Defect Scheelite-Type Lanthanoid(III) Ortho-Oxomolybdates(VI) Ln0.667[MoO4] (Ln = Ce, Pr, Nd, and Sm) and Their Relationship to Zircon and the NaTl-Type Structure
Abstract
: The rare-earth metal(III) ortho-oxomolybdates with the formula Ln0.667[MoO4] (Ln = Ce, Pr, Nd, and Sm) and defect scheelite-type structure crystallize in the tetragonal space group I41/a (a = 533−525, c = 1183−1158 pm) with four formula units per unit cell. The Ln3+ cations at Wyckoff position 4b exhibit a coordination sphere of eight oxygen atoms in the shape of a trigonal dodecahedron. The same site symmetry (4̄..) is observed for the tetrahedral oxomolybdate(VI) entities [MoO4]2−, since their central Mo6+ cation is situated at the 4a position. Due to this equal site multiplicity, the lanthanoid(III) cations have to be statistically under-occupied to maintain electroneutrality, thus a defect scheelite structure emerges. The partial structure of both the Ln3+ cations and the [MoO4]2− anions (if shrunk to their centers of gravity) can be best described as distorted diamond-like arrangements. Therefore, these two interpenetrating partial structures exhibit a similar setup as found in the zircon-type as well as in the NaTl-type structure.1. Introduction
The mineral scheelite (Ca[WO4]) is named after the German-Swedish pharmacist and chemist Carl Wilhelm Scheele, who, besides other elements, also discovered oxygen (independently from Joseph Priestly) and tungsten, and was able to synthesize tungstic acid from this mineral in the first place. The X-ray crystal structure of Ca[WO4] was originally published 1920 by Dickinson [1], but the positions of the oxygen atoms have not been determined. Besides zircon Zr[SiO4] [2], the scheelite structure is nature's favorite structure type for compounds containing larger cations (C.N. = 8 in case of both structure types) together with tetrahedral oxoanions. For trivalent rare-earth metal compounds, tetrahedral entities with pentavalent central atoms as counteranions, such as phosphates, arsenates and vanadates, are widely known. Besides compounds containing the larger lanthanide cations, which crystallize in the monazite-type (C.N.(Ln3+) = 9) [3-8], rare-earth metal phosphates, arsenates and vanadates prefer the xenotime- (Ln[PO4]) [4,7-11] and the wakefieldite-type Ln[VO4] [12,13], which are both equal to the zircon-type (C.N.(Ln3+) = 8); only a high-pressure modification of Sm[AsO4] is known to crystallize in the scheelite-type [14]. Switching from tri- to [MoO4]2− dianions, the trivalent lanthanide cations have to be either mixed with monovalent, mostly alkali metal, cations (e.g. NaLa[MoO4]2 [15]) or a deficiency on the atomic site prevails, which is known to the literature so far only for Nd0.667[MoO4] [16]. In this paper, we focus on the close relationship between the scheelite-type (Ca[WO4], here: the title compounds Ln0.667[MoO4], Ln = Ce, Pr, Nd, and Sm) and the zircon-type structure (Zr[SiO4]), which can both be derived from the crystal structure of sodium thallide (NaTl) [17].
2. Results and Discussion
2.1. Structure Description of Scheelite-Type Ln0.667[MoO4]
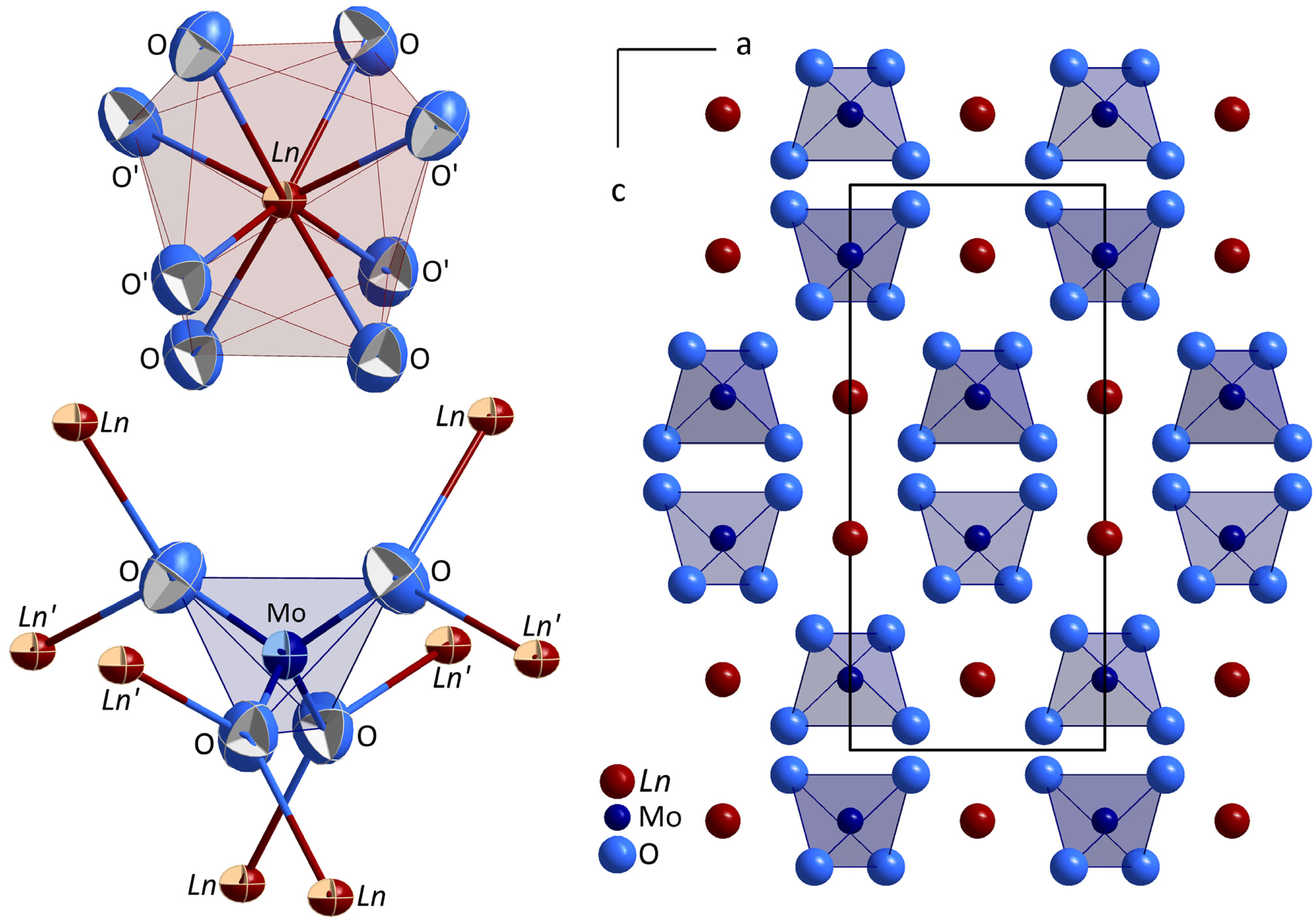
The rare-earth metal(III) ortho-oxomolybdates(VI) of the empirical formula Ln0.667[MoO4] (Ln = Ce, Pr, Nd, and Sm) in the defect scheelite-type structure crystallize tetragonally with the space group I41/a (a = 533−525, c = 1183−1158 pm) and four formula units per unit cell. In their crystal structure one crystallographically unique lanthanoid trication is present at Wyckoff position 4b (see Table 1 site symmetry: 4̄..), showing a coordination sphere of eight oxygen atoms in the shape of a trigonal dodecahedron (Figure 1, left top). The distances between the Ln3+ cations and their surrounding O2− anions range between 255 pm for the cerium compound, the largest of the four lanthanoid representatives, and 249 pm in the samarium derivative, the smallest one in this case (see Table 2). These distances are in good agreement with those of other rare-earth metal compounds with complex oxoanions such as the monazite-type phosphates with the formula Ln[PO4] (Ce[PO4]: d(Ce3+−O2−) = 245–265 pm; Sm[PO4]: d(Sm3+−O2−) = 239–259 pm) [4]. To maintain electroneutrality, the atom site of the Ln3+ cations cannot be fully occupied, but by about two thirds, which is the case for all four title compounds (see Table 1). The molybdenum(VI) cations are also crystallographically unique and situated at the Wyckoff position 4b (see Table 1, site symmetry: 4̄..). They are surrounded by four oxygen atoms forming bisphenoidally distorted tetrahedra whose molybdenum–oxygen bond lengths, as well as their O–Mo–O angles, do not vary much throughout the presented series (d(Mo6+−O2−) = 175–177 pm, (∢)(O2−−Mo6+−O2−) = 107–108°, 4×, and 114–116°, 2×, see Table 2). For comparison, the Mo–O bond lengths and angles found in powellite (scheelite-type Ca[MoO4]) lie at 176 pm, 107° (4×), and 115° (2×) [18] and thus agree very well with the herein presented data for the title compounds. The isolated [MoO4]2− tetrahedra (see Figure 1, left bottom) are exclusively vertex-connected to the polyhedra around the Ln3+ cations, therefore the crystallographically unique O2− anions are surrounded by one molydenum and two lanthanoid cations. The crystal structure of the Ln0.667[MoO4] series (Ln = Ce, Pr, Nd, and Sm) is shown in Figure 1, on the right.
2.2. The Structural Relationship Between the Scheelite-Type, the Zircon-Type, and the NaTl-Type Structure
A simple structure type for compounds containing complex building blocks can usually be determined when the complex unit is shrunk to its center of gravity. In the case of the scheelite-type structure, the result can be considered as an AB structure with a coordination number ratio of 8:8. The first structure type that comes to mind with these “real” coordination numbers would be cesium chloride (CsCl) [20], but no further similarity can be found between these two structures. Interconnecting the Ln3+ and the Mo6+ cations with themselves, they show a tetrahedral coordination environment towards each other and; thus, the structure ends up in two interpenetrating diamond-like lattices [21] (Figure 2, middle), which is the description of the NaTl-type structure [17] (Figure 2, left). The same is also true considering zircon-type structures [2] if stripped off the ligand O2− anions (Figure 2, right).
In the crystal structure of sodium thallide each Tl− anion is surrounded by ten Na+ cations and four Tl− anions resulting in an overall coordination number of 14. If only the sodium cations are considered, the thallium atoms are enclosed by adamantane-like cage with four shorter (323 pm, Na in Figure 3, left) and six longer distances (373 pm, Na′ in Figure 3, left). While the longer-bonded (Na′)+ cations form an octahedron around the central Tl− anion, the shorter connected (Na)+ cations arrange themselves tetrahedrally, building up a heterocuban cage together with the four next thallium neighbors, which show the same distance to the center as the four closest Na+ cations. Vice versa, the same is of course true, if the environment of Na+ is described. This symmetrically ideal setup (NaTl: cubic, Fd3̄, a = 748.8(3) pm [17], Na at Wyckoff position 8a, Tl in 8b, both with site symmetry 4̄3m) is tetragonally distorted in the scheelite-type structure (site symmetry: 4̄.. for both Ln3+ and Mo6+). Therefore eight short (≈ 375 pm, 4× and ≈ 400 pm, 4×) and two long distances (≈ 590 pm, 2×) between Mo6+ and Ln3+ are determined in the alike adamantane cage of Ln3+ around the Mo6+ (and thus around the [MoO4]2− anions) with the four closest neighbors (Ln in Figure 3, middle) forming a square plane around the central Mo6+ cation and the four slightly more distant ones (Ln′ in Figure 3, middle) arrange tetrahedrally like the (Na)+ cations in NaTl. Here also the description of the Ln3+ environment around the oxomolybdate unit can be interchanged to the alternative situation. In the case of the the zircon-type structure (Zr[SiO4]: tetragonal, I41/amd, a = 660.7(1), c = 598.2(1) pm [22], Zr at the Wyckoff position 4a, Si at 4b, both with site symmetry 4̄m2) a further distortion of the aforementioned arrangement is detectable. Again, four out of the ten Zr4+ cations of the adamantane cage build a tetrahedron around the central Si4+ cation (and thus around the [SiO4]4− anion). However, in this structure type once again these four are not the nearest surrounding atoms (363 pm, Zr′ in Figure 3, right), but two of the remaining six show a very short Zr4+⋯Si4+ distance of 299 pm (Zr in Figure 3, right). The other four are about 170 pm further away (467 pm, Zr″ in Figure 3, right). This distortion is easily explained by the interconnection of the tetrahedral complex oxoanion with the anionic polyhedra around the Zr4+ cations. While in the scheelite structure these are exclusively vertex-connected (at all eight O2− anions), in the zircon structure two edges and four vertices are the joining links, and the aforementioned short zirconium–silicon distances of 299 pm are those running through the connecting edges. Here again the partial structures around Zr4+ and [SiO4]4− can be interchanged.
In general, the adamantane cage can be dismembered resulting in an octahedron with an interpenetrating tetrahedron. While the tetrahedron contributes to the linking in all three cases (Figure 3: Na in NaTl, Ln′ in scheelite-type Ln0.667[MoO4], and Zr′ in zircon-type Zr[SiO4]), in the case of the octahedron, only for NaTl do all six members show the same distance to the central Tl− anion. For scheelite-type compounds the octahedron is stretched, leaving two very far (Ln″) and four short (Ln) contacts behind, and in zircon-type compounds it is compressed, comprising two very short (Zr) and four long (Zr″) distances to the central unit. In all cases the structures can also be described vice versa.
3. Experimental Section
3.1. Synthesis
All four representatives of the short Ln0.667[MoO4] series (Ln = Ce, Pr, Nd, and Sm) were only obtained as by-products so far. The direct synthesis using Ln2O3 and MoO3 in 1:3 molar ratios experiences a direct competition with the rare-earth metal “sesquimolybdates” Ln2[MoO4]3 (better: Ln2Mo3O12 since not all of the these structures contain isolated [MoO4]2− units), which are known in literature for some of the rare-earth elements, comprising Ln = Ce [23], Nd [16], and Sm [24], although in different structure types, depending on the size of the lanthanoid. In the case of Ce0.667[MoO4], the single crystals emerged from an unsuccessful attempt to synthesize Ce[MoO4]2. Pr0.667[MoO4] and Sm0.667[MoO4] were obtained in experiments planned to prepare the respective fluoride oxodimolybdates PrFMo2O7 and SmFMo2O7 [25], and the neodymium representative Nd0.667[MoO4] occurred as by-product in the synthesis of NdBr[MoO4] [26]. The single crystals of all four title compounds are coarse, transparent and remain stable when exposed to air and water. They show the color of the respective Ln3+ cation, i. e. green in the case of Pr, violet for Nd, and pale yellow for Sm. The crystals of the cerium derivative display an orange color, which is not quite uncommon, since very often in cerium(III) compounds the transition energy is lowered and the compound exhibits a color in the range between yellow and red, depending on the actual chemical surrounding of the Ce3+ cations. This can be assigned to the effect that the orbital, which contains the single f-electron lies within the band gap between the valence and the conduction band [27,28].
3.2. X-ray Structure Analysis
Intensity data sets for single crystals of all four Ln0.667[MoO4] representatives (Ln = Ce, Pr, Nd, and Sm) were collected on a Nonius Kappa-CCD diffractometer using graphite-monochromatized Mo-Kα radiation (wavelength: λ = 71.07 pm). A numerical absorption correction was performed with the help of the program HABITUS [29]. The structure solutions and refinements were carried out by using the program package SHELX-97 [30]. Details of the data collections and the structure refinements [31] are summarized in Table 3, atomic positions and coefficients of the equivalent isotropic displacement parameters [19] can be found in Table 1, while interatomic distances and selected bond angles are listed in Table 2. Further details of the crystal structure investigations can be obtained from the Fachinformationszentrum (FIZ) Karlsruhe, D-76344 Eggenstein-Leopoldshafen, Germany (Fax: +497247-808-666; E-Mail: crysdata@fiz-karlsruhe.de), on quoting the depository numbers CSD-423509 for Ce0.667[MoO4], CSD-423510 for Pr0.667[MoO4], CSD-423511 for Nd0.667[MoO4], and CSD-423512 for Sm0.667[MoO4].
4. Conclusions
Single crystals of four representatives of lanthanoid(III) oxomolybdates(VI) with deficient scheelite-type structure according to Ln0.667[MoO4] (Ln = Ce, Pr, Nd, and Sm) were obtained from the corresponding oxides (Ln2O3 and MoO3) as by-products in various synthetic experiments. Their crystal structure was determined and described in detail. Furthermore, the structural setup of the scheelite-type (Ca[WO4]) was compared to that of the zircon-type (Zr[SiO4]), which are both distortion varieties of the NaTl-type structure with two interpenetrating diamond-like sublattices.


| Ln = Ce | Wyckoff position | x/a | y/b | z/c | s. o. f.b) | occupation percentage | Ueqa) |
|---|---|---|---|---|---|---|---|
| Ce | 4b | 0 | ¼ | ⅝ | 0.1678(3) | 67.12(3) % | 121(2) |
| Mo | 4a | 0 | ¼ | ⅛ | 0.25 | 100 % | 139(2) |
| O | 16f | 0.1406(3) | 0.0112(3) | 0.2067(2) | 1.0 | 100 % | 288(5) |
| Ln = Pr | |||||||
| Pr | 4b | 0 | ¼ | ⅝ | 0.1654(6) | 66.16(6) % | 80(3) |
| Mo | 4a | 0 | ¼ | ⅛ | 0.25 | 100 % | 124(3) |
| O | 16f | 0.1406(6) | 0.0096(7) | 0.2062(3) | 1.0 | 100 % | 299(10) |
| Ln = Nd | |||||||
| Nd | 4b | 0 | ¼ | ⅝ | 0.1685(7) | 67.40(7) % | 107(3) |
| Mo | 4a | 0 | ¼ | ⅛ | 0.25 | 100 % | 132(4) |
| O | 16f | 0.1458(9) | 0.0099(9) | 0.2049(4) | 1.0 | 100 % | 256(10) |
| Ln = Sm | |||||||
| Sm | 4b | 0 | ¼ | ⅝ | 0.1632(4) | 65.28(4) % | 102(3) |
| Mo | 4a | 0 | ¼ | ⅛ | 0.25 | 100 % | 128(3) |
| O | 16f | 0.1477(4) | 0.0097(4) | 0.2078(2) | 1.0 | 100 % | 254(6) |
a)Ueq is defined as the ⅓ of the trace of the orthogonalized Uij tensor [19];b)s. o. f. = site occupation factor.
| Ce0.667[MoO4] | |||
|---|---|---|---|
| d(Ce3+−O2−) | 4 × 254.3(2) pm | d(Mo6+−O2−) ∢;(O2−−Mo6+−O2−) | 4 × 176.6(2) pm |
| 4 × 255.8(2) pm | 4 × 107.5(1)° | ||
| d(Ce3+⋯Mo6+) | 4 × 376.9(2) pm | 2 × 113.6(1)° | |
| 4 × 398.2(2) pm | |||
| Pr0.667[MoO4] | |||
| d(Pr3+−O2−) | 4 × 253.5(4) pm | d(Mo6+−O2−) ∢(O2−−Mo6+−O2−) | 4 × 176.5(3) pm |
| 4 × 254.7(3) pm | 4 × 107.1(1)° | ||
| d(Pr3+⋯Mo6+) | 4 × 376.4(3) pm | 2 × 114.3(2)° | |
| 4 × 397.0(3) pm | |||
| Nd0.667[MoO4] | |||
| d(Nd3+−O2− | 4 × 250.0(4) pm | d(Mo6+−O2−) ∢(O2−−Mo6+−O2−) | 4 × 175.2(5) pm |
| 4 × 253.6(5) pm | 4 × 106.5(2)° | ||
| d(Nd3+⋯Mo6+) | 4 × 373.3(4) pm | 2 × 115.6(3)° | |
| 4 × 393.8(4) pm | |||
| Sm0.667[MoO4] | |||
| d(Sm3+−O2−) | 4 × 249.0(2) pm | d(Mo6+–O2−) ∢(O2−−Mo6+−O2−) | 4 × 176.4(2) pm |
| 4 × 249.3(2) pm | 4 × 107.2(1)° | ||
| d(Sm3+⋯Mo6+) | 4 × 371.3(2) pm | 2 × 114.2(1)° | |
| 4 × 390.9(2) pm | |||
| Ln | Ce | Pr | Nd | Sm |
|---|---|---|---|---|
| Lattice constants, a/pm | 533.07(3) | 532.27(3) | 527.94(3) | 525.09(3) |
| c/pm | 1183.33(7) | 1178.56(7) | 1169.12(7) | 1158.38(7) |
| c/a | 2.220 | 2.214 | 2.214 | 2.206 |
| Calculated density, Dx/g·cm−3 | 5.005 | 5.051 | 5.220 | 5.411 |
| Molar volume, Vm/cm3·mol−1 | 50.63 | 50.27 | 49.06 | 48.08 |
| F(000) | 450.7 | 453.4 | 456.0 | 461.4 |
| Index range, ±h/±k/±l | 7/7/15 | 7/7/15 | 6/7/14 | 6/6/15 |
| Theta range, θmin − θmax/deg | 4.2 – 28.3 | 4.2 – 28.2 | 4.2 – 28.3 | 4.3 – 28.1 |
| Absorption coefficient, μ/mm−1 | 12.53 | 13.25 | 14.25 | 15.94 |
| Collected/unique reflections/parameters | 2298/209/16 | 2290/206/16 | 1444/202/16 | 2492/196/16 |
| Rint/Rσ | 0.039/0.016 | 0.082/0.030 | 0.073/0.042 | 0.063/0.020 |
| R1 for (n) refletions with | 0.014 | 0.020 | 0.019 | 0.016 |
| |Fo| > 4σ(Fo) | (n = 173) | (n = 121) | (n = 96) | (n = 164) |
| R1/wR2 for all reflections | 0.018/0.030 | 0.045/0.040 | 0.068/0.040 | 0.021/0.036 |
| Goodness of Fit (GooF) | 1.082 | 1.038 | 0.925 | 1.107 |
| Extinction, g | 0.0103(6) | 0.0040(6) | 0.0033(4) | 0.0123(8) |
| Residual electron density, ρ/e−·10−6 pm−3, min./max. | 0.34/−0.35 | 0.51/−0.43 | 0.46/−0.57 | 0.33/−0.41 |
Acknowledgments
The financial support of the German Research Foundation (DFG, Bonn, Germany) and the State of Baden-Württemberg (Stuttgart, Germany) is gratefully acknowledged.
References and Notes
- Dickinson, R.G. The Crystal Structure of Wulfenite and Scheelite. J. Am. Chem. Soc. 1920, 42, 85–93. [Google Scholar]
- Hassel, O. Die Kristallstruktur einiger Verbindungen von der Zusammensetzung MRO4. I. Zirkon ZrSiO4. Z Kristallogr. 1926, 63, 247–254. [Google Scholar]
- Mooney, R.C.L. Crystal Structures of a Series of Rare Earth Phosphates. J. Chem. Phys. 1948, 16, 1003–1003. [Google Scholar]
- Ni, Y.-X.; Hughes, J.M.; Mariano, A.N. Crystal Chemistry of the Monazite and Xenotime Structures. Amer. Miner. 1995, 80, 21–26. [Google Scholar]
- Brusset, H.; Madaule-Aubry, F.; Mahe, R.; Boursier, C. Chimie Structural - Étude de la Structure de l'Orthovanadate de Lanthane. C. R. Hebd. Seances Acad. Sci. 1971, 273, 455–458. [Google Scholar]
- Brahimi, A.; Ftini, M.M.; Amor, H. Cerium Arsenate, CeAsO4. Acta Crystallogr. 2002, E58, 98–99. [Google Scholar]
- Schmidt, M.; Müller, U.; Cardoso-Gil, R.; Milke, E.; Binnewies, M. Zum chemischen Transport und zur Kristallstruktur von Seltenerdarsenaten(V). Z. Anorg. Allg. Chem. 2005, 631, 1154–1162. [Google Scholar]
- Kang, D.-H.; Schleid, Th. Einkristalle von La[AsO4] im Monazit- und Sm[AsO4] im Xenotim-Typ. Z. Anorg. Allg. Chem. 2005, 631, 1799–1802. [Google Scholar]
- Vegard, L. Structure of Xenotime and Relations Between Chemical Constitution and Crystal Structure. Philos. Mag. 1927, 4, 511–525. [Google Scholar]
- Schäfer, W.; Will, G. Neutron Diffraction Study of Antiferromagnetic DyAsO4. J. Phys. 1971, C 4, 3224–3233. [Google Scholar]
- Kang, D.-H.; Höss, P.; Schleid, Th. Xenotime-Type Yb[AsO4]. Acta Crystallogr. 2005, E61, i270–i272. [Google Scholar]
- Broch, E. Die Kristallstruktur von Yttriumvanadat. Z Phys. Chem. B. 1933, 20, 345–350. [Google Scholar]
- Milligan, W.O.; Vernon, L.W. Crystal Structure of Heavy Metal Orthovanadates. J. Phys. Chem. 1952, 56, 145–148. [Google Scholar]
- Kang, D.-H.; Schleid, Th. Einkristalle von Sm[AsO4] im Scheelit-Typ. Z Anorg. Allg. Chem. 2006, 632, 2147–2147. [Google Scholar]
- Stevens, S.B.; Morrison, C.A.; Allik, T.H.; Rheingold, A.L.; Haggerty, B.S. NaLa(MoO4)2 as a Laser Host Material. Phys. Rev. B: Condens. Matter. 1991, 43, 7386–1394. [Google Scholar]
- Jamieson, P.B.; Abrahams, S.C.; Bernstein, J.L. Crystal Structure of the Transition-Metal Molybdates and Tungstates. V. Paramagnetic α-Nd2[MoO4]3. J. Chem. Phys. 1969, 50, 86–94. [Google Scholar]
- Zintl, E.; Dullenkopf, W. Über den Gitterbau von NaTl und seine Beziehung zu den Strukturen vom Typus des β-Messings. Z Phys. Chem. B. 1932, 16, 195–205. [Google Scholar]
- Aleksandrov, V.B.; Gorbatyii, L.V.; Ilyukhin, V.V. Crystal Structure of Powellite Ca[MoO4]. Kristallografiya 1968, 13, 512–513. [Google Scholar]
- Fischer, R.X.; Tillmanns, E. The Equivalent Isotropic Displacement Factor. Acta Crystallogr. 1988, C44, 775–776. [Google Scholar]
- Davey, W.P.; Wick, F.G. Crystal Structures of CsCl and TlCl. Phys. Rev. 1921, 17, 403–404. [Google Scholar]
- Hull, W.H.; Bragg, W.L. Structure of some Crystals. Proc. R Soc. London Ser. A. 1913, 33, 277–277. [Google Scholar]
- Robinson, K.; Gibbs, K.V.; Ribbe, P.H. The Structure of Zircon: A Comparison with Garnet. Am. Mineral. 1971, 56, 782–789. [Google Scholar]
- Hartenbach, I. Crystal Structure of Ce2Mo3O12,; University of Stuttgart: Germany, Unpublished Results; 2010. [Google Scholar]
- Hartenbach, I. Die Kristallstruktur von Samarium-Sesquimolybdat Sm2[MoO4]3. Z. Anorg. Allg. Chem. 2008, 634, 2044–2044. [Google Scholar]
- Müller, S.L. Synthese und Charakterisierung von Selten-Erd-Metall Fluorid Dimolybdaten. Exam Thesis, University of Stuttgart, 2012; In Preparation. [Google Scholar]
- Schustereit, T. Die Reihe der Seltenerdmetall(III)-Bromid-Oxomolybdate(VI) und die Kristallstrukturen zweier Nebenprodukte. Exam Thesis, University of Stuttgart, 2010. [Google Scholar]
- Dieke, G.H. Spectra and Energy Levels of Rare Earth Ions in Crystals; Interscience Publishers: New York, NY, USA, 1969. [Google Scholar]
- Dorenbos, P. Absolute Location of Lanthanide Energy Levels and the Performance of Phosphors. J. Lumin. 2007, 122-123, 315–317. [Google Scholar]
- Herrendorf, W.; Bärnighausen, H. HABITUS: Program for the Optimization of the Crystal Shape for Numerical Absorption Correction in X-SHAPE, version 1.06; Fa. Stoe: Darmstadt/Karlsruhe/Gießen, Germany, 1999/1993/1996. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar]
- Hahn, T.; Wilson, A.J.C. International Tables for Crystallography, 2nd ed.; Kluwer Academic Publishers: Boston, MA, USA, 1992; Volume C. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schustereit, T.; Müller, S.L.; Schleid, T.; Hartenbach, I. Defect Scheelite-Type Lanthanoid(III) Ortho-Oxomolybdates(VI) Ln0.667[MoO4] (Ln = Ce, Pr, Nd, and Sm) and Their Relationship to Zircon and the NaTl-Type Structure. Crystals 2011, 1, 244-253. https://doi.org/10.3390/cryst1040244
Schustereit T, Müller SL, Schleid T, Hartenbach I. Defect Scheelite-Type Lanthanoid(III) Ortho-Oxomolybdates(VI) Ln0.667[MoO4] (Ln = Ce, Pr, Nd, and Sm) and Their Relationship to Zircon and the NaTl-Type Structure. Crystals. 2011; 1(4):244-253. https://doi.org/10.3390/cryst1040244
Chicago/Turabian StyleSchustereit, Tanja, Sabine L. Müller, Thomas Schleid, and Ingo Hartenbach. 2011. "Defect Scheelite-Type Lanthanoid(III) Ortho-Oxomolybdates(VI) Ln0.667[MoO4] (Ln = Ce, Pr, Nd, and Sm) and Their Relationship to Zircon and the NaTl-Type Structure" Crystals 1, no. 4: 244-253. https://doi.org/10.3390/cryst1040244
APA StyleSchustereit, T., Müller, S. L., Schleid, T., & Hartenbach, I. (2011). Defect Scheelite-Type Lanthanoid(III) Ortho-Oxomolybdates(VI) Ln0.667[MoO4] (Ln = Ce, Pr, Nd, and Sm) and Their Relationship to Zircon and the NaTl-Type Structure. Crystals, 1(4), 244-253. https://doi.org/10.3390/cryst1040244





