1969, 31, 632. [Google Scholar]
- Eda, G.; Chhowalla, M. Chemically derived graphene oxide: Towards large-area thin-film electronics and optoelectronics. Adv. Mater. 2010, 22, 2392. [Google Scholar] [CrossRef]
- Sun, L.; Yu, H.; Fugetsu, B. Graphene oxide adsorption enhanced by in situ reduction with sodium hydrosulfite to remove acridine orange from aqueous solution. J. Hazard. Mater. 2012, 203–204, 101. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud, R.K.; Aksay, I.A.; Car, R. Raman Spectra of Graphite Oxide and Functionalized Graphene Sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar]
- Gu, J.; She, J.; Yue, Y. Micro/nanoscale thermal characterization based on spectroscopy techniques. ES Energy Environ. 2020, 9, 15–27. [Google Scholar] [CrossRef]
- Khalili, D. Graphene oxide: A promising carbocatalyst for the regioselective thiocyanation of aromatic amines, phenols, anisols and enolizable ketones by hydrogen peroxide/KSCN in water. N. J. Chem. 2016, 40, 2547. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon N. Y. 2013, 53, 38. [Google Scholar] [CrossRef]
- Mishima, K.; Du, X.; Sekiguchi, S.; Kano, N. Experimental and theoretical studies on the adsorption and desorption mechanisms of chromate ions on cross-linked chitosan. J. Funct. Biomater. 2017, 8, 3. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol. 2011, 45, 10454. [Google Scholar] [CrossRef]
- Shirkhanloo, H.; Khaligh, A.; Mousavi, H.Z.; Rashidi, A. Graphene oxide-packed micro-column solid-phase extraction combined with flame atomic absorption spectrometry for determination of lead (II) and nickel (II) in water. J. Environ. Anal. Chem. 2015, 95, 16–32. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Sun, Q.; Yang, C.; Lu, A.-H. Synthesis of magnetic hollow carbon nanospheres with superior microporosity for efficient adsorption of hexavalent chromium ions. Sci. China Mater. 2015, 58, 611. [Google Scholar] [CrossRef]
- Hu, J.; Chen, C.; Zhu, X.; Wang, X. Removal of chromium from aqueous solution by using oxidized multiwalled carbon nanotubes. J. Hazard. Mater. 2009, 162, 1542. [Google Scholar] [CrossRef] [PubMed]
- Matei, E.; Predescu, A.M.; Râpă, M.; Tarcea, C.; Pantilimon, C.M.; Favier, L.; Berbecaru, A.C.; Sohaciu, M.; Predescu, C. Removal of Chromium(VI) from Aqueous Solution Using a Novel Green Magnetic Nanoparticle–Chitosan Adsorbent. Anal. Lett. 2019, 52, 2416. [Google Scholar] [CrossRef]
- Hu, J.; Lo, I.M.C.; Chen, G. Removal of Cr(VI) by magnetite nanoparticle. Water Sci. Technol. 2004, 50, 139. [Google Scholar] [CrossRef]
- Liu, W.; Yang, L.; Xu, S.; Chen, Y.; Liu, B.; Li, Z.; Jiang, C. RSC Advances Efficient removal of hexavalent chromium from water by an adsorption-reduction mechanism with sandwiched nanocomposites. RSC Adv. 2018, 8, 15087–15093. [Google Scholar] [CrossRef]
- Samuel, M.S.; Bhattacharya, J.; Raj, S.; Santhanam, N.; Singh, H.; Singh, N.D.P. Efficient removal of Chromium(VI) from aqueous solution using chitosan grafted graphene oxide (CS-GO) nanocomposite. Int. J. Biol. Macromol. 2019, 121, 285. [Google Scholar] [CrossRef] [PubMed]
- Konicki, W.; Aleksandrzak, M.; Mijowska, E. Equilibrium and kinetics studies for the adsorption of Ni2+ and Fe3+ ions from aqueous solution by graphene oxide. Polish J. Chem. Technol. 2017, 19, 120. [Google Scholar] [CrossRef]
- Donga, C.; Mishra, S.; Aziz, A.; Ndlovu, L.; Kuvarega, A.; Mishra, A.K. 3-Aminopropyl) Triethoxysilane (APTES) Functionalized Magnetic Nanosilica Graphene Oxide (MGO) Nanocomposite for the Comparative Adsorption of the Heavy Metal [Pb(II), Cd(II) and Ni(II)] Ions from Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2235–2248. [Google Scholar] [CrossRef]
- Ma, M.; Li, H.; Xiong, Y.; Dong, F. Rational design, synthesis, and application of silica/graphene-based nanocomposite: A review. Mater. Des. 2021, 198, 109367. [Google Scholar] [CrossRef]
- Kalidhasan, S.; Kumar, A.S.K.; Rajesh, V.; Rajesh, N. Enhanced adsorption of hexavalent chromium arising out of an admirable interaction between a synthetic polymer and an ionic liquid. Chem. Eng. J. 2013, 222, 454. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Rajesh, N.; Kalidhasan, S.; Rajesh, V. An enhanced adsorption methodology for the detoxification of chromium using n-octylamine impregnated Amberlite XAD-4 polymeric sorbent. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2011, 46, 1598. [Google Scholar] [CrossRef]
- Zhang, L.; Song, F.; Wang, S.; Wang, H.; Yang, W.; Li, Y. Efficient removal of hexavalent chromium and congo red by graphene oxide/silica nanosheets with multistage pores. ACS Publ. 2020, 65, 4368. [Google Scholar] [CrossRef]
- Hu, J.; Lo, I.M.C.; Chen, G. Fast removal and recovery of Cr(VI) using surface-modified jacobsite (MnFe2O4) nanoparticles. Langmuir 2005, 21, 11173. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, G.; Lo, I.M.C. Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Res. 2005, 39, 4528. [Google Scholar] [CrossRef]
- Najafabadi, H.H.; Irani, M.; Rad, L.R.; Haratameh, A.H.; Haririan, I. Removal of Cu2+, Pb2+ and Cr6+ from aqueous solutions using a chitosan/graphene oxide composite nanofibrous adsorbent. RSC Adv. 2015, 5, 16532. [Google Scholar] [CrossRef]
- Liu, Y.; Shan, H.; Zeng, C.; Zhan, H.; Pang, Y. Removal of Cr(VI) from Wastewater Using Graphene Oxide Chitosan Microspheres Modified with α–FeO(OH). Materials 2022, 15, 4909. [Google Scholar] [CrossRef] [PubMed]



| Sample Code | ID/IG |
|---|---|
| Graphite | 0.05 |
| GO | 0.60 |
| GO/SiO2 | 0.94 |
| pH of the Sample | Initial Concentration (Ci) (ppm) | Final Concentration (Ce) (ppm) | Adsorbance Percentage (%) | ||
|---|---|---|---|---|---|
| GO | Si/GO | GO | Si/GO | ||
| 3 | 30 | 8.08331 | 5.77240 | 73.06 ± 0.6 | 80.76 ± 0.125 |
| 5 | 30 | 10.09871 | 10.97567 | 66.34 ± 0.3 | 63.41 ± 0.312 |
| 7 | 30 | 10.00400 | 10.68623 | 66.65 ± 0.025 | 64.38 ± 0.421 |
| 9 | 30 | 10.11465 | 11.00769 | 66.28 ± 0.532 | 60.97 ± 0.25 |
| Initial Concentration of the Sample (Ci) (ppm) | Concentration after Adsorption (Ce) (ppm) | Percentage of Adsorption (%) | ||
|---|---|---|---|---|
| GO | Si/GO | GO | Si/GO | |
| 1 | 0.61504 | 0.29760 | 38.49 ± 0.25 | 70.24 ± 0.65 |
| 10 | 5.94674 | 2.46630 | 40.53 ± 0126 | 75.34 ± 35 |
| 30 | 8.08331 | 5.77240 | 73.06 ± 0.6 | 80.76 ± 0.69 |
| 50 | 6.92333 | 3.85825 | 86.15 ± 0.8 | 92.28 ± 0.45 |
| 100 | 65.34009 | 52.31453 | 34.66 ± 0.58 | 47.69 ± 0.52 |
| 200 | 132.2878 | 118.8226 | 33.86 ± 0.87 | 40.59 ± 0.85 |
| No. | Adsorbent | Optimum pH | Removal % (a)/Adsorption Capacity (mg/g) (b) | Reference |
|---|---|---|---|---|
| 1 | Graphene oxide/silica nanosheets with multistage pores | 2 | 90 (a) | [57] |
| 2 | Surface-modified Jacobsite (MnFe2O4) nanoparticles | 2 | 31.55 (b) | [58] |
| 3 | GO prepared from Graphite | 4 | 92.8 (a) | [32] |
| 4 | Maghemite nanoparticles | 2.5 | 97.3 (a) | [59] |
| 5 | Magnetite nanoparticle | 2.5 | 99.8 (a) | [49] |
| 6 | Nanostructured graphite oxide | 3 | 63 (a) | [31] |
| 7 | Silica/graphite oxide composite | 3 | 60.24 (b) | [31] |
| 8 | Chitosan-grafted graphene oxide (CS-GO) nanocomposite | 2 | 96 (a) | [51] |
| 9 | Chitosan GO | 3 | 310.4 (b) | [60] |
| 10 | Graphene oxide chitosan microspheres modified with α–FeO(OH) | 3 | 97.69 (a) | [61] |
| 11 | Trioctylamine-exfoliated graphene oxide (TOA–EGO) | 2.5–3 | 96.3 | [23] |
| 12 | Graphene oxide (GO) | 3 | 86.15 ± 0.8 (a) | Present study |
| 13 | GO-SiO2 | 3 | 92.28 ± 0.45 (a) | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jibin, K.P.; Augustine, S.; Velayudhan, P.; George, J.S.; Krishnageham Sidharthan, S.; Paulose, S.V.; Thomas, S. Unleashing the Power of Graphene-Based Nanomaterials for Chromium(VI) Ion Elimination from Water. Crystals 2023, 13, 1047. https://doi.org/10.3390/cryst13071047
Jibin KP, Augustine S, Velayudhan P, George JS, Krishnageham Sidharthan S, Paulose SV, Thomas S. Unleashing the Power of Graphene-Based Nanomaterials for Chromium(VI) Ion Elimination from Water. Crystals. 2023; 13(7):1047. https://doi.org/10.3390/cryst13071047
Chicago/Turabian StyleJibin, Keloth Paduvilan, Silpa Augustine, Prajitha Velayudhan, Jesiya Susan George, Sisanth Krishnageham Sidharthan, Sylas Variyattel Paulose, and Sabu Thomas. 2023. "Unleashing the Power of Graphene-Based Nanomaterials for Chromium(VI) Ion Elimination from Water" Crystals 13, no. 7: 1047. https://doi.org/10.3390/cryst13071047
APA StyleJibin, K. P., Augustine, S., Velayudhan, P., George, J. S., Krishnageham Sidharthan, S., Paulose, S. V., & Thomas, S. (2023). Unleashing the Power of Graphene-Based Nanomaterials for Chromium(VI) Ion Elimination from Water. Crystals, 13(7), 1047. https://doi.org/10.3390/cryst13071047







