Abstract
A reaction of YI3, dibenzo-24-crown-8 and iodine in ethanol yielded, as a by-product, red single crystals of (I2)@(db24c8). In the triclinic crystal, P-1, a = 485.0(1), b = 1203.7(3), c = 1280.4(2) pm, a = 64.56(2)°, β = 86.82(2)°, γ = 83.89(2)°, V = 671.1(2) × 106.pm3, Z = 1, R1= 0.0301 for 1965 reflections with I0 > 2σ(I0), iodine molecules with an I–I distance of 268.39(7) pm, slightly longer than in the gas phase, are included in a matrix of db24c8 molecules.1. Introduction
Dibenzo-24-crown-8 (db24c8) is a large crown ether which may encapsulate rather large cations in a nest-like conformation, such as in [K(db24c8)](I3) [1]. Three of these molecules may also form the second coordination shell of the large cation [Y(H2O)8(db24c8)3]3+ in which the first coordination sphere is built from eight water molecules. This cation was recently found in the polyiodide salt [Y(H2O)8(db24c8)3]2(I3)3(I5)3 [1]. In the course of the reactions that led to this mixed tri-/pentaiodide, we have also observed a small amount of red needles which turned out to be of the inclusion compound (I2)@(db24c8). Similar inclusion compounds of halogens and common polyethers seem not to be known. However, there are inclusion compounds of polyethers with compounds that are capable of forming hydrogen bonds, as for example in (PicH)2(db24c8) [2] (PicH = picric acid) and [Y(NO3)3(H2O)3](db24c8) [3].
2. Results and Discussion
The crystal structure of (I2)@(db24c8) is triclinic, space group P-1, with a pseudo-hexagonal unit cell, a = 485.0(1), b = 1203.7(3), c = 1280.4(2) pm, α = 64.56(2)°, β = 86.82(2)°, γ = 83.89(2)°, hence with ahex = bhex → b ≈ c, chex = a; αhex = βγhex →β ≈ γ ≈ 90°, γhex ≈ γ* 120°. There is one formula unit in the unit cell, the volume amounts to 671.1(2) × 106.pm3 (= Å3). One iodine molecule resides in the center of the unit cell, surrounded by four db24c8 molecules, see Figure 1. The I2 molecules are stacked almost on top of each other with the shortest intermolecular I–I distance of 485.0(1) pm = a. The intramolecular I–I distance is surprisingly short, 268.39(7) pm, only slightly larger than in gaseous iodine, 267 pm [4,5], and shorter than in solid iodine at 170 K, 271.5(6) pm [6,7]. Shortest distances of I to neighboring O and C atoms are 292.8(2) (I1-O4) and 374.7(2) pm (I1-C3), well and far beyond the sum of the van der Waals radii [8], 264 (I + O) and 275 (I + C) pm, respectively.
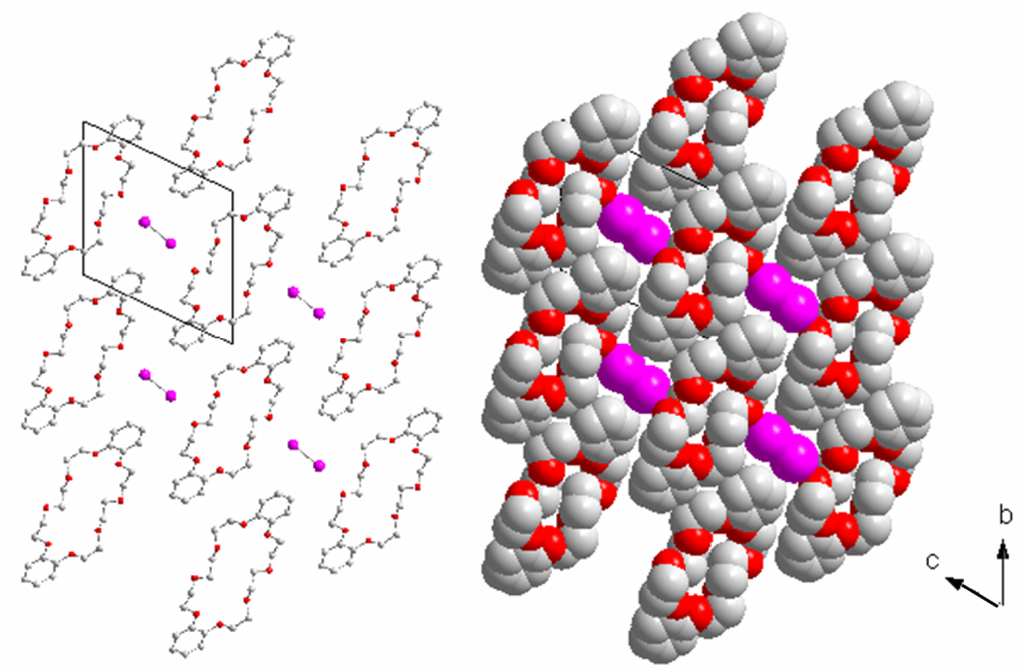
Thus, (I2)@(db24c8) can be considered as a true inclusion compound. The crystal structure of db24c8 [9] contains a rather dense peanut packing with a volume per db24c8 molecule of 577 × 106.pm3, 94 × 106.pm3 less than the volume of one formula unit of (I2)@(db24c8). In the crystal structure of iodine [6, 7], there are four molecules per unit cell with a cell volume of 327.2 × 106.pm3. One iodine molecule, therefore, affords a volume of 82 × 106.pm3 in solid iodine. In (I2)@(db24c8), the volume per iodine molecule is, with 94 × 106.pm3, considerably larger. This is in agreement with the more gas-like I–I distance in (I2)@(db24c8), hence with much less (I2) to (db24c8) interactions in the inclusion compound, compared with those in solid iodine where the shortest intermolecular distances, 353.8 pm, are much shorter than twice the van der Waals radius, 2 × 198 = 396 pm.
Structurally, rows of db24c8 molecules appear in the [001] direction in the crystal structure of db24c8, see Figure 2, right. These rows are arranged almost parallel to each other, with a shift according to the angle β = 104.93(1)°. The layers that occur parallel (010) are stacked on top of each other in the [010] direction of monoclinic db24c8. Space for one iodine molecule per db24c8 molecule (I2)@(db24c8) is provided through some rotation of the db24c8 molecules and a larger shift of the rows in the [010] direction of (I2)@(db24c8), see the comparison of the structures in Figure 2.

The conformation of the db24c8 molecules in both structures, (I2)@(db24c8) and the parent (db24c8), and their arrangement in both crystal structures are very similar. This may be seen from Figure 2 and from a comparison of the db24c8 molecules in both structures as shown in Figure 3. The C–C and C–O distances are identical within the error limits.

3. Experimental Section
In a typical reaction, 0.02 g (0.1 mmol) yttrium triiodide, YI3, previously synthesized from the elements [10], 0.09 g (0.02 mmol) dibenzo-24-crown-8 (db24c8) and 0.08 g (0.3 mmol) iodine were dissolved in 40 mL of ethanol. Slow evaporation of the solvent (beaker with perforated Parafilm®) yielded single crystals of [Y(H2O)8(db24c8)3]2(I3)3(I5)3 together with a small amount of (I2)@(db24c8).
Single crystals were selected under a microscope and sealed in thin-walled glass capillaries. After their quality had been checked by Laue diffraction patterns, the single crystals were transferred to a single-crystal X-ray diffractometer (Stoe Image Plate Diffraction System, IPDS II) to collect a complete intensity data set at ambient temperature. Structure solution and refinement was performed with the programs SHELXS-97 (direct methods) [11,12] and SHELXL-97 [11,13], scattering factors were from International Tables for X-ray Crystallography [14]. Data corrections were carried out for Lorentz and polarization factors and absorption (numerical with the aid of the programs X-RED [15] and X-SHAPE [16]). Further details of the crystal structure determination may be obtained from the Cambridge Crystallography Data center, CCDC-837899, free of charge, on application from CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44 1223 336033 or e-mail: deposit@ccdc.cam.ac.uk).
Crystal data for (I2)@(db24c8) (720.30 g mol−1); diffractometer IPDS-II, Stoe, Darmstadt; Mo-Kα (graphite monochromator, λ = 71.073 pm); T = 293(2) K; 2θmax = 59.32°; 180 images, 0° ≤ ω ≤ 180°; φ = 0°, 0° ≤ ω ≤ 180°; φ = 90°, Δω = 2°; indices: −5 ≤ h ≤ 6, −16 ≤ k ≤ 16, −17 ≤ l ≤ 17; transmission (min, max) = 0.5359, 0.7874; ρcalc = 1.738 g.cm−3; 13396 reflection intensities measured of which 3765 were symmetrically independent, Rint = 0.0446, F(000) = 346, μ = 2.387 mm−1. Triclinic, P-1 (no. 2), a = 485.0(1), b = 1203.7(3), c = 1280.4(2) pm, α = 64.56(2)°, β = 86.82(2)°, γ = 83.89(2)°, V = 671.1(2) × 106.pm3, Z = 1. R values: R1/wR2 for 1965 reflections with [I0 > 2σ(I0)]: 0.0301/0.0625 and for all data: 0.0804/0.0751; Sall = 0.835.
4. Conclusions
Iodine molecules with I–I distances of 268.39(7) pm, between those in gaseous iodine and solid iodine, 267 and 271.5 pm, respectively, are included in the solid-state structure of dibenzo-24-crown-8. The molecular structures of the db24c8 molecules are practically unchanged; there is a volume increase of about 16% relative to the parent crystal structure of db24c8. This increase amounts to 94 × 106.pm3 which is considerably larger than the volume of 82 × 106.pm3 of an iodine molecule in solid iodine. Only very weak interactions between the iodine and the db24c8 molecules in (I2)@(db24c8) are thereby attested.
References
- Walbaum, C. Neue Poly(inter)halogenide mit Kronenether-stabilisierten Kationen. PhD Dissertation, Universität zu Köln, Köln, Germany, 2009. [Google Scholar]
- Colquhoun, H.M.; Doughty, S.M.; Stoddart, J.F.; Slawin, A.M.Z.; Williams, D.J. Isolation and X-Ray Crystal Structure of a 2:1 Complex between Picric Acid and Dibenzo-24-crown-8; an Example of a Sandwich Structure. J. Chem. Soc. Perkin Trans. 1986, II, 253–257. [Google Scholar]
- Lu, T.; Gan, X.; Tan, M.; Yu, K. Studies on Crown Ether Complexes—XXVII. Synthesis, Characterization and Crystal Structure of [Y(NO3)3(OH2)3]·(Dibenzo-24-crown-8). Polyhedron 1993, 12, 621–625. [Google Scholar]
- Karle, I.L. Anomalous Electron Scattering from Iodine Vapor. J. Chem. Phys. 1955, 23, 1739–1740. [Google Scholar]
- Ukaji, T.; Kuchitsu, K. Effect of Temperature on the Molecular Structure of Iodine Observed by Gas Electron Diffraction. Bull. Chem. Soc. Japn. 1966, 39, 2153–2156. [Google Scholar]
- Harris, P.M.; Mack, E.; Blake, F.C. The atomic arrangement in the crystal of orthorhombic iodine. J. Am. Chem. Soc. 1928, 50, 1583–1600. [Google Scholar]
- Van Bolhuis, F.; Koster, P.B.; Migchelsen, T. Refinement of the crystal structure of iodine at 110 K. Acta Crystallogr. 1967, 23, 90–91. [Google Scholar]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar]
- Hanson, I.R.; Hughes, D.L.; Truter, M.R. Crystal and Molecular Structure of 6,7,9,10,12,13,20,21,23,24,26,27-Dodecahydrodibenzo[b,n][1,4,7,10,13,16,19,22]octaoxacyclotetracosin (Dibenzo-24-crown-8). J. Chem. Soc. Perkin Trans. 1976, II, 972–976. [Google Scholar]
- Meyer, G. Binary Lanthanide(III) Halides, MX3 (X = Cl, Br, I). In Synthesis of Lanthanide and Actinide Compounds; Meyer, G., Morss, L.R., Eds.; Kluwer Acad. Publ.: Dordrecht, The Netherlands, 1991; pp. 135–144. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst 2008, A64, 112–122. [Google Scholar]
- Sheldrick, G.M. SHELXS-97, Program for Structure Analysis; University of Göttingen: Göttingen, Germany, 1998. [Google Scholar]
- Sheldrick, G.M. SHELXL-93, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1993. [Google Scholar]
- Wilson, A.J.C. International Tables for Crystallagraphy; Kluwer Acad. Publ.: Dordrecht, The Netherlands, 1992; Volume C. [Google Scholar]
- Stoe. X-RED 1.22, Stoe Data Reduction Program (C); Stoe & Cie GmbH: Darmstadt, Germany, 1999. [Google Scholar]
- X-Shape 1.06, Crystal Optimisation for Numerical Absorption Correction (C); Stoe & Cie GmbH: Darmstadt, Germany, 1999.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).