Synthesis and Crystal Structure of 1-(3-Fluorophenyl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one
Abstract
: The base catalyzed intramolecular nucleophilic cyclization of 1-(2-bromobenzoyl)-3-(2-fluorophenyl)thiourea (1) in the presence of N,N-dimethyl formamide (DMF) afforded the 1-(3-fluorophenyl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one (2) by an intramolecular nucleophilic substitution SNAr mechanism. The structure was supported by the spectroscopic data and unambiguously confirmed by the single crystal X-ray diffraction data. It crystallizes in the orthorhombic space group P na21 with unit cell dimensions a = 22.430(4), b = 8.1478(16), c = 13.522(3) Å, V = 2471.2(9) Å3. There are two independent molecules per asymmetric unit that are linked to centrosymmetric AB-dimers via intermolecular N-H…S bonds.1. Introduction
Quinazolinone is the building unit of nearly 150 naturally occurring alkaloids isolated from microorganisms, plants and animals [1]. It is a very important heterocycle exhibiting excellent pharmacological activities such as antimicrobial [2], antifungal [3], antitumor [4], anticancer [5], antiinflammatory [6], antidepressant [7] and anticonvulsant [8] activities. 3-Aryl-2-thioxo-2,3-dihydroquinazolin-4(1H)-one are a subclass quinazolinones having a wide range of applications including pharmacological and biological activities and important building blocks a variety of heterocycles [9]. Thus altanserin (3-(2-(4-(4-fluorobenzoyl)-l-piperidinyl)-ethyl)-2,3-dihydro-2-thioxo-1H-quinazolin-4-one) and nitroaltanserin are used as drugs for 5-HT2A receptor antagonists [10]. 2-Thioxo-1H-4-quinazolinones are also versatile intermediates for fused heterocycloquinazolines like 2-phenyl-5H-[1,3,4]thiadiazolo[2,3-b]quinazolin-5-one, 3-(4-bromo phenyl)-2H,6H-[1,3,4]thiadiazino- [2,3-b] quinazolin-6-one, 4-amino-2-phenyl-3a,4-dihydro-2H-thiazolo[3,2-a]quinazoline-1,5-dione and 2-phenyl-[1,3,4]thiadiazino[2,3-b] quinazoline-3,6(2H,4H)-diones [11]. Two approaches for the solution-phase parallel synthesis of 2-thioxoquinazolin-4-ones include the reaction of methyl anthranilates with isothiocyanates in refluxing pyridine or DMF and reacting 2-(methylcarboxy)-benzene isothiocyanates in isopropyl alcohol with different aliphatic amines or anilines [12]. Herein we report a convenient method for synthesis of these compounds by base catalyzed intramolecular nucleophilic cyclization of 1-aroyl-3-arylthioureas.
2. Results and Discussion
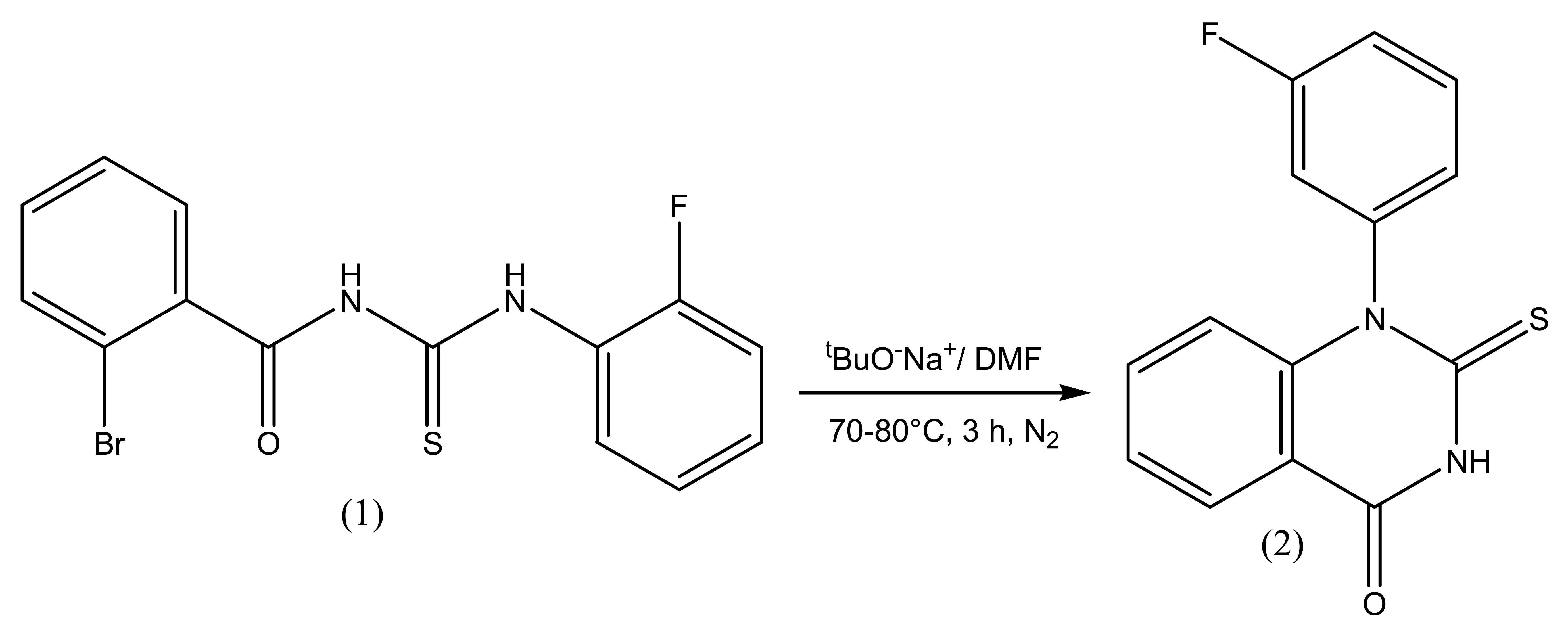
The reaction sequence leading to the formation of title heterocycle is outlined in Figure 1. The base catalyzed intramolecular nucleophilic cyclization of 1-(2-bromobenzoyl)-3-(2-fluorophenyl)thiourea (1) was achieved using sodium tertiary butoxide in the presence of dry DMF by heating at 70–80 °C for 3 h under nitrogen to afford (2) which was recrystallized from ethanol as colorless crystals.
IR spectral data of (2) shows the characteristic single broad N-H peak in the range 3265 cm−1 and a sharp C=O peak at 1650 cm−1 which are relatively at higher wave number compared to the parent thiourea; aromatic absorptions appear at 1603 cm−1, while those for C-S and C-N at 1249 cm−1 and 1136 cm-1 respectively. 1H-NMR shows characteristic broad singlet for N-H at δ 10.8 in addition to those due to aromatic protons at δ 6.55–8.3 ppm [13]. In 13C-NMR spectrum characteristic peaks for C=S appears downfield as compared to C=O in contrast to the trend in thioureas where the C=O resonates at relatively low field while the C=S resonates up field; thus C=S appears at δ 177, C=O appears at δ 168.9 and C-F couplings appear in the range 1JC-F = 247 Hz, 2JC-F = 22–24 Hz, 3JC-F = 6–14 Hz and 4JC-F = 3.75 Hz.
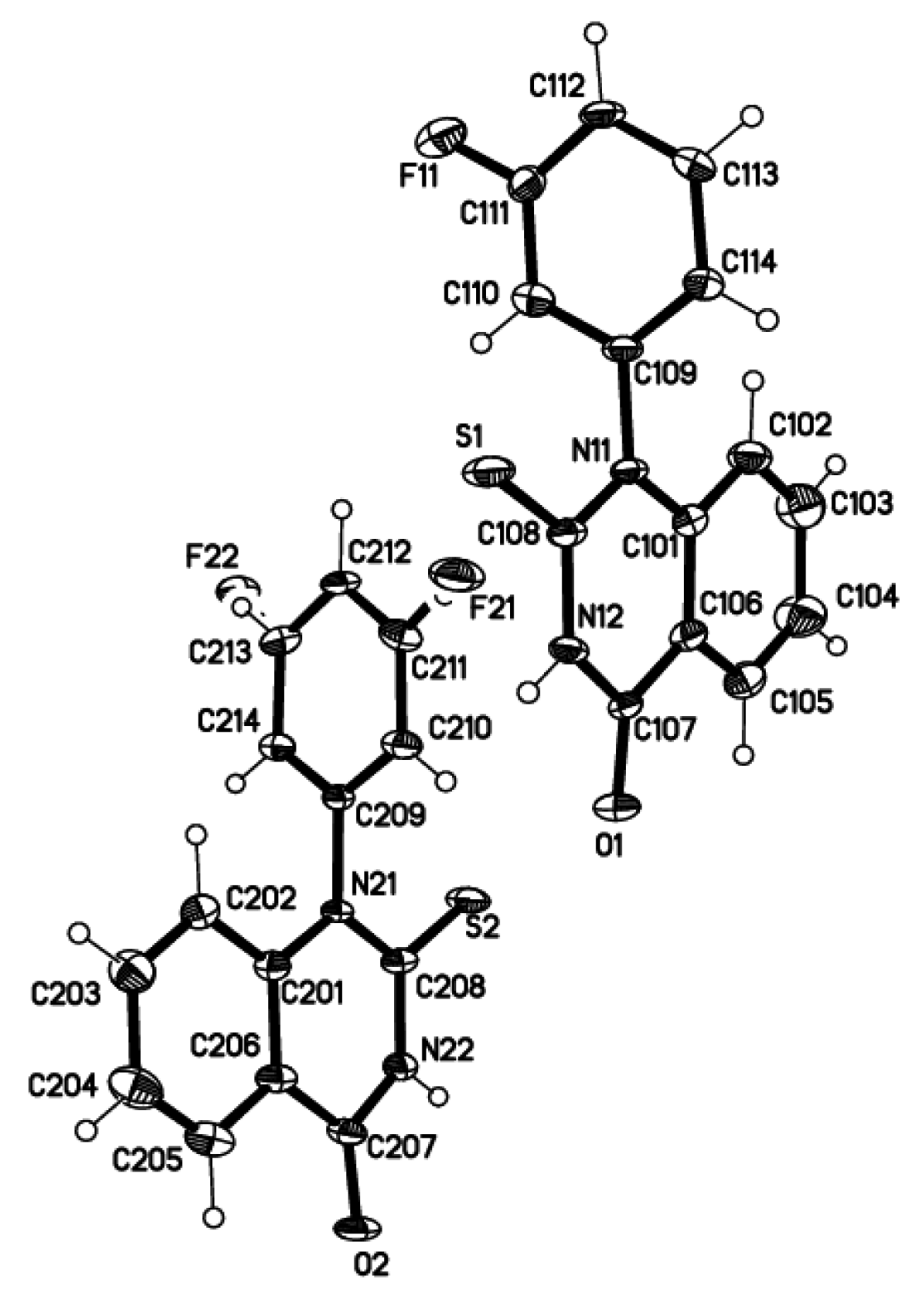
There are two crystallographically independent molecules A and B per asymmetric unit which exhibit almost equal geometries. Both the fluorophenyl rings make dihedral angles of 86.3(1)° (A) and 82.6(1)° (B) with the corresponding quinazoline planes. C=O and S=O bond lengths of av. 1.231(4) and 1.666(4) Å, respectively, correspond well to those from CCDC reference JESWEK [14] with equal thioxo group (Figure 2).
In the crystal packing molecules A and B are linked to centrosymmetric AB-dimers (Figure 3) via intermolecular N-H…S hydrogen bonds N12-H…S2 (−x + 0.5, y + 0.5,z − 0.5) with N…S of 3.374(3) Å and N22-H…S1 (−x + 0.5, y − 0.5, z + 0.5) with N…S of 3.272(3) Å, resp.
2.1. Crystal Structure Determination
Data were collected at 120(2) K on a Bruker AXS SMART APEX CCD diffractometer using MoKα radiation. Multi-scan absorption correction with SADABS [15]. Structure solved by direct methods [16], full-matrix least-squares refinement [16] on F² for 5877 unique intensities and 354 parameters, all but H atoms refined anisotropically, H atoms from difference Fourier maps refined with riding model on idealized positions with Uiso = 1.2 Ueq(C/N) and C-H/N-H distances of 0.95/0.88 Å. There are two chemically equal but crystallographically independent molecules A and B per asymmetric unit. Molecule B shows disorder of the fluoro substituent over both meta-positions with site occupation factors of 0.671(7) and 0.329(7) for F21 and F22, resp. Experimental data are listed in Table 1.
3. Experimental Section
Melting points were recorded using a digital Gallenkamp (SANYO) model MPD BM 3.5 apparatus and are uncorrected. 1H NMR spectra were determined as CDCl3 solutions at 300 MHz using a Bruker Mass Spectra (EI, 70 eV) on a GC-MS instrument. All compounds were purified by thick layer chromatography using silica gel from Merck.
Synthesis of 1-(3-Fluorophenyl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one (2)
1-(2-Bromobenzoyl)-3-(2-fluorophenyl)thiourea (1) (0.0015 mol), sodium tertiary butoxide (0.0072 mol) and dry DMF (20 mL) were taken in 100 ml three neck round bottom flask fitted with reflux condenser and nitrogen assembly. The reaction mixture was stirred at 70–80 °C for 1–3 h. The progress of reaction was monitored with the help of TLC. When the reaction was completed, the reaction mixture was diluted with water and extracted with dichloromethane. The organic layer was washed with water, dried and evaporated at rotary evaporator and the solid obtained was recrystallized from ethanol to afford (2) as colourless crystals. (80%): m.p. 203 °C; Rf 0.38 (a); IR (KBr): ν/cm−1 3265 (N-H), 1650 (C=O), 1603 (Ar-C=C), 1249 (C-S), 1136 (C-N); 1H NMR (300 MHz, DMSO-d6) δ 10.08 (1H, br s, NH), 8.29 (1H, dd, J = 1.5, 7.8 Hz, Ar-H), 7.67−7.29 (4H, m, Ar-H), 7.15 (1H, d, J = 8.1 Hz, Ar-H), 7.09 (1H, td, J = 2.1, 8.7 Hz, Ar-H), 6.55 (1H, d, J = 8.4 Hz, Ar-H); 13C NMR (75 MHz, d6-DMSO): δ 177.1 (1C, C=S), 168.9 (1C, C=O), 157.7 (1C, d, 1JC-F = 247 Hz, Ar-C), 143.4 (1C, Ar-C), 137.1(1C, d, 4J = 3.75, Ar-C), 132.5−122.2 (3C, 3Ar-C), 121.1 (1C, d, 3J = 6.75Hz, Ar-C), 119.8 (1C, d, 2JC-F = 22.5 Hz, Ar-C), 118.9−117.9 (3C, 3Ar-C), 117.6 (1C, d, 3JC-F = 13.5 Hz, 1C, Ar-C), 117.3 (1C, d, 2JC-F = 23.25 Hz, Ar-C); Anal. Calcd. for C14H9N2OSF: C 61.75; H 3.33; N 10.29; S 11.78%. Found: C 61.79; H 3.35; N 10.21; S 11.71%; GC-MS (m/z): 272 (M͘+) (100%), 213, 185, 149, 92, 75.
4. Conclusions
Synthesis, characterization, crystal and molecular structure of a novel medicinally and synthetically important heterocycle have been described.

| Empirical formula | C14 H9 F N2 O S |
| Formula weight | 272.29 |
| Temperature | 120(2) K |
| Wavelength | 0.71073 Å |
| Density (calculated) | 1.464 Mg/m3 |
| Absorption coefficient | 0.266 mm−1 |
| F(000) | 1120 |
| Crystal size | 0.39 × 0.31 × 0.29 mm3 |
| Theta range for data collection | 1.82 to 27.88°. |
| Index ranges | −29 ≤ h ≤ 29, −10 ≤ k ≤ 10, −17 ≤ l ≤ 17 |
| Reflections collected | 18748 |
| Independent reflections | 5877 [R(int) = 0.0701] |
| Completeness to theta = 27.88° | 100.0% |
| Absorption correction | Semi-empirical from equivalents |
| Max. and min. transmission | 0.9268 and 0.9033 |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 5877/1/354 |
| Goodness-of-fit on F2 | 1.038 |
| Final R indices [I > 2σ(I)] | R1 = 0.0649, wR2 = 0.1439 |
| R indices (all data) | R1 = 0.1001, wR2 = 0.1638 |
| Absolute structure parameter | 0.89(12) |
| Largest diff. peak and hole | 0.971 and −0.325 e Å−3 |
| CCDC No. | 822489 |
Acknowledgments
The author gratefully acknowledges a research grant from the Higher Education Commission of Pakistan under project No. 4-279/PAK-US/HEC 2010-917.
References and Notes
- Mhaske, S.B.; Argade, N.P. The chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron 2006, 62, 9787–9826. [Google Scholar]
- Patel, B.N.; Patel, C.J. Synthesis and Antimicrobial Activity of 3-(1,3,4-Oxadiazol-2-yl)quinazolin-4(3H)-ones. Sci. Pharm. 2010, 78, 171–193. [Google Scholar]
- Bartroli, J.; Turmo, E.; Alguero, M.; Boncompte, E.; Vericat, M.L.; Conte, L.; Ramis, J.; Merlos, M.; Garcia-Rafanell, J.; Forn, J. New Azole Antifungals. 3. Synthesis and Antifungal Activity of 3-Substituted-4(3H)-quinazolinones. J. Med. Chem. 1998, 41, 1869–1882. [Google Scholar]
- Al-Obaid, A.M.; Abdel-Hamide, S.G.; El-Kashef, H.A.; Abdel-Aziz, A.A.-M.; El-Azab, A.S.; Al-Khamees, H.A.; El-Subbagh, H.I. Substituted quinazolines, part 3. Synthesis, in vitro antitumor activity and molecular modeling study of certain 2-thieno-4(3H)-quinazolinone analogs. Eur. J. Med. Chem. 2009, 44, 2379–2391. [Google Scholar]
- Kamal, A.; Vijaya, B.E.; Janaki Ramaiah, M.; Dastagiri, D.; Surendranadha, R.J.; Viswanath, A.; Sultana, F.; Pushpavalli, S.N.C.V.L.; Pal-Bhadra, M.; Srivastava, H.K.; Narahari, S.G.; Juvekar, A.; Sen, S.; Zingde, S. Quinazolinone linked pyrrolo[2,1-c][1,4]benzodiazepine (PBD) conjugates: Design, synthesis and biological evaluation as potential anticancer agents. Bioorg. Med. Chem. 2010, 18, 526–542. [Google Scholar]
- Laddha, S.S.; Bhatnagar, P.S. A new therapeutic approach in Parkinson's disease: Some novel quinazoline derivatives as dual selective phosphodiesterase 1 inhibitors and anti-inflammatory agents. Bioorg. Med. Chem. 2009, 17, 6796–6802. [Google Scholar]
- Gökhan-Kelekçi, N.; Koyunoglu, S.; Yabanoğlu, S.; Yelekçi, K.; Özgen, Ö.; Uçar, G.; Erol, K.; Kendi, E.; Yesilada, A. New pyrazoline bearing 4(3H)-quinazolinone inhibitors of monoamine oxidase: Synthesis, biological evaluation, and structural determinants of MAO-A and MAO-B selectivity. Bioorg. Med. Chem. 2009, 17, 675–689. [Google Scholar]
- Mohsen, M.A.; Yahia, A.M.; Elkhairy, A.M.; Wahid, M.B.; Samir, Y.A. Synthesis of some new 4(3H)-quinazolinone-2-carboxaldehyde thiosemicarbazones and their metal complexes and a study on their anticonvulsant, analgesic, cytotoxic and antimicrobial activities - Part-1. Eur. J. Med. Chem. 2010, 45, 3365–3373. [Google Scholar]
- Abdel Gawad, N.M.; Georgey, H.H.; Youssef, R.M.; El Sayed, A.N. Design, synthesis, and anticonvulsant activity of novel quinazolinone analogues. Med. Chem. Res. 2011, 20, 1280–1286. [Google Scholar]
- Liu, K.C.; Shih, B.J.; Chern, J.W. Condensed 1,3-benzothiazinones. Facile synthesis of 2-amino-1,2,4-triazolo[5,1-b][1,3]-benzothiazin-9-one. J. Heterocycl. Chem. 1988, 25, 1215–1217. [Google Scholar]
- Bowman, W.R.; Harry Heaney, H.; Smith, P.H.G. Synthesis of 1H-quinazoline-4-ones using intramolecular aromatic nucleophilic substitution. ARKIVOC 2003, (x), 434–442. [Google Scholar]
- Al-Khalil, S.I.; Bowman, W.R. Effects of methanol solvation on the nucleophilic reactions of thiolates with 2-substituted-2-nitropropanes. Tetrahedron Lett. 1984, 25, 461–464. [Google Scholar]
- Saeed, A.; Shaheen, U.; Bolte, M. Synthesis, Characterization and Crystal Structure of Some Novel 1-Aryl-2-thioxo-2,3-dihydro-1H-quinazolin-4-ones. J. Chinese Chem. Soc. 2010, 5, 82–88. [Google Scholar]
- Xue, S.-J.; Wang, Q.-D.; Li, J.-Z. 1-(4,6-Diethoxypyrimidin-2-yl)-2-thioxo-2,3-dihydropyrido [2,3-d]pyrimidin-4(1H)-one. Acta Cryst. 2006, C62, o666–o668. [Google Scholar]
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 2004. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
© 2011 by the authors, licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saeed, A.; Flörke, U. Synthesis and Crystal Structure of 1-(3-Fluorophenyl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one. Crystals 2011, 1, 254-259. https://doi.org/10.3390/cryst1040254
Saeed A, Flörke U. Synthesis and Crystal Structure of 1-(3-Fluorophenyl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one. Crystals. 2011; 1(4):254-259. https://doi.org/10.3390/cryst1040254
Chicago/Turabian StyleSaeed, Aamer, and Ulrich Flörke. 2011. "Synthesis and Crystal Structure of 1-(3-Fluorophenyl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one" Crystals 1, no. 4: 254-259. https://doi.org/10.3390/cryst1040254





