The Role of SO3 Poisoning in CU/SSZ-13 NH3-SCR Catalysts
Abstract
:1. Introduction
2. Results
2.1. Structural Characterization
2.1.1. BET and XRD Results
2.1.2. Ex-Situ Diffused Reflectance Infrared Fourier Transform Spectroscopy (DRIFTs) Results
2.1.3. NMR Results
2.2. Copper Species Variation on Cu/SSZ-13
2.2.1. TG Results
2.2.2. H2-TPR Results
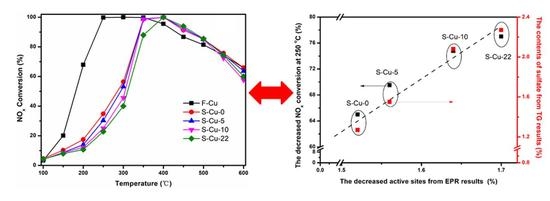
2.2.3. EPR Results
2.3. NH3-SCR Reactions over Cu/SSZ-13
2.3.1. NH3-SCR Activity
2.3.2. Activation Energy (Ea) Measurements
3. Discussion
3.1. The Variation of the CHA Structure Under SO3 Poisoning
3.2. The Structural-activity Relationship Over Cu/SSZ-13
4. Materials and Methods
4.1. Materials
4.2. Methods
5. Conclusions
- As a good acid-resistant ability, even at a high ratio of SO3/SOx flux, the CHA structure of Cu/SSZ-13 keep intact.
- Copper sulfate form during sulfation with SO2 alone or SOx at 250 °C and the contents of sulfate show the linear relationship with the SO3 contents in SOx.
- Cu(OH)+ show the stronger response to sulfate, compared with Cu2+ ions, and no trace of Cu(OH)+ has been found during sulfation. The declined contents of copper ions depend on the ratio of SO3/SOx, and only ~13% copper ions remain after sulfation in 22% SO3/SOx stream.
- For sulfated catalysts, the loss of Cu2+ contents contributed to the inferior SCR activity at low temperatures.
Author Contributions
Funding
Conflicts of Interest
References
- Gao, F.; Walter, E.D.; Kollar, M.; Wang, Y.; Szanyi, J.; Peden, C.H.F. Understanding ammonia selective catalytic reduction kinetics over Cu/SSZ-13 from motion of the Cu ions. J. Catal. 2014, 319, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Crandell, D.W.; Zhu, H.; Yang, X.; Hochmuth, J.; Baik, M.H. The mechanism of selective catalytic reduction of NOx on Cu-SSZ-13—A computational study. Dalton Trans. 2017, 46, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Leistner, K.; Xie, K.; Kumar, A.; Kamasamudram, K.; Olsson, L. Ammonia desorption peaks can be assigned to different copper sites in Cu/SSZ-13. Catal. Lett. 2017, 147, 1882–1890. [Google Scholar] [CrossRef]
- Beale, A.M.; Lezcano-Gonzalez, I.; Slawinksi, W.A.; Wragg, D.S. Correlation between Cu ion migration behaviour and deNOx activity in Cu-SSZ-13 for the standard NH3-SCR reaction. Chem. Commun. 2016, 52, 6170–6173. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, Y.; Walter, E.D.; Washton, N.M.; Mei, D.; Kovarik, L.; Engelhard, M.H.; Prodinger, S.; Wang, Y.; Peden, C.H.F.; et al. Toward rational design of Cu/SSZ-13 selective catalytic reduction catalysts: Implications from atomic-level understanding of hydrothermal stability. ACS Catal. 2017, 7, 8214–8227. [Google Scholar] [CrossRef]
- Gao, F.; Mei, D.; Wang, Y.; Szanyi, J.; Peden, C.H. Selective catalytic reduction over Cu/SSZ-13: Linking homo- and heterogeneous catalysis. J. Am. Chem. Soc. 2017, 139, 4935–4942. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, C.; Khurana, I.; Parekh, A.A.; Li, S.; Shih, A.J.; Li, H.; di Iorio, J.R.; Albarracin-Caballero, J.D.; Yezerets, A.; Miller, J.T.; et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 2017, 357, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Varga, T.; Peden, C.H.F.; Gao, F.; Hanson, J.C.; Szanyi, J. Following the movement of Cu ions in a SSZ-13 zeolite during dehydration, reduction and adsorption: A combined in situ TP-XRD, XANES/DRIFTS study. J. Catal. 2014, 314, 83–93. [Google Scholar] [CrossRef]
- Jangjou, Y.; Sampara, C.S.; Gu, Y.; Wang, D.; Kumar, A.; Li, J.; Epling, W.S. Mechanism-based kinetic modeling of Cu-SSZ-13 sulfation and desulfation for NH3-SCR applications. React. Chem. Eng. 2019, 4, 1038–1049. [Google Scholar] [CrossRef]
- Dahlin, S.; Lantto, C.; Englund, J.; Westerberg, B.; Regali, F.; Skoglundh, M.; Pettersson, L.J. Chemical aging of Cu-SSZ-13 SCR catalysts for heavy-duty vehicles—Influence of sulfur dioxide. Catal. Today 2018, 320, 72–83. [Google Scholar] [CrossRef]
- Xie, K.; Leistner, K.; Wijayanti, K.; Kumar, A.; Kamasamudram, K.; Olsson, L. Influence of phosphorus on Cu-SSZ-13 for selective catalytic reduction of NOx by ammonia. Catal. Today 2017, 297, 46–52. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, C.; Pang, L.; Ming, S.; Liu, P.; Li, T. The influence of phosphorus on the catalytic properties, durability, sulfur resistance and kinetics of Cu-SSZ-13 for NOx reduction by NH3-SCR. Appl. Catal. B Environ. 2018, 237, 116–127. [Google Scholar] [CrossRef]
- Xie, L.; Liu, F.; Shi, X.; Xiao, F.-S.; He, H. Effects of post-treatment method and Na co-cation on the hydrothermal stability of Cu–SSZ-13 catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2015, 179, 206–212. [Google Scholar] [CrossRef]
- Fan, C.; Chen, Z.; Pang, L.; Ming, S.; Dong, C.; Brou Albert, K.; Liu, P.; Wang, J.; Zhu, D.; Chen, H.; et al. Steam and alkali resistant Cu-SSZ-13 catalyst for the selective catalytic reduction of NOx in diesel exhaust. Chem. Eng. J. 2018, 334, 344–354. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Wang, J.; Wang, Z.; Chen, Z.; Li, X.; Shen, M.; Yan, W.; Kang, X. The Role of Impregnated Sodium Ions in Cu/SSZ-13 NH3-SCR Catalysts. Catalysts 2018, 8, 593. [Google Scholar] [CrossRef]
- Houel, V.; Millington, P.; Pollington, S.; Poulston, S.; Rajaram, R.R.; Tsolakis, A. Chemical deactivation of Ag/Al2O3 by sulphur for the selective reduction of NOx using hydrocarbons. Catal. Today 2006, 114, 334–339. [Google Scholar] [CrossRef]
- Cheng, Y.; Lambert, C.; Kim, D.H.; Kwak, J.H.; Cho, S.J.; Peden, C.H.F. The different impacts of SO2 and SO3 on Cu/zeolite SCR catalysts. Catal. Today 2010, 151, 266–270. [Google Scholar] [CrossRef]
- Kumar, A.; Smith, M.A.; Kamasamudram, K.; Currier, N.W.; An, H.; Yezerets, A. Impact of different forms of feed sulfur on small-pore Cu-zeolite SCR catalyst. Catal. Today 2014, 231, 75–82. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Liu, Y.; Kamasamudram, K.; Li, J.; Epling, W. SO2 poisoning impact on the NH3-SCR reaction over a commercial Cu-SAPO-34 SCR catalyst. Appl. Catal. B Environ. 2014, 156, 371–377. [Google Scholar] [CrossRef]
- Wijayanti, K.; Leistner, K.; Chand, S.; Kumar, A.; Kamasamudram, K.; Currier, N.W.; Yezerets, A.; Olsson, L. Deactivation of Cu-SSZ-13 by SO2 exposure under SCR conditions. Catal. Sci. Technol. 2016, 6, 2565–2579. [Google Scholar] [CrossRef]
- Wijayanti, K.; Xie, K.; Kumar, A.; Kamasamudram, K.; Olsson, L. Effect of gas compositions on SO2 poisoning over Cu/SSZ-13 used for NH3 -SCR. Appl. Catal. B Environ. 2017, 219, 142–154. [Google Scholar] [CrossRef]
- Luo, J.; Wang, D.; Kumar, A.; Li, J.; Kamasamudram, K.; Currier, N.; Yezerets, A. Identification of two types of Cu sites in Cu/SSZ-13 and their unique responses to hydrothermal aging and sulfur poisoning. Catal. Today 2016, 267, 3–9. [Google Scholar] [CrossRef]
- Shih, A.J.; Khurana, I.; Li, H.; González, J.; Kumar, A.; Paolucci, C.; Lardinois, T.M.; Jones, C.B.; Albarracin Caballero, J.D.; Kamasamudram, K.; et al. Spectroscopic and kinetic responses of Cu-SSZ-13 to SO2 exposure and implications for NOx selective catalytic reduction. Appl. Catal. A Gen. 2019, 574, 122–131. [Google Scholar] [CrossRef]
- Jangjou, Y.; Do, Q.; Gu, Y.; Lim, L.-G.; Sun, H.; Wang, D.; Kumar, A.; Li, J.; Grabow, L.C.; Epling, W.S. Nature of Cu Active Centers in Cu-SSZ-13 and Their Responses to SO2 Exposure. ACS Catal. 2018, 8, 1325–1337. [Google Scholar] [CrossRef]
- Lai Wei, D.Y. Feng Wu, Biao Liu, Xiaohan Hu, Xingwen Li, and Xinlei Wang, Impact of Hydrothermal Aging on SO2 Poisoning over Cu-SSZ-13 Diesel Exhaust SCR Catalysts. IECR 2019, 58, 3949–3958. [Google Scholar] [CrossRef]
- Hammershøi, P.S.; Jangjou, Y.; Epling, W.S.; Jensen, A.D.; Janssens, T.V.W. Reversible and irreversible deactivation of Cu-CHA NH3-SCRcatalysts by SO2 and SO3. Appl. Catal. B Environ. 2018, 226, 38–45. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Wang, J.; Wang, C.; Wang, J. Nature of SO3 poisoning on Cu/SAPO-34 SCR catalysts. J. Catal. 2018, 358, 277–286. [Google Scholar] [CrossRef]
- Jangjou, Y.; Ali, M.; Chang, Q.; Wang, D.; Li, J.; Kumar, A.; Epling, W.S. Effect of SO2 on NH3 oxidation over a Cu-SAPO-34 SCR catalyst. Catal. Sci. Technol. 2016, 6, 2679–2685. [Google Scholar] [CrossRef]
- Shan, Y.; Shi, X.; Yan, Z.; Liu, J.; Yu, Y.; He, H. Deactivation of Cu-SSZ-13 in the presence of SO2 during hydrothermal aging. Catal. Today 2019, 320, 84–90. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Wang, J.; Yu, T.; Shen, M.; Wang, W.; Li, W. The effect of sulfate species on the activity of NH3-SCR over Cu/SAPO-34. Appl. Catal. B Environ. 2017, 204, 239–249. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.K.; Min, K.M.; Hong, S.B.; Nam, I.-S.; Cho, B.K. Hydrothermal stability of CuSSZ13 for reducing NOx by NH3. J. Catal. 2014, 311, 447–457. [Google Scholar] [CrossRef]
- Leistner, K.; Kumar, A.; Kamasamudram, K.; Olsson, L. Mechanistic study of hydrothermally aged Cu/SSZ-13 catalysts for ammonia-SCR. Catal. Today 2018, 307, 55–64. [Google Scholar] [CrossRef]
- Paolucci, C.; Di Iorio, J.R.; Ribeiro, F.H.; Gounder, R.; Schneider, W.F. Catalysis Science of NOx Selective Catalytic Reduction With Ammonia Over Cu-SSZ-13 and Cu-SAPO-34. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 2016; Volume 59, pp. 1–107. [Google Scholar]
- Gao, F.; Szanyi, J. On the hydrothermal stability of Cu/SSZ-13 SCR catalysts. Appl. Catal. A Gen. 2018, 560, 185–194. [Google Scholar] [CrossRef]
- Shen, M.; Li, X.; Wang, J.; Wang, C.; Wang, J. Nature Identification of Cu Active Sites in Sulfur-Fouled Cu/SAPO-34 Regeneration. Ind. Eng. Chem. Res. 2018, 57, 3501–3509. [Google Scholar] [CrossRef]
- Shen, M.; Wang, Z.; Li, X.; Wang, J.; Wang, J.; Wang, C.; Wang, J. Effects of regeneration conditions on sulfated CuSSZ-13 catalyst for NH3-SCR. Korean J. Chem. Eng. 2019, 36, 1249–1257. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Z.; Qiao, H.; Yu, H.; Hu, Y.; Chang, L.; Bao, W. Cerium-Stabilized Cu-SSZ-13 Catalyst for the Catalytic Removal of NOx by NH3. Ind. Eng. Chem. Res. 2016, 55, 1174–1182. [Google Scholar] [CrossRef]
- Hammershøi, P.S.; Vennestrøm, P.N.R.; Falsig, H.; Jensen, A.D.; Janssens, T.V.W. Importance of the Cu oxidation state for the SO2 -poisoning of a Cu-SAPO-34 catalyst in the NH3-SCR reaction. Appl. Catal. B Environ. 2018, 236, 377–383. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Wang, J.; Wang, J.; Shen, M.; Li, W. Effects of Na(+) on Cu/SAPO-34 for ammonia selective catalytic reduction. J. Environ. Sci. 2018, 70, 20–28. [Google Scholar] [CrossRef]
- Wang, J.; Shao, L.; Wang, C.; Wang, J.; Shen, M.; Li, W. Controllable preparation of various crystal size and nature of intra-crystalline diffusion in Cu/SSZ-13 NH3-SCR catalysts. J. Catal. 2018, 367, 221–228. [Google Scholar] [CrossRef]










| Samples’ Name | Specific Surface Area (m2/g) | ΔS (%) 1 |
|---|---|---|
| F-Cu | 801 | — |
| S-Cu-0 | 740 | 7.6 |
| S-Cu-5 | 725 | 9.5 |
| S-Cu-10 | 710 | 11.4 |
| S-Cu-22 | 700 | 12.6 |
| CuSSZ-13s’ Name | Specific Surface Area (m2/g) | ΔSBET (%) 1 | ΔSXRD (%) 2 | CuSAPO-34s’ Name | Specific Surface Area (m2/g) | ΔSBET (%) 1 | ΔSXRD (%) 2 |
|---|---|---|---|---|---|---|---|
| F-Cu | 791 | — | — | F-Cu | 733 | — | — |
| Cu-80-2 | 763 | 3.5 | 2.1% | Cu-80-2 | 430 | 41.3 | 62.0 |
| Cu-80-1 | 750 | 5.2 | 3.5% | Cu-80-1 | 306 | 58.3 | 83.5 |
| Cu-80-0.5 | 600 | 24.1 | 20.1% | Cu-80-0.5 | 8 | 98.9 | 100.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Hou, Y.; Yan, W.; Zhang, Y.; Wang, J.; Wang, J.; Shen, M.; Kang, X. The Role of SO3 Poisoning in CU/SSZ-13 NH3-SCR Catalysts. Catalysts 2019, 9, 741. https://doi.org/10.3390/catal9090741
Wang C, Hou Y, Yan W, Zhang Y, Wang J, Wang J, Shen M, Kang X. The Role of SO3 Poisoning in CU/SSZ-13 NH3-SCR Catalysts. Catalysts. 2019; 9(9):741. https://doi.org/10.3390/catal9090741
Chicago/Turabian StyleWang, Chen, Yaqin Hou, Wenjun Yan, Yun Zhang, Jun Wang, Jianqiang Wang, Meiqing Shen, and Xue Kang. 2019. "The Role of SO3 Poisoning in CU/SSZ-13 NH3-SCR Catalysts" Catalysts 9, no. 9: 741. https://doi.org/10.3390/catal9090741