Biodiesel Production by Esterification Reaction on K+ Modified MgAl-Hydrotalcites Catalysts
Abstract
:1. Introduction
2. Results and Discussion
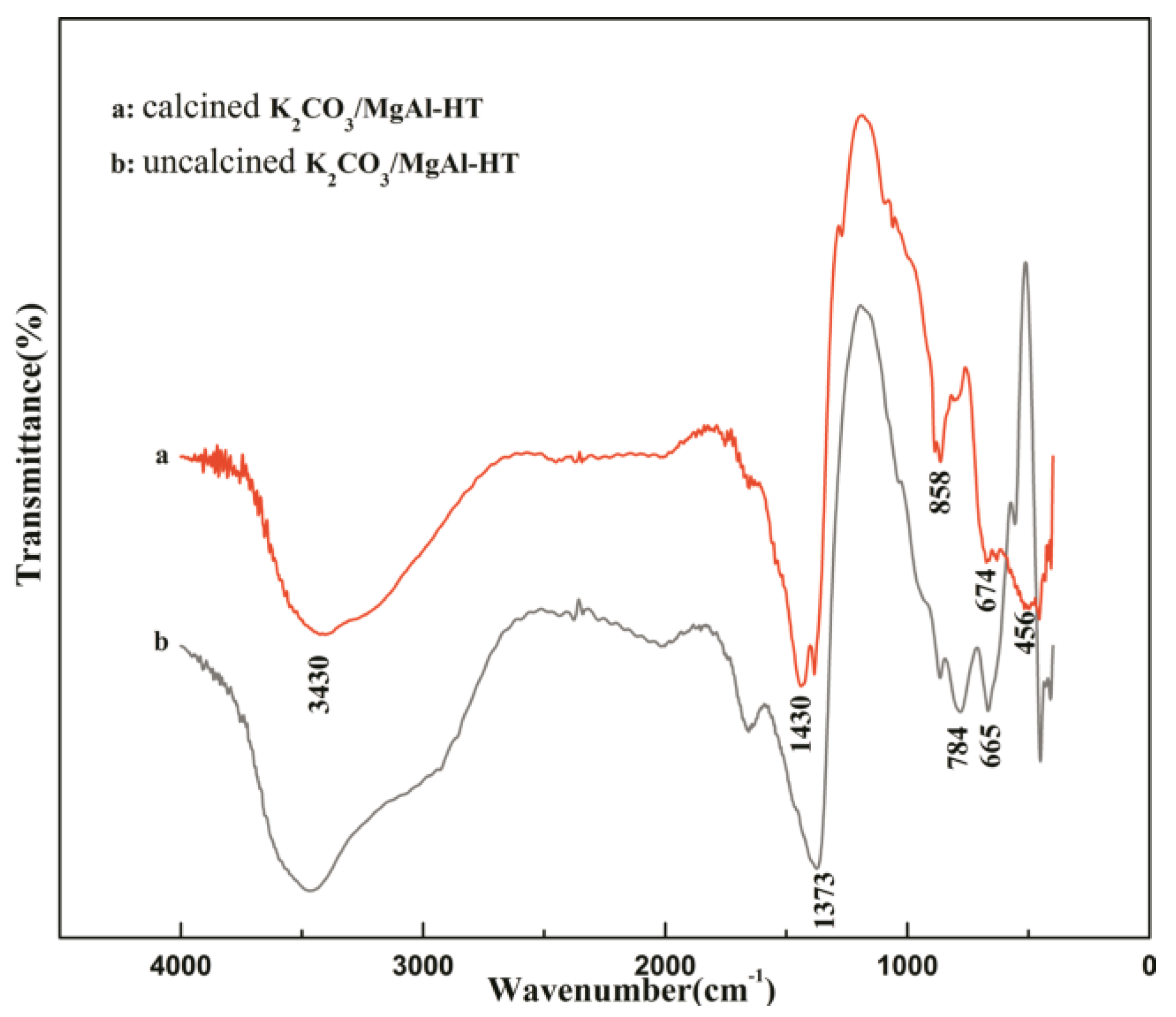
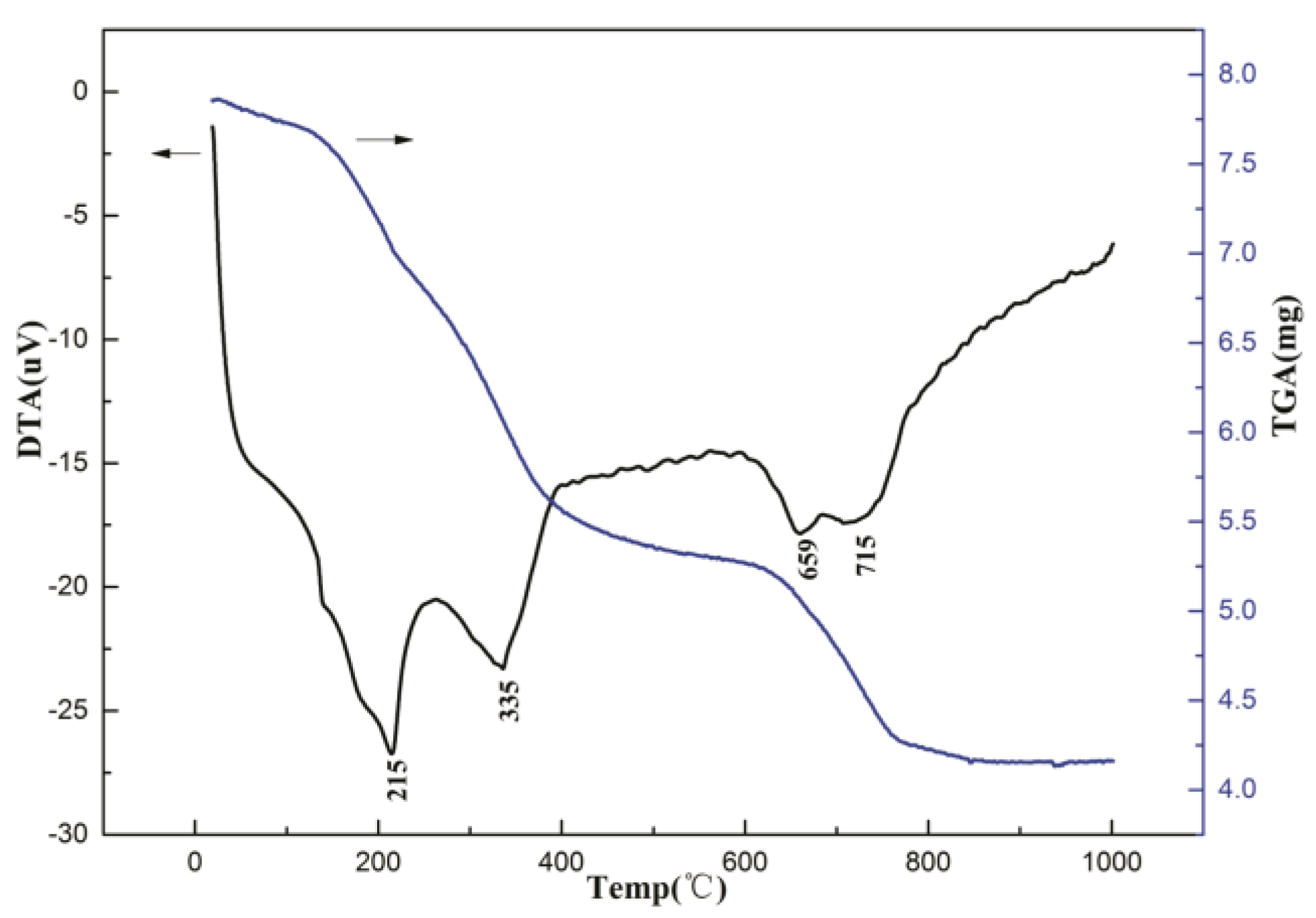
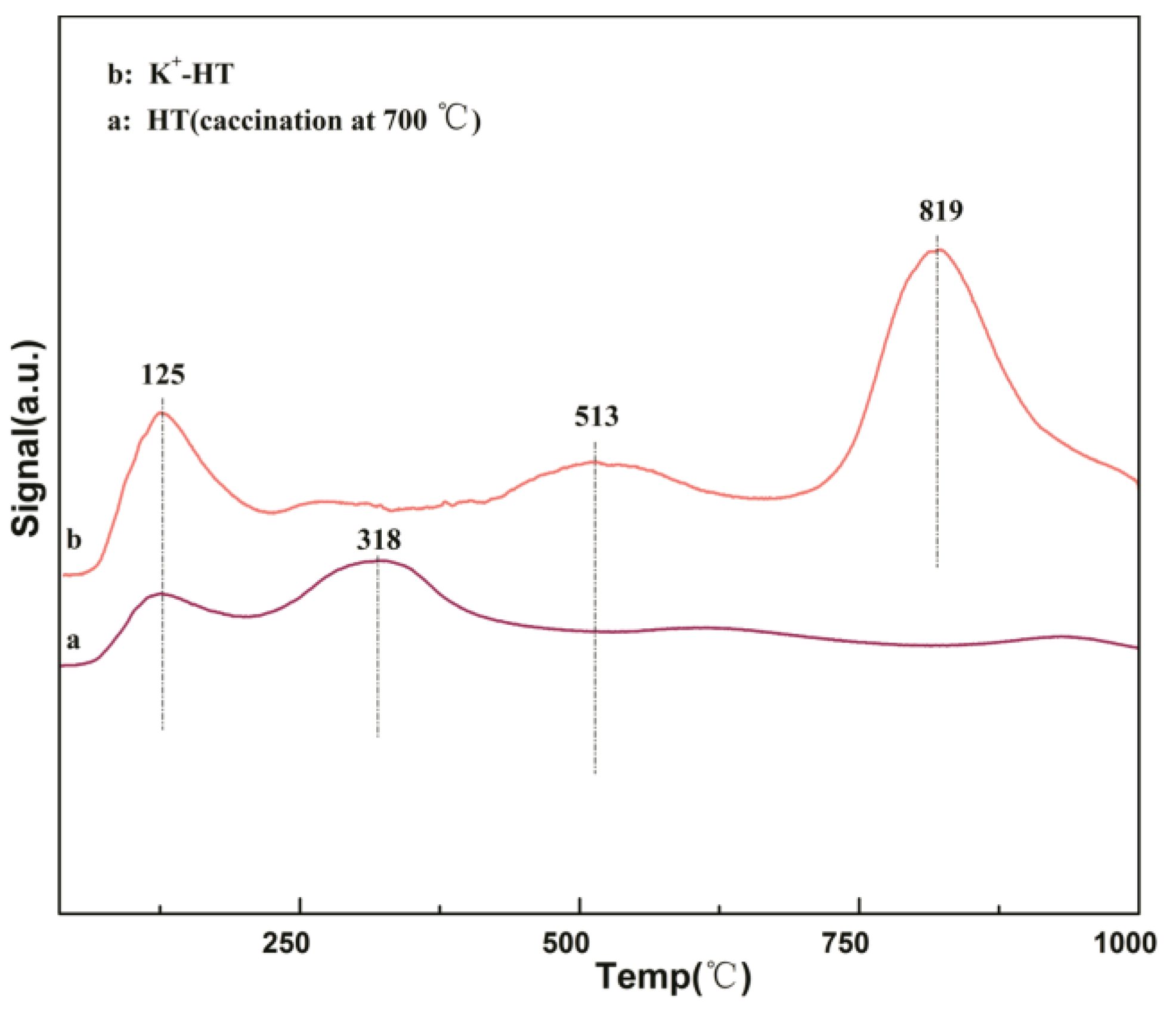
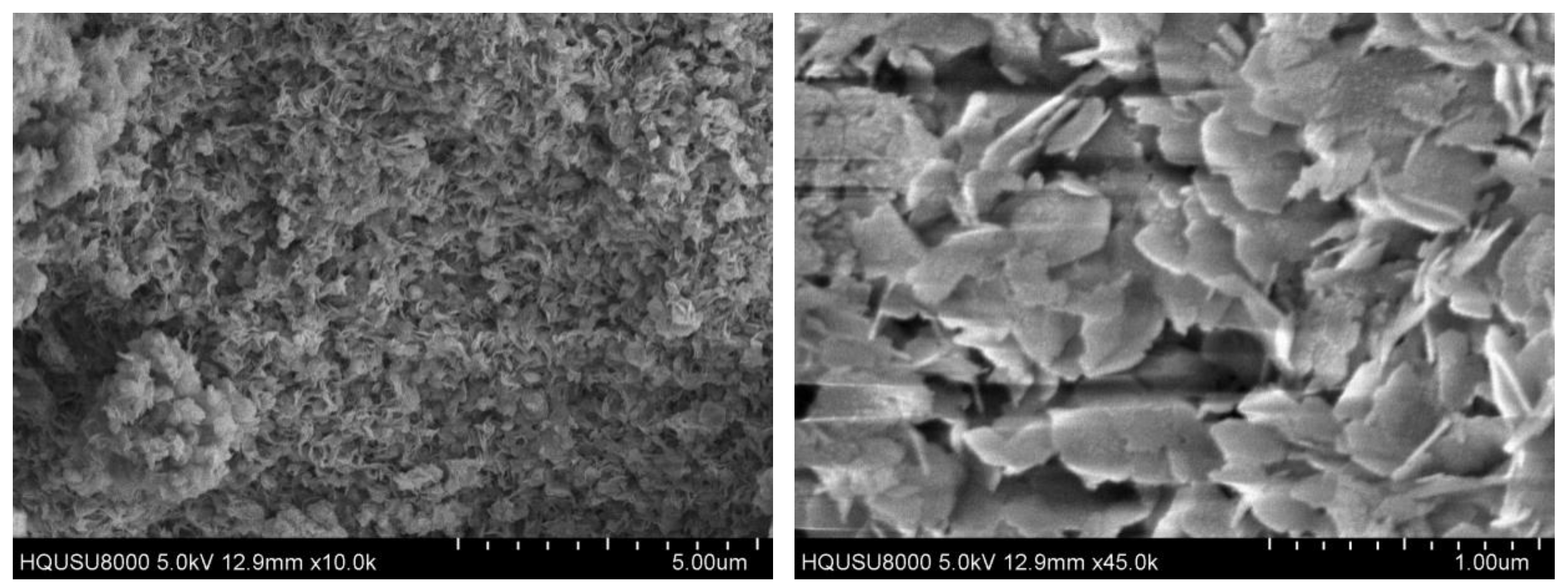
2.1. Catalysts Characterization
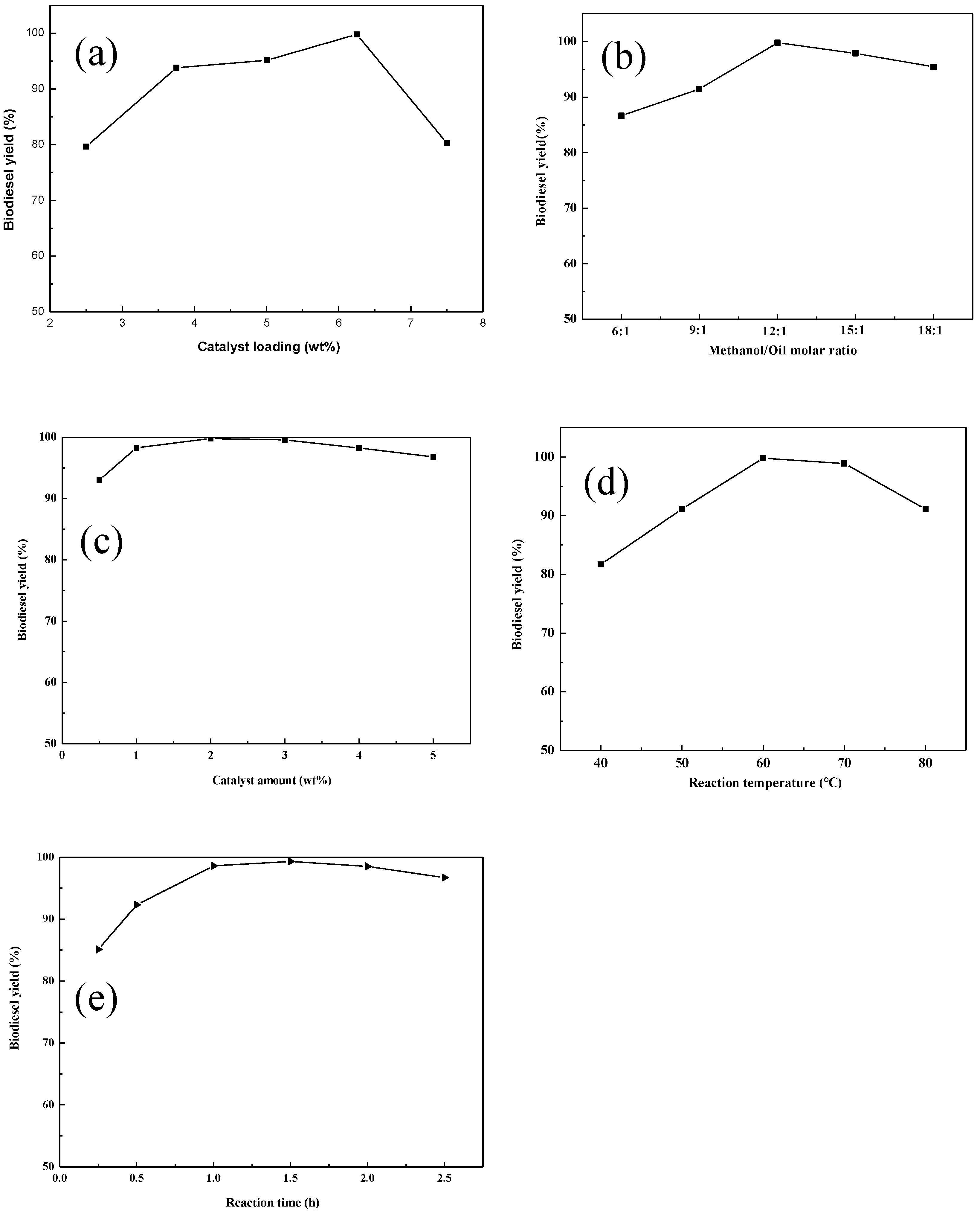
2.2. Catalytic Performance for Biodiesel Production
2.2.1. Effect of K+ Loading Ratio
2.2.2. Effect of Methanol/Oil Molar Ratio
2.2.3. Effect of the Amount of Added Catalyst
2.2.4. Effect of the Reaction Temperature
2.2.5. Effect of the Reaction Time
2.2.6. Effect of Ultrasound Aid
3. Experimental Methods
3.1. Materials
3.2. Catalysts Preparation
3.3. Catalysts Characterization
3.4. Transesterification Reaction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef] [PubMed]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Biodiesel from microalgae: a critical evaluation from laboratory to large scale production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Almeida, E.L.; Andrade, C.M.G.; dos Santos, O.A. Production of biodiesel via catalytic processes: A brief review. Int. J. Chem. React. Eng. 2018, 16, 13. [Google Scholar] [CrossRef]
- Avhad, M.R.; Marchetti, J.M. A review on recent advancement in catalytic materials for biodiesel production. Renew. Sustain. Energy Rev. 2015, 50, 696–718. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Lee, H.V. A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sustain. Energy Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Pourzolfaghar, H.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review of the enzymatic hydroesterification process for biodiesel production. Renew. Sustain. Energy Rev. 2016, 61, 245–257. [Google Scholar] [CrossRef]
- Canakci, M.; Van Gerpen, J. Biodiesel production viaacid catalysis. Trans. ASAE 1999, 42, 1203. [Google Scholar] [CrossRef]
- Buasri, A.; Chaiyut, N.; Loryuenyong, V.; Rodklum, C.; Chaikwan, T.; Kumphan, N. Continuous process for biodiesel production in packed bed reactor from waste frying oil using potassium hydroxide supported on Jatropha curcas fruit shell as solid catalyst. Appl. Sci. 2012, 2, 641–653. [Google Scholar] [CrossRef]
- Pastore, C.; Barca, E.; Del Moro, G.; Lopez, A.; Mininni, G.; Mascolo, G. Recoverable and reusable aluminium solvated species used as a homogeneous catalyst for biodiesel production from brown grease. Appl. Catal. A: Gen. 2015, 501, 48–55. [Google Scholar] [CrossRef]
- Evangelista, J.P.; Chellappa, T.; Coriolano, A.C.; Fernandes, V.J., Jr.; Souza, L.D.; Araujo, A.S. Synthesis of alumina impregnated with potassium iodide catalyst for biodiesel production from rice bran oil. Fuel Process. Technol. 2012, 104, 90–95. [Google Scholar] [CrossRef]
- Badday, A.S.; Abdullah, A.Z.; Lee, K.T. Ultrasound-assisted transesterification of crude Jatropha oil using alumina-supported heteropolyacid catalyst. Appl. Energy 2013, 105, 380–388. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, M.; Liu, Y.; Fan, M.; Jiang, P.; Dong, Y. Sr doping magnetic CaO parcel ferrite improving catalytic activity on the synthesis of biodiesel by transesterification. Fuel 2016, 186, 787–791. [Google Scholar] [CrossRef]
- Shibasaki-Kitakawa, N.; Tsuji, T.; Chida, K.; Kubo, M.; Yonemoto, T. Simple continuous production process of biodiesel fuel from oil with high content of free fatty acid using ion-exchange resin catalysts. Energy Fuels 2010, 24, 3634–3638. [Google Scholar] [CrossRef]
- Dias, A.P.S.; Bernardo, J.; Felizardo, P.; Correia, M.J.N. Biodiesel production over thermal activated cerium modified Mg-Al hydrotalcites. Energy 2012, 41, 344–353. [Google Scholar] [CrossRef]
- Conterosito, E.; Gianotti, V.; Palin, L.; Boccaleri, E.; Viterbo, D.; Milanesio, M. Facile preparation methods of hydrotalcite layered materials and their structural characterization by combined techniques. Inorg. Chim. Acta 2018, 470, 36–50. [Google Scholar] [CrossRef]
- Park, M.; Lee, C.I.; Lee, E.J.; Choy, J.H.; Kim, J.E.; Choi, J. Layered double hydroxides as potential solid base for beneficial remediation of endosulfan-contaminated soils. J. Phys. Chem. Solids 2004, 65, 513–516. [Google Scholar] [CrossRef]
- Winter, F.; Xia, X.; Hereijgers, B.P.; Bitter, J.H.; van Dillen, A.J.; Muhler, M.; de Jong, K.P. On the nature and accessibility of the brønsted-base sites in activated hydrotalcite catalysts. J. Phys. Chem. B 2006, 110, 9211–9218. [Google Scholar] [CrossRef]
- Hájek, M.; Tomášová, A.; Kocík, J.; Podzemná, V. Statistical evaluation of the mutual relations of properties of Mg/Fe hydrotalcites and mixed oxides as transesterification catalysts. Appl. Clay Sci. 2018, 154, 28–35. [Google Scholar] [CrossRef]
- Benedictto, G.P.; Sotelo, R.M.; Dalla Costa, B.O.; Fetter, G.; Basaldella, E.I. Potassium-containing hydroxylated hydrotalcite as efficient catalyst for the transesterification of sunflower oil. J. Mater. Sci. 2018, 53, 12828–12836. [Google Scholar] [CrossRef]
- Dossin, T.F.; Reyniers, M.F.; Berger, R.J.; Marin, G.B. Simulation of heterogeneously MgO-catalyzed transesterification for fine-chemical and biodiesel industrial production. Appl. Catal. B: Environ. 2006, 67, 136–158. [Google Scholar] [CrossRef]
- Noiroj, K.; Intarapong, P.; Luengnaruemitchai, A.; Jai-In, S. A comparative study of KOH/Al2O3 and KOH/NaY catalysts for biodiesel production via transesterification from palm oil. Renew. Energy 2009, 34, 1145–1150. [Google Scholar] [CrossRef]
- Trakarnpruk, W.; Porntangjitlikit, S. Palm oil biodiesel synthesized with potassium loaded calcined hydrotalcite and effect of biodiesel blend on elastomer properties. Renew. Energy 2008, 33, 1558–1563. [Google Scholar] [CrossRef]
- Xie, W.; Peng, H.; Chen, L. Transesterification of soybean oil catalyzed by potassium loaded on alumina as a solid-base catalyst. Appl. Catal. A: Gen. 2006, 300, 67–74. [Google Scholar] [CrossRef]
- Lukić, I.; Krstić, J.; Jovanović, D.; Skala, D. Alumina/silica supported K2CO3 as a catalyst for biodiesel synthesis from sunflower oil. Bioresour. Technol. 2009, 100, 4690–4696. [Google Scholar] [CrossRef]
- Alonso, D.M.; Mariscal, R.; Moreno-Tost, R.; Poves, M.Z.; Granados, M.L. Potassium leaching during triglyceride transesterification using K/γ-Al2O3 catalysts. Catal. Commun. 2007, 8, 2074–2080. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, F.; Yang, D.; Li, X.; Sun, P. Preparation, characterization and application of heterogeneous solid base catalyst for biodiesel production from soybean oil. Biomass Bioenergy 2011, 35, 2787–2795. [Google Scholar] [CrossRef]
- Ferreira, O.P.; Alves, O.L.; Gouveia, D.X.; Souza Filho, A.G.; de Paiva, J.A.; Mendes Filho, J. Thermal decomposition and structural reconstruction effect on Mg–Fe-based hydrotalcite compounds. J. Solid State Chem. 2004, 177, 3058–3069. [Google Scholar] [CrossRef]
- Xie, W.; Peng, H.; Chen, L. Calcined Mg–Al hydrotalcites as solid base catalysts for methanolysis of soybean oil. J. Mol. Catal. A: Chem. 2006, 246, 24–32. [Google Scholar] [CrossRef]
- Bhangu, S.K.; Gupta, S.; Ashokkumar, M. Ultrasonic enhancement of lipase-catalysed transesterification for biodiesel synthesis. Ultrason. Sonochem. 2017, 34, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles–an overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.R.; Yen, T.F. An upgrading process through cavitation and surfactant. Energy Fuels 1993, 7, 111–118. [Google Scholar] [CrossRef]



| Sample | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| K2CO3/MgAl-HT | 109.286 | 0.421 | 3.825 |
| MgAl-HT (calcination at 700 °C) | 123.885 | 0.513 | 3.834 |
| MgAl-HT | 91.153 | 0.323 | 3.822 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.-Y.; Shao, W.-L.; Zhou, W.-X.; Liu, Y.; Han, Y.-Y.; Zheng, Y.; Liu, Y.-J. Biodiesel Production by Esterification Reaction on K+ Modified MgAl-Hydrotalcites Catalysts. Catalysts 2019, 9, 742. https://doi.org/10.3390/catal9090742
Zhang C-Y, Shao W-L, Zhou W-X, Liu Y, Han Y-Y, Zheng Y, Liu Y-J. Biodiesel Production by Esterification Reaction on K+ Modified MgAl-Hydrotalcites Catalysts. Catalysts. 2019; 9(9):742. https://doi.org/10.3390/catal9090742
Chicago/Turabian StyleZhang, Chen-Yang, Wen-Li Shao, Wei-Xia Zhou, Yang Liu, Yuan-Yuan Han, Yi Zheng, and Yong-Jun Liu. 2019. "Biodiesel Production by Esterification Reaction on K+ Modified MgAl-Hydrotalcites Catalysts" Catalysts 9, no. 9: 742. https://doi.org/10.3390/catal9090742




