A New Open-framework Iron Borophosphate from Ionic Liquids: KFe[BP2O8(OH)]
Abstract
: A new open-framework iron borophosphate, KFe[BP2O8(OH)], has been obtained by ionothermal synthesis from KH2PO4, FeCl3·4H2O, H3BO3 and [C4mpyr]Br (1-butyl-1-methylpyrrolidinium bromide). Single-crystal X-ray diffraction analysis shows that KFe[BP2O8(OH)] (monoclinic, P21/c, a = 9.372(2) Å , b = 8.146(2)Å , c = 9.587(2) Å, β = 101.18(3)°, V = 718.0(2)Å3 and Z= 4) has a three-dimensional (3-D) framework structure composed by {Fe(III)O5(OH)} octahedra as well as {BO3(OH)} and {PO4} tetrahedra. As anionic structural sub-unit, KFe[BP2O8(OH)], contains an infinite open-branched {[BP2O8(OH)]4-} chain which is formed by alternating {BO3(OH)} and {PO4} tetrahedra. {Fe(III)O5(OH)} octahedra share common O corners with five phosphate tetrahedra and the OH corner links to the hydrogen borate group to give a 3D framework. The negative charges of the inorganic framework are balanced by K+ ions.1. Introduction
Open-framework iron borophosphates have received much attention for their potential magnetic properties. A broad spectrum of borophosphates with various dimensionalities and stoichiometries has been prepared using hydro-/solvothermal, as well as boric acid flux methods with or without the employment of organic amines as templates [1-7]. So far, the ionothermal synthesis of iron borophosphates has not been explored [8-11].
The known borophosphate anionic frameworks can be derived from a few typical borophosphate fundamental building units (FBUs) with B/P ratios of 6/1, 5/1, 3/1, 3/2, 1/1, 3/4, 2/3, 1/2, 2/5, 1/3 and 1/4 [12,13]. Among them, nonameric FBUs, [B3P6X26] [14] and [BP2X8] [15] (X = O, OH) with a B/P ratio of 1/2, form the largest group. Borophosphates of the general formula AM[BP2O8(OH)] (A = K, Rb, Cs or NH4, M = V, Fe, Al, Ga, Sc) have been described in the past several years [12]. Here we report on a new member of this family, KFe[B(PO4)2OH], obtained by ionothermal synthesis.
2. Results and Discussion
KFe[BP2O8(OH)] was prepared by reacting ionothermally FeCl3·4H2O, KH2PO4, H3BO3 and 1-butyl-1-methylpyrrolidinium bromide, [C4mpy]Br, in a ratio of 3:3:1;1.5 at 200 °C for 5 days. Upon cooling crystals of KFe[BP2O8(OH)] formed. The crystal structure of KFe[BP2O8(OH)] (monoclinic, P21/c, a = 9.372(2) Å, b = 8.146(2) Å, c = 9.587(2) Å, β = 101.18(3)°, V = 718.0(2) Å3 and Z = 4) is built up by a 3-D framework of {Fe(III)O6} octahedra, {BO3(OH)} and {PO4} tetrahedra. It is isotypic with KAl[BP2O8(OH)] [16], (NH4)M[BP2O8(OH)] (M, Al, Ga, Fe, V) [17-20], RbM[BP2O8(OH)] (M = Al, Ga, V, Fe) [21-25], Cs[BP2O8(OH)] (M = Al, Ga, Fe) [26-28].
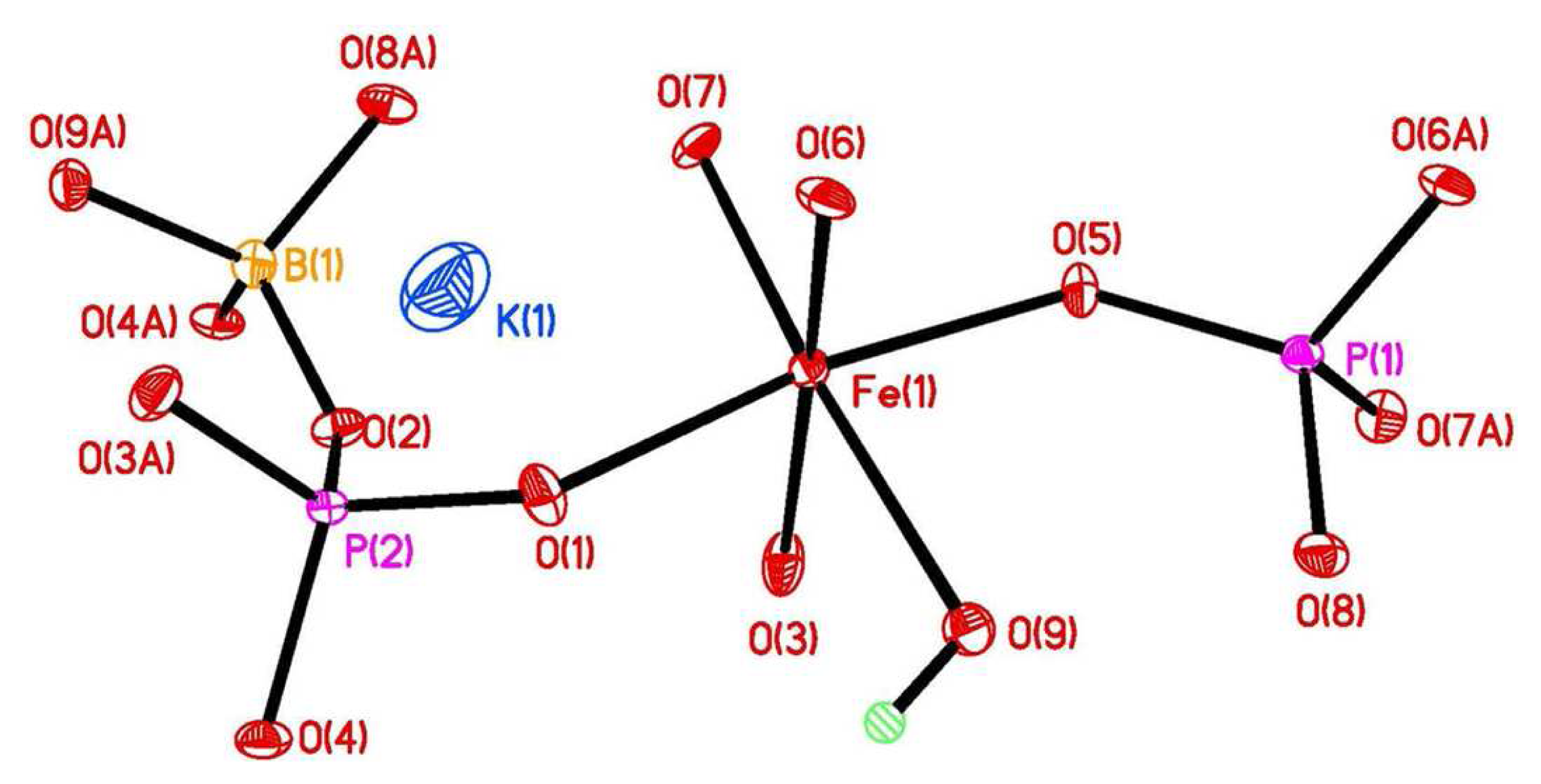
The asymmetric unit of KFe[BP2O8(OH)] contains one crystallographically distinct Fe3+ cation, one B3+ cation, one K+ cation, two crystallographically distinct P5+ cations, which are all coordinated by oxygen (Figure 1).
In KFe[BP2O8(OH)], the Fe(1) atom connects three O atoms to three P(1), two O atoms to two P(2) atoms, and one O(9)H to the B(1) atom. The Fe–O internuclear distances vary in the range of 1.954(4)–2.143(4) Å. All boron and phosphorus atoms are tetrahedrally coordinated by oxygen atoms. The P(1) atom connects via three oxygen atoms to Fe(1) and one O atom to B(1) atom; the P(2) atom connects two Fe(1) atoms and two B(1) atoms via four oxygen atoms, respectively. The P–O bond lengths vary in the range of 1.505(4)–1.562(3) Å. The B(1) atom connects via one oxygen atom to a P(1) atom, two oxygen atoms to P(2) atoms and one O(9)H to Fe(1) atom, respectively. The B–O bond lengths vary in the range of 1.457(6)–1.475(7) Å.
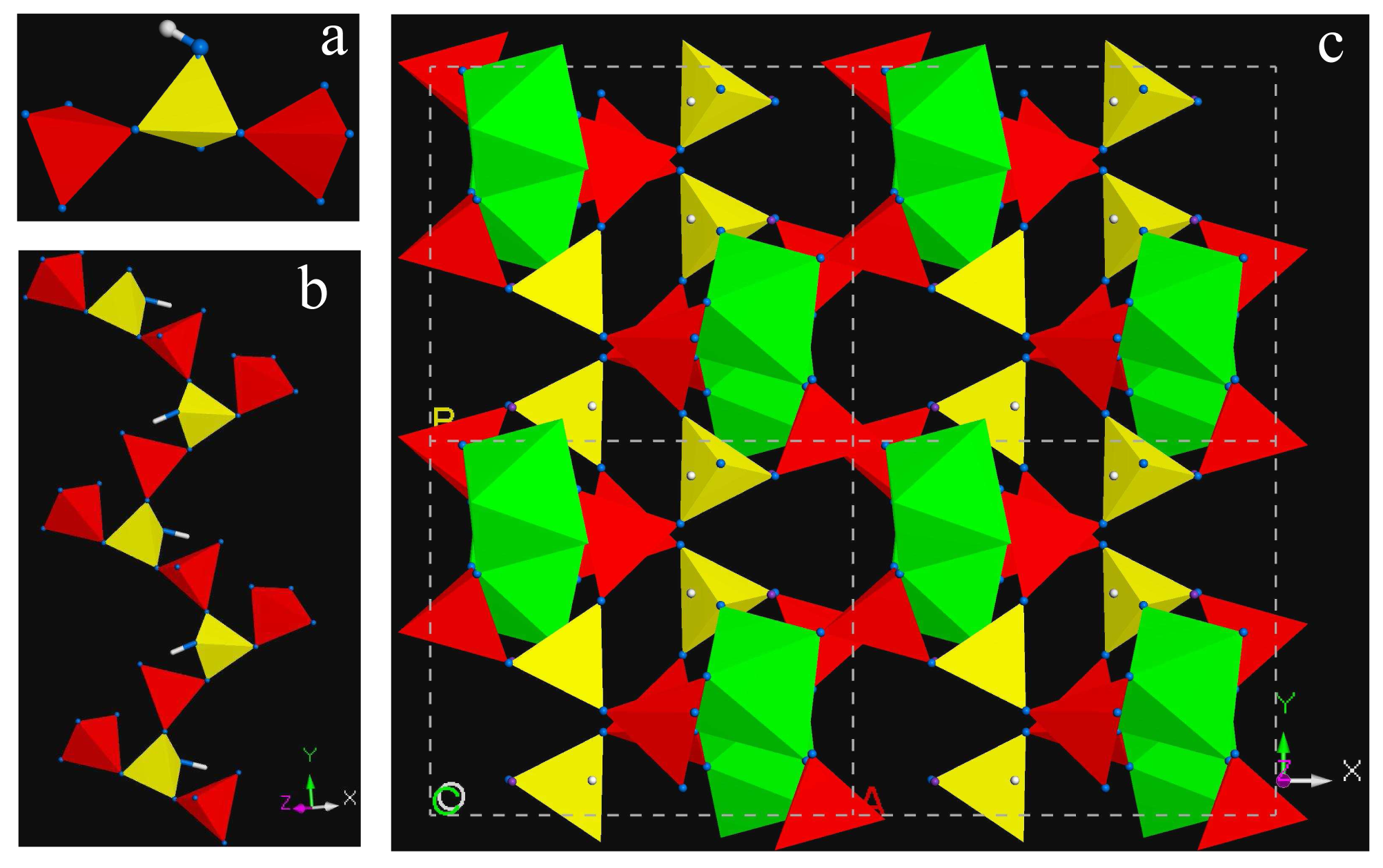
KFe[BP2O8(OH)] features an unbranched trimer, 3□:3□, as the basic building unit (BBU) (Figure 2a). These BBUs are connected to an infinite open-branched (oB) vierer 1∞{[BP2Φ9]2 (Φ = O, OH) chain (Figure 2b). The condensation of this borophosphate FBU with {Fe(III)O5(OH)} octahedra by sharing common oxygen atoms with five phosphate tetrahedra and an OH group with a hydrogenborate group link to a 3-D framework (Figure 2c). It features 8-ring channels along the [100] direction enclosed by two FeO5(OH) octahedra, two BO3(OH) and four PO4 tetrahedra.
3. Experimental Section
KFe[BP2O8(OH)] is prepared under ionothermal synthesis conditions using the ionic liquid 1-butyl-1-methylpyrrolidinium bromide, [C4mpyr]Br, as the solvent. A mixture of FeCl3·4H2O (99%, Fluka), KH2PO4 (99%, J.T. Baker), H3BO3 (99.8%, Appl. Chem.) and [C4mpy]Br (99%, Merck) was reacted in a 3 mL Teflon-lined stainless steel container at 200 °C for 5 days followed by cooling to room temperature. The products were filtered off, washed with deionized water and acetone, filtered by suction, and dried at 60 °C for one day.
A suitable single crystal of KFe[BP2O8(OH)] was selected for single-crystal X-ray diffraction (XRD) analysis. The data were collected at ambient temperature using graphite-monochromated Mo-Kα radiation on an Image Plate Diffraction System, IPDS I, (Stoe, Darmstadt, Germany). The data were corrected for Lorentz and polarization effects. Data correction was carried out with the program X-RED [29]. A face-indexed numerical absorption correction (X-SHAPE) was applied [30]. The structure was solved by direct methods and refined by full-matrix least-squares techniques with the SHELXTL crystallographic software package [31]. The Fe, B, P, and O atoms could be unambiguously located. The K+ ions were subsequently located from a difference Fourier map. In the final refinement cycles H atoms associated with the hydroxyl groups were added computationally.
Further details of the crystal structure investigations may be obtained from the Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (Fax: +49-7247-808-666; E-Mail: crysdata@fiz-karlsruhe.de, http://www.fiz-karlsruhe.de/request_for_deposited_data.hmtl) on quoting ICSD-422900.
Crystal data for KFe[BP2O8(OH)], (312.71 gmol−1); diffractometer IPDS-I, Stoe, Darmstadt; Mo-Kα (graphite monochromator, λ = 71.073 pm); T = 293(2) K; 2θmax = 56.18°; 100 images, 0° ≤ φ ≤ 200°; Δφ = 2°; indices: -12 ≤ h ≤12, -10 ≤ k ≤ 10, -12 ≤ l ≤12; transmission (min, max) = 0.0307, 0.0929; ρcalc = 2.893 g cm−3; 8321 reflection intensities measured of which 1726 were symmetrically independent, Rint = 0.0653, F(000) = 612, μ = 27.547 mm−1. Monoclinic, P21/c (no. 62), a = 9.372(2) Å, b = 8.146(2) Å, c = 9.587(2) Å, β = 101.18(3)°, V = 718.0(2) Å3 and Z = 4. R values: R1/wR2 for final indices with [Io > 2σ(Io)]: 0.0400/ 0.1095 and for all data: 0.0541/0.1122; Sall = 1.071.
4. Conclusions
A new open-framework iron borophosphate KFe[BP2O8(OH)], has been prepared under ionothermal conditions using the ionic liquid 1-butyl-1-methyl pyrrolidinium bromide, [C4mpy]Br, as the solvent. The successful preparation of the new iron borophosphate in an ionic liquid demonstrates not only that many more open-framework borophosphates could be obtained, but also that the ionothermal method is a promising method to synthesize new kinds of open-framework materials.
Acknowledgments
This work is supported by the European Research Council (ERC) under the contract 200475.
References and Notes
- Kniep, R.; Will, H.G.; Boy, I.; Röhr, C. 61 Helices from Tetrahedral Ribbons 1∞[BP2O3-8 ] Isostructural Borophosphates MIMII(H2O)2[BP2O8]H2O (MI = Na, K; MII = Mg, Mn, Fe, Co, Ni, Zn) and Their Dehydration to Microporous Phases MIMII(H2O)[BP2O8]. Angew. Chem. Int. Ed. Engl. 1997, 36, 1013–1014. [Google Scholar]
- Yang, T.; Li, G.B.; Ju, J.; Liao, F.H.; Xiong, M.; Lin, J.H. A series of borate-rich metalloborophosphates Na2[MIIB3P2O11(OH)]·0.67H2O (MII = Mg, Mn, Fe, Co, Ni, Cu, Zn): Synthesis, structure and magnetic susceptibility. J. Solid State Chem. 2006, 179, 2513. [Google Scholar]
- Yilmaz, A.; Bu, X.H.; Kizilyalli, M.; Stucky, G.D. Fe(H2O)2BP2O8·H2O, a First Zeotype Ferriborophosphate with Chiral Tetrahedral Framework Topology. Chem. Mater. 2000, 12, 3243–3245. [Google Scholar]
- Kniep, R.; Schäfer, G. Isotype Borophosphate MII(C2H10N2)[B2P3O12(OH)] (MII = Mg, Mn, Fe, Ni, Cu, Zn): Verbindungen mit Tetraeder-Schichtverbänden. Z. Anorg. Allg. Chem. 2000, 626, 141–147. [Google Scholar]
- Huang, Y.X.; Ewald, B.; Schnelle, W.; Prots, Y.; Kniep, R. Chirality and Magnetism in a Novel Series of Isotypic Borophosphates: MII[BPO4(OH)2] (MII = Mn, Fe, Co). Inorg. Chem. 2006, 45, 7578–7580. [Google Scholar]
- Yang, W.T.; Li, J.Y.; Pan, Q.H.; Xing, H.H.; Chen, Y.; Yu, J.H.; Xu, R.R. Synthesis, structure and magnetic property of a new organo-templated mixed-valent iron(II, III) borophosphate. J. Mater. Chem. 2009, 19, 4523–4528. [Google Scholar]
- Yang, T.; Sun, J.L.; Li, G.B.; Wang, Y.X.; Christensen, J.; He, Z.B.; Christensen, K.E.; Zou, X.D.; Liao, F.H.; Lin, J.H. Fe5O5[B6O10(OH)3]nH2O: Wave-Layered Iron Borate and Frustrated Antiferromagnetism. Inorg. Chem. 2009, 48, 11209–11214. [Google Scholar]
- Shi, H.; Shan, Y.; He, M.; Liu, Y. Impetus for solvothermal synthesis technique: Synthesis and structure of a novel 1-D borophosphate using ionic liquid as medium. J. Solid State Chem. 2003, 176, 33–36. [Google Scholar]
- Lin, Z.; Wragg, D.S.; Lightfoot, P.; Morris, R.E. A novel non-centrosymmetric metallophosphate- borate compound via ionothermal synthesis. Dalton Trans. 2009, 5287–5289. [Google Scholar]
- Xing, H.; Li, Y.; Su, T.; Xu, J.; Yang, W.; Zhu, E.; Yu, J.; Xu, R. Spontaneous crystallization of a new chiral open-framework borophosphate in the ionothermal system. Dalton Trans. 2010, 39, 1713–1715. [Google Scholar]
- Yang, M.; Xu, F.F.; Liu, Q.S.; Yan, P.F.; Liu, X.F.; Wang, C.; Welz-Biermann, U. Chelated orthoborate ionic liquid as a reactant for the synthesis of a new cobalt borophosphate containing extra-large 16-ring channels. Dalton Trans. 2010, 39, 10571–10573. [Google Scholar]
- Kniep, R.; Engelhardt, H.; Hauf, C. A First Approach to Borophosphate Structural Chemistry. Chem. Mater. 1998, 10, 2930–2934, and references therein. [Google Scholar]
- Ewald, B.; Huang, Y.X.; Kniep, R. Structural Chemistry of Borophosphates, Metalloborophosphates, and Related Compounds. Z. Anorg. Allg. Chem. 2007, 633, 1517–1540, and references therein. [Google Scholar]
- Huang, Y.X.; Prots, Y.; Schnelle, W.; Kniep, R. A new borophosphate chain anion in an organo-templated iron(III) borophosphate: Synthesis, crystal structure and magnetic properties of (C4H12N2)3FeIII6(H2O)4[B6P12O50(OH)2]·2H2O. Sci. Technol. Adv. Mater. 2007, 8, 399–405. [Google Scholar]
- Kniep, R.; Engelhardt, H. Na1.89Ag0.11[BP2O7(OH)] und Na2[BP2O7(OH)] – Isotype Borophosphate mit Tetraeder-Schichtpaketen. Z. Anorg. Allg. Chem. 1998, 624, 1291–1297. [Google Scholar]
- Kniep, R.; Koch, D.; Hartmann, T. Crystal structure of potassium aluminum catena- (monohydrogenmonoborate)-bis(monophosphate), KAl[BP2O8(OH)]. Z. Kristallogr. NCS 2002, 217, 186. [Google Scholar]
- Mi, J.; Huang, Y.X.; Deng, J.F.; Borrmann, H.; Zhao, J.T.; Kniep, R. Crystal structure of ammonium aluminum catena-[monohydrogenmonoborate-bis(monophosphate)], (NH4)Al [BP2O8(OH)]. Z. Kristallogr. NCS 2002, 217, 305–306. [Google Scholar]
- Li, M.R.; Mao, S.Y.; Huang, Y.X.; Mi, J.X.; Wei, Z.B.; Zhao, J.T.; Kniep, R. Crystal structure of ammonium gallium (monophosphate-hydrogenmonoborate-mono-phosphate), (NH4)Ga[BP2O8(OH)]. Z. Kristallogr. NCS 2002, 217, 165–166. [Google Scholar]
- Kriticos, M.; Wikstad, E.; Walldén, K. Hydrothermal synthesis, characterization and magnetic properties of three isostructural chain borophosphates; NH4M(III)[BP2O8(OH)] with M = V or Fe and NH4(Fe(III)0.53V(III)0.47)[BP2O8(OH)]. Solid State Sci. 2001, 3, 649–658. [Google Scholar]
- Huang, Y.X.; Schäfer, G.; Carrillo Cabrera, W.; Cardoso, R.; Schnelle, W.; Zhao, J.T.; Kniep, R. Open-Framework Borophosphates: (NH4)0.4FeII0.55FeIII0.5(H2O)2[BP2O8]-·0.6H2O and NH4FeIII[BP2O8(OH)]. Chem. Mater. 2001, 13, 4348–4354. [Google Scholar]
- Mi, J.; Zhao, J.T.; Huang, Y.X.; Deng, J.F.; Borrmann, H.; Kniep, R. Crystal structure of rubidium aluminum catena-[monohydrogen-monoborate-bis(monophosphate)], RbAl[BP2O8(OH)]. Z. Kristallogr. NCS 2002, 217, 171–172. [Google Scholar]
- Yakubovicha, O.V.; Steeleb, I.M.; Dimitrovab, O.V. Polymorphism of the Borophosphate Anion in K(Fe,Al)[BP2O8(OH)] and Rb(Al, Fe)[BP2O8(OH)] Crystal Structures. Kristallografiya 2010, 55, 810–817. [Google Scholar]
- Mi, J.; Borrmann, H.; Mao, S.Y.; Huang, Y.-X.; Zhang, H.; Zhao, J.T.; Kniep, R. Crystal structure of rubidium gallium catena-[monohydrogen-monoborate-bis(monophosphate)] RbGa[BP2O8(OH)], from a twinned crystal. Z. Kristallogr. NCS 2003, 218, 17–18. [Google Scholar]
- Engelhardt, H.; Borrmann, H.; Kniep, R. Crystal structure of rubidium vanadium(III) catena-[monohydrogenmonoborate-bis(monophosphate)], RbV[BP2O8(OH)]. Z. Kristallogr. NCS 2000, 215, 203–204. [Google Scholar]
- Kniep, R.; Boy, I.; Engelhard, H. RbFe[BP2O8(OH)]: A New Borophosphate Containing Open-Branched Tetrahedral Vierer-Einfach Chains. Z. Anorg. Allg. Chem. 1999, 625, 1512–1516. [Google Scholar]
- Mi, J.; Huang, Y.X.; Deng, J.F.; Borrmann, H.; Zhao, J.T.; Kniep, R. Crystal structure of caesium aluminum catena-[monohydrogen-monoborate-bis(monophosphate)], CsAl[BP2O8(OH). Z. Kristallogr. NCS 2002, 217, 169–170. [Google Scholar]
- Mi, J.; Borrmann, H.; Huang, Y.X.; Mao, S.Y.; Zhao, J.T.; Kniep, R. Crystal structure of caesium gallium(III) catena-[monohydrogenmonoborate-bis(monophosphate)], CsGa[BP2O8(OH)]. Z. Kristallogr. NCS 2003, 218, 171–172. [Google Scholar]
- Engelhard, H.; Kniep, R. Crystal structure of caesium iron(III) catena-[monohydrogenmonoborate- bis(monophosphate)], CsFe[BP2O8(OH)]. Z. Kristallogr. NCS 1999, 214, 443–444. [Google Scholar]
- X-RED 1.22, Stoe Data Reduction Program (C); Stoe & Cie GmbH: Darmstadt, Germany, 2001.
- X-Shape 1.06, Crystal Optimization for Numerical Absorption Correction (C); Stoe & Cie GmbH: Darmstadt, Germany, 1999.
- SHELXTL Program; version 5.1; Siemens Industrial Automation, Inc.: Madison, WI, USA, 1997.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, G.; Mudring, A.-V. A New Open-framework Iron Borophosphate from Ionic Liquids: KFe[BP2O8(OH)]. Crystals 2011, 1, 22-27. https://doi.org/10.3390/cryst1020022
Wang G, Mudring A-V. A New Open-framework Iron Borophosphate from Ionic Liquids: KFe[BP2O8(OH)]. Crystals. 2011; 1(2):22-27. https://doi.org/10.3390/cryst1020022
Chicago/Turabian StyleWang, Guangmei, and Anja-Verena Mudring. 2011. "A New Open-framework Iron Borophosphate from Ionic Liquids: KFe[BP2O8(OH)]" Crystals 1, no. 2: 22-27. https://doi.org/10.3390/cryst1020022
APA StyleWang, G., & Mudring, A.-V. (2011). A New Open-framework Iron Borophosphate from Ionic Liquids: KFe[BP2O8(OH)]. Crystals, 1(2), 22-27. https://doi.org/10.3390/cryst1020022



