1. Introduction
During the last few years, there has been increasing interest in the study of spinel ZnFe
2O
4 (ZFO) as a photoanode material for photoelectrochemical water oxidation [
1,
2,
3]. As new scientific investigations in this field are reported, differences concerning the photoelectrochemical activity of ZFO photoanodes prepared by different routes have become evident [
4,
5,
6,
7,
8,
9,
10,
11]. It is well known that ZFO exhibits a variable structure where the distribution of Zn
2+ and Fe
3+ cations between octahedral and tetrahedral sites within the crystal lattice depends on the synthetic conditions [
12,
13,
14,
15,
16,
17,
18]. Therefore, the reason behind the broad variety of results found in the scientific literature for ZFO photoanodes might be related to the cation distribution. The parameter used to quantify the cation distribution is the degree of inversion,
x, which is defined as
T[Zn
1−xFe
x]
O[Zn
xFe
2−x]O
4 with 0 ≤
x ≤ 1 (where the superscripts T and O denote the tetrahedral and octahedral sites, respectively). When
x = 0 and
x = 1, ZFO adopts the so-called normal (
T[Zn]
O[Fe
2]O
4) and inverse (
T[Fe]
O[ZnFe]O
4) structure, respectively. The degree of inversion of bulk ZFO can be controlled by the calcination of the samples at temperatures higher than 737 K and subsequent quenching [
12,
13,
14,
15]. Thus, bulk ZFO samples with
x ranging from approximately 0.02 to 0.20 can be prepared. Pavese et al. [
13] reported degrees of inversion up to
x ≈ 0.34 at 1600 K for bulk ZFO by in situ high-temperature neutron powder diffraction measurements. Nevertheless, as shown by O’Neill [
12], degrees of inversion higher than
x ≈ 0.20 cannot be experimentally accessed for bulk ZFO samples prepared by means of a solid-state reaction and subsequent quenching. Calcination temperatures higher than 1200 K are required to increase the degree of inversion above this upper limit and, under these conditions, the rate of re-ordering is too fast to quench the sample. For nanoparticulate ZFO, higher degrees of inversion are likely to be obtained [
16,
17,
18], and the synthesis of ZFO nanoparticles having an almost completely inverted structure (
x = 0.94) has been reported [
19].
In a recent publication, Zhu et al. [
8] reported that the cation distribution in partially reduced ZFO anodes affected the performance of light-induced water oxidation. The authors showed that partially reduced ZFO had a relatively poor crystallinity, but a high degree of inversion exhibited superior photogenerated charge carrier transport when compared to ZFO with a high crystallinity but a low degree of inversion. The research of Zhu et al. pioneered the investigation of the effect of the cation distribution on the photoelectrochemical activity of ZFO. However, a study of the effect of the degree of inversion on the photoelectrochemical activity of pristine ZFO samples exhibiting uniform particle size, crystallinity, and crystallite size is, to the best of our knowledge, missing.
Recently, we reported the preparation of ZFO by a solid-state reaction and its further processing into pellets with varying degrees of inversion [
14,
20]. The elemental analysis of the ZFO pellets revealed a Fe to Zn ratio of 2:1 within the limit of the experimental error, as expected for ZFO [
14]. The absence of non-reacted α-Fe
2O
3 and ZnO as well as the absence of secondary iron oxide phases, were confirmed by XRD and Raman spectroscopy [
14]. Mössbauer spectroscopy confirmed the absence of Fe
2+ and, hence, of oxygen vacancies for all the ZFO pellets [
14]. The crystallite size values deduced from the Rietveld refinements and the particle size distribution obtained from the SEM confirmed that the pellets exhibited similar crystallite and particle sizes independently of the degree of inversion [
14,
20]. Thus, the degree of inversion was found to be the only independent variable between the different ZFO pellets. These characteristics made it possible to investigate the impact of the degree of inversion on the photoelectrochemical activity of ZFO photoanodes unaffected by other variable parameters such as impurities, the number of oxygen vacancies, the particle size, the crystallite size, and the crystallinity.
In the present work, the photoelectrochemical activity of photoanodes made of pristine ZFO with degrees of inversion increasing from
x ≈ 0.07 to
x ≈ 0.20 is reported. Furthermore, the effect of the cation distribution on electronic properties such as the Fermi level and the electronic structure was investigated for the first time. The electronic structure was studied by means of time-averaged and transient room-temperature photoluminescence spectroscopy. It is well known that the photoluminescence spectrum of a material depends on its particle size, crystallinity, and the presence of point defects [
21,
22]. Therefore, the crystallinity and crystallite size homogeneity of the synthesized ZFO pellets is of utmost importance in order to access meaningful information concerning the effect of the degree of inversion on the photoluminescence properties. The nature of the observed transitions as well as their lifetime and the impact of the degree of inversion are discussed.
2. Results
ZFO pellets with degrees of inversion of
x ≈ 0.07 (ZFO_773),
x ≈ 0.10 (ZFO_873),
x ≈ 0.13 (ZFO_973),
x ≈ 0.16 (ZFO_1073), and
x ≈ 0.20 (ZFO_1173) were prepared by employing a spinel zinc ferrite synthesized by a solid-state reaction as reported previously [
14]. In order to access information concerning the porosity of the ZFO pellets, N
2 and Ar physisorption isotherms were measured. Total pore volumes below 10 cm
3 g
−1 were obtained for all pellets. These values were at the lower limit of quantification, suggesting that the samples did not exhibit a considerable porosity. This is an expected result for dense pellets pressed at high pressure. Other consequences of the low porosity were small BET surface areas below 10 m
2 g
−1. The pellets exhibited values ranging from 3.0 to 9.9 m
2 g
−1 with an average of 5.3 m
2 g
−1 and no systematic trend concerning the degree of inversion. Thus, independent of the degree of inversion, negligible total pore volumes and sizes were obtained for the different ZFO pellets.
The photoelectrochemical activity of the photoanodes was evaluated by measuring the photocurrent for the methanol oxidation reaction.
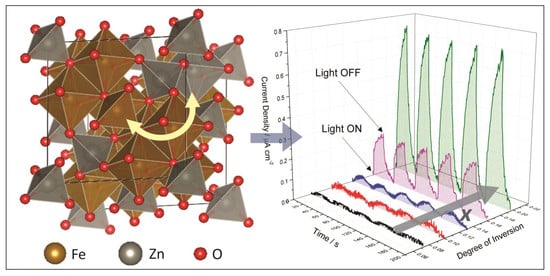
Figure 1a shows the current density – voltage (j-V) curves measured under chopped solar simulated light for the ZFO photoanodes with increasing degrees of inversion. The light was turned on and off at 20 s intervals. Onset photocurrents for the methanol oxidation were observed at an anodic bias potential of around +0.9 V vs. RHE. At a bias potential of +1.2 V vs. RHE, the dark currents were still negligible and the photocurrents were high enough to allow a direct comparison between the different photoanodes.
Figure 1b shows the chopped light chronoamperometry measured at an applied bias of +1.2 V vs. RHE. It was observed that the photocurrent for the methanol oxidation increased as the degree of inversion rose from
x ≈ 0.07 to
x ≈ 0.20. The photoanodes with degrees of inversion of
x ≈ 0.07,
x ≈ 0.10, and
x ≈ 0.13 showed current densities below 0.05 μA cm
−2. There was a significant increase in the current density from 0.05 to 0.24 μA cm
−2 as the degree of inversion increased from
x ≈ 0.13 to
x ≈ 0.16. The current density further increased up to 0.77 μA cm
−2 as the degree of inversion increased from
x ≈ 0.16 to
x ≈ 0.20.
Figure 2 shows the result of an incident photon-to-current efficiency (IPCE) measurement performed with the ZFO photoanode with a degree of inversion of
x ≈ 0.20 (ZFO_1173). This photoanode showed the highest photocurrent density for the methanol oxidation (0.77 μA cm
−2,
Figure 1b). Considering the optical properties of the ZFO_1173 pellet reported previously [
14], the absorbed photon-to-current efficiency (APCE) was calculated. It can be observed from
Figure 2 that the APCE and, therefore, the ratio between the number of photogenerated holes reacting with methanol and the number of absorbed photons increased as the wavelength of the incident light became shorter. Thus, the photoanode converts the incident light into an electrical current more efficiently as the energy of the photons emitted by the excitation source increases.
Figure 3 shows the work function measured by the Kelvin probe technique for the ZFO pellet samples with different degrees of inversion. It was observed that the work function exhibited values ranging from 5.28 to 5.47 eV. Considering the experimental uncertainty (±0.13 eV), no significant changes in the Fermi level were observed as the degree of inversion of the ZFO pellets increased.
Time-averaged room-temperature photoluminescence measurements were carried out to investigate the effect of the degree of inversion on the electronic structure of ZFO. It becomes obvious from
Figure 4 that all pellets exhibited three emission signals at approximately 464, 547, and 625 nm. The relative fluorescence quantum yield of the photoluminescence was determined by using quinine hemisulfate as a standard. Fluorescence quantum yields of 0.05% for the ZFO pellets with degrees of inversion of
x ≈ 0.07,
x ≈ 0.10, and
x ≈ 0.13, 0.04% for the pellet with
x ≈ 0.15, and 0.03% for the pellets with
x ≈ 0.20 were obtained.
Transient room-temperature photoluminescence measurements were conducted to study the lifetime of the electronic transitions.
Figure 5a shows the photoluminescence spectra of the ZFO pellets with different degrees of inversion measured at 28 ns after the 355 nm laser excitation. Since 10 nm steps were used to record the transient signals, the spectral resolution was lower in comparison to the time-averaged measurements. Thus, the emission observed at approximately 625 nm in the time-averaged photoluminescence measurements (
Figure 4) was not detected in the transient measurements. However, the emissions centered at 547 and 464 nm were clearly observed. The emission centered at 464 nm showed a higher intensity than the emission centered at 547 nm.
Figure 5b shows the room-temperature photoluminescence spectra of the ZFO pellet with a degree of inversion of
x ≈ 0.07 (ZFO_773) measured at different times after the laser excitation. The emission signal centered at 464 nm was weakly observed at 208 ns after the excitation, while the emission signal centered at 547 nm was no longer observed at 148 ns after the excitation.
3. Discussion
A series of ZFO photoanodes with different cation distributions between the tetrahedral and octahedral sites of the oxygen lattice were investigated to study the impact of the degree of inversion on the photoelectrochemical activity of ZFO. Due to the high-temperature calcination steps carried out during the preparation of the samples, large crystallite sizes of approximately 300 nm were obtained [
14]. Large crystallite sizes imply large diffusion lengths for the photogenerated charge carriers [
23,
24]. Consequently, the charge carrier recombination rate increases and the photoelectrochemical efficiency of the material decreases [
25]. Furthermore, low active surface areas are expected as the crystallite size increases [
23,
24]. Therefore, the large crystallite size of the ZFO photoanodes were responsible for the low photocurrent values obtained for the photoelectrochemical methanol oxidation (
Figure 1). The large thickness of the films (approximately 750 μm) also had a negative impact on the photoelectrochemical activity for the same reasons above-mentioned. Higher photocurrent densities are reported in the literature for ZFO photoelectrodes [
4,
6,
26,
27]. In fact, the current benchmark in the performance of a partially reduced ZFO photoanode for solar water oxidation is 1.0 mA cm
−2 at 1.23 V [
8]. However, the aim of this work was to understand the impact of the degree of inversion on both the electronic properties and the photoelectrochemical activity of ZFO. The large particle size and thickness of the prepared ZFO electrodes as well as the absence of oxygen vacancies were the main reasons behind the observed low photocurrents. These drawbacks cannot be avoided when the synthesis of pristine ZFO samples in which the degree of inversion is the only independent variable is intended. However, although the measured photocurrents were below 1.0 μA cm
−2, an impact of the degree of inversion on the current density was clearly observed. It becomes obvious from
Figure 1 that an increase of the cation distribution results in an increase in the photoelectrochemical activity of ZFO. The electrical conductivity of the pellets used in the present work was reported in a previous study [
20]. An increase in the conductivity from 9.28 × 10
−9 to 1.82 × 10
−5 S cm
−1 was observed as the degree of inversion increased from
x ≈ 0.07 to
x ≈ 0.20 [
20]. Interestingly, a significant increase of two orders of magnitude was observed in the conductivity by increasing the degree of inversion from
x ≈ 0.13 to
x ≈ 0.16. This increase in the conductivity agreed with the large increase in the photocurrent from 0.05 to 0.24 μA cm
−2 that was observed for methanol oxidation as the degree of inversion increased from
x ≈ 0.13 to
x ≈ 0.16 (
Figure 1b). It becomes evident from the preceding discussion that the increase in the electrical conductivity of the pellets with a higher degree of inversion is closely related to the improvement of the photoelectrochemical activity. However, the interrelation between the photoelectrochemical activity and the degree of inversion might also be related to other physicochemical properties of ZFO. Therefore, the impact of the cation distribution on the work function and the electronic transitions of ZFO was investigated.
As mentioned in the previous section, no significant changes in the work function were observed as the degree of inversion of the ZFO pellets increased. In a semiconductor, the work function represents the minimum energy required to remove an electron from the Fermi level into the free space [
28]. The work function values obtained for the ZFO pellets corresponded, considering the IUPAC recommended value of −4.44 V for the absolute electrode potential of the hydrogen electrode [
29], to Fermi levels around +0.94 V vs. SHE. Sun et al. [
5] reported work function values ranging from 5.46 to 5.55 eV (+1.02 and +1.11 V vs. SHE) for mesoporous ZFO nanoparticles obtained via an evaporation-induced self-assembly method and calcined at different temperatures. The results were in good agreement with the values obtained in the present work. The work function of a semiconductor strongly depends on its doping level [
30]. As well as for many n-type metal oxide semiconductors [
31], the mechanism for n-type doping of ZFO is the formation of oxygen vacancies [
32], which results in the reduction of Fe
3+ to Fe
2+ [
33]. As no Fe
2+ was detected by Mössbauer spectroscopy for the ZFO pellets used in this work [
14], a low donor density was expected. The high work function values obtained and the large anodic potential necessary for the methanol photoelectrochemical oxidation were the consequences of the low donor density. Furthermore, as similar work functions were observed for the different pellets, it was concluded that their donor densities were in the same order of magnitude and did not depend on the degree of inversion. For highly doped ZFO samples, work function values between 4.23 and 4.97 eV (−0.21 and +0.53 V vs. SHE) have been reported in the literature [
27,
34,
35,
36].
The optical band gap and the nature of the optical transitions of the pellets used for the present work were determined by measuring the diffuse reflectance and applying the derivation of absorption spectrum fitting (DASF) method, as was shown in a previous report [
14]. Independent of the degree of inversion, the pellets exhibited an indirect band gap transition at approximately 614 nm (2.02 eV), and a direct band gap transition at approximately 532 nm (2.33 eV) [
14]. These values were in good agreement with the 1.9 and 2.3 eV reported by Guijarro et al. [
27] for the indirect and direct band gap, respectively. From density functional theory calculations, Yao et al. [
37] showed that the valence band of ZFO consisted of O
2− 2
s, Zn
2+ 3
d, Fe
3+ 3
d, and O
2− 2
p states. However, the valence band edge consisted of Fe
3+ 3
d and mainly O
2− 2
p states [
37]. The conduction band edge consisted of O
2− 2
p and mainly Fe
3+ 3
d states [
37]. At higher energies, the contribution from the Zn
2+ 4
s states was observed in the density of states. Lv et al. [
38] claimed that the energy band structures of ZFO were defined by considering the O
2− 2
p levels as the valence band edge and the Fe
3+ 3d levels as the conduction band edge. The emissions observed in
Figure 4 at approximately 547 and 625 nm were in reasonable agreement with the direct and indirect band gap transitions at 532 and 614 nm, respectively [
14]. Thus, these bands were ascribed to near-band-edge emissions due to the electron relaxation from Fe
3+ 3
d levels located in the conduction band edge to O
2− 2
p levels in the valence band edge [
39,
40]. The emission centered at 464 nm might be related to transitions involving the Zn
2+ cations [
41,
42]. It is well known for Fe-doped ZnO that the near-band-edge emission of ZnO centered at approximately 379 nm becomes red-shifted as the amount of Fe increases [
42,
43]. If the amount of Fe is high enough and spinel ZFO is formed as a secondary phase, a new emission centered at 464 nm is observed [
42]. Therefore, the transition observed at approximately 464 nm could be ascribed to the electron relaxation from Zn
2+ 4
s levels within the conduction band to O
2− 2
p in the valence band edge. This assignment agrees with the density of states presented by Yao et al. [
37] Regardless of the nature of the electronic transitions, it is important to stress that the conduction band of ZFO does not exhibit a continuous density of empty electronic energetic states. Contradicting the semiconductor band theory [
44], the conduction band of photoexcited electrons were delocalized in confined densities of states involving either Fe
3+ 3
d levels or Zn
2+ 4
s levels. Whether the photoexcited electron is delocalized in Fe
3+ 3
d levels or Zn
2+ 4
s levels depends on the energy of the excitation source. A scheme of the electronic excitation mechanism of ZFO is shown in
Figure 6.
The influence of the O
2− 2
p to Fe
3+ 3
d (indirect and direct) and the O
2− 2
p to Zn
2+ 4
s electronic transitions on the photoelectrochemical efficiency of ZFO was deduced from the APCE measurement. According to
Figure 2, when the ZFO photoanode is irradiated with light at wavelengths longer than 600 nm, the APCE is approximately zero. Thus, the methanol oxidation efficiency of the charge carriers generated via the indirect O
2− 2
p to Fe
3+ 3
d transition (625 nm) is negligible. As also reported by Guijarro et al. [
27], the indirect transition of ZFO does not effectively drive photoelectrochemical processes. The APCE increases as wavelengths ranging from 475 to 600 nm are used for the excitation of the photoanode. Under these irradiation conditions, the direct O
2− 2
p to Fe
3+ 3
d transition occurs and the generated charge carriers can convert the incident light into an electrical current. Interestingly, a significant increase in the APCE values can be observed when the photoanode is irradiated with light at wavelengths shorter than 475 nm. This phenomenon could be attributed to two reasons. One is the increase in the absorptivity of the material as is observed from the diffuse reflectance measurements of the ZFO pellets reported elsewhere [
14]. The second reason is the contribution of the charge carriers generated by the electronic transition from O
2− 2
p to Zn
2+ 4
s levels (464 nm). The absorption coefficient of ZFO has approximately the same magnitude for wavelengths ranging from 400 to 500 nm [
45,
46]. Hence, the significant increase in the APCE should be observed at wavelengths longer than 475 nm if the larger light absorption of the material is responsible for this effect. Therefore, the increase in the APCE values at wavelengths shorter than 475 nm must be mainly due to the contribution to the methanol photooxidation of the charge carriers generated via the O
2− 2
p to Zn
2+ 4
s electronic transition (464 nm).
The data presented in
Figure 4 revealed that the degree of inversion affected the relative intensity of the room-temperature photoluminescence bands of ZFO. As the cation disorder increased, the intensity of the near-band-edge emission centered at 547 nm decreased, and the intensity of the emission centered at 464 nm increased. These emission bands were the result of electron-hole recombination processes and, thus, it is reasonable to assume that the observed increase or decrease in the emission intensity is due to an increase or decrease, respectively, in the number of generated electron-hole pairs. Therefore, as Zn
2+ cations located in tetrahedral sites are interchanged by Fe
3+ cations from octahedral sites, the probability of the electronic transition at 464 nm (from O
2− 2
p to Zn
2+ 4
s levels) increases and the probability of the near-band-edge electronic transitions (from O
2− 2
p to Fe
3+ 3
d levels) decreases. From the time-averaged photoluminescence measurements, it was observed that the intensity of the emission centered at 547 nm was higher than that of the emission centered at 464 nm (
Figure 4). However, transient photoluminescence measurements showed a higher intensity for the emission centered at 464 nm (
Figure 5a). This can be explained by a faster decay of the emission centered at 547 nm. In fact, the signal centered at 547 nm was no longer observed at 148 ns after the excitation while the signal centered at 464 nm (due to the relaxation of the O
2− 2
p to Zn
2+ 4
s electron excitation) was observed even 208 ns after the excitation (
Figure 5b).
As discussed above, the electron-hole pairs generated by the electronic transitions observed at 547 and 464 nm are involved in the photoelectrochemical process occurring at the ZFO electrodes. It was shown that the transition centered at 464 nm had a higher efficiency for photoelectrochemical methanol oxidation than the transition centered at 547 nm (
Figure 2). However, the valence band holes generated via both transitions had the same redox potential and, therefore, the same oxidizing activity. The higher efficiency of the transition centered at 464 nm was due to the longer lifetime of the generated charge carriers (
Figure 5b). As the degree of inversion of the ZFO pellets increased, both, the amount of O
2− 2
p to Zn
2+ 4
s electronic transitions and the photoelectrochemical activity increased.
4. Materials and Methods
Polycrystalline ZFO samples with degrees of inversion increasing from
x ≈ 0.07 to
x ≈ 0.20 were synthesized by means of a solid-state reaction as reported previously [
14]. Briefly, stoichiometric amounts of ZnO (60 mmoles, Sigma Aldrich, Taufkirchen, Germany, ≥ 99.0%) and α-Fe
2O
3 (60 mmoles, Sigma Aldrich, Taufkirchen, Germany, ≥99.0%) powders were ground in an agate mortar. The mixture was calcined in air at 1073 K for 12 h, cooled down to room temperature, and ground once again. Aliquots of 0.500 g were pressed into 13 mm diameter pellets applying a pressure of 55 MPa. The pellets were calcined at 1273 K for 2 h, cooled down to 1073 K and kept at this temperature for 12 h, then cooled down to 773 K and kept at this temperature for 50 h, and finally quenched in cold water. Some of the pellets (referred as ZFO_773) were separated and the rest were divided into four sets of pellets. These pellets were heated up with a rate of 300 K h
−1 and calcined at 873, 973, 1073, and 1173 K for 25, 20, 12, and 10 h, respectively. As reported by O’Neill [
12], these calcination times were sufficiently long for the ZFO pellets to reach a steady-state value of the degree of inversion. After this period of time, the calcined pellets were immediately quenched in cold water. These pellets are referred as ZFO_873, ZFO_973, ZFO_1073, and ZFO_1173. The ZFO pellets were sanded with Al
2O
3 sandpaper (KK114F, grit size P320, VSM Abrasives, Hannover, Germany) to a final thickness of 0.75 mm ± 0.02 mm.
Molecular nitrogen physisorption isotherms were measured at 77 K on a Quantachrome Autosorb-3MP instrument and argon physisorption isotherms were measured at 87 K on a Quantachrome Autosorb-1 instrument (3P Instruments GmbH & Co. KG, Odelzhausen, Germany). The pellets were outgassed in vacuum at 423 K for 24 h prior to the sorption measurements. Surface areas were estimated by applying the Brunauer–Emmett–Teller (BET) equation [
47] and the total pore volumes were estimated by the single-point method at
p/
p0 = 0.95.
Electrochemical and photoelectrochemical measurements were performed by employing a ZENNIUM Electrochemical Workstation (Zahner Scientific Instruments, Kronach, Germany) equipped with a three-electrode electrochemical cell with a Pt counter electrode and an Ag/AgCl/NaCl (3 mol kg−1) reference electrode. ZFO working electrodes were prepared by attaching a copper wire with silver paint (Ferro GmbH, Frankfurt am Main, Germany) and conductive epoxy (Chemtronics, Kennesaw, GA, USA) to one face of the pellet sample. Photoelectrochemical measurements were performed in a 50% v/v methanol aqueous solution containing 0.1 mol L−1 KNO3. A solar simulator (LOT-Quantum Design GmbH, Darmstadt, Germany ) consisting of a 300 W xenon-arc lamp provided with an AM 1.5-global filter was used as the irradiation source. An intensity output of 680 W m−2 at the position of the working electrode was measured using a SpectraRad Xpress spectral irradiance meter (B&W Tek, Newark, DE, USA). For the IPCE measurements, a TLS03 tunable light source (Zahner Scientific Instruments, Kronach, Germany) consisting of an array of monochromatic LEDs with emission wavelengths ranging from 400 to 650 nm was used. A monochromator was employed for fine tuning the wavelengths to a final resolution of 1 nm.
A scheme of the set-up used for the transient photoluminescence spectroscopy is shown in
Figure 7. The third harmonic (355 nm) of a Brilliant Nd:YAG laser (Quantel, Lannion, France) with a pulse duration of 6 ns was used as the excitation source. A laser intensity of 3.0 mJ per pulse was selected for the measurements. The intensity of the laser was measured using a Maestro laser power meter (Gentec-EO, Québec, Canada). The angle of the laser beam path was adjusted by rotating a Pellin-Broca prism beam steering module. The illumination area of the laser beam was approximately 0.5 cm
2. The light emitted by the pellets after the laser excitation was collected by a Spectrosil lens and directed toward the monochromator by a folding mirror. The monochromator was connected with a PMT R928 photomultiplier detector (Hamamatsu Photonics, Hamamatsu, Japan). To avoid the overloading of the photomultiplier, a 370 nm cut off filter was introduced in front of the monochromator entrance. For the transient photoluminescence measurements, a constant voltage of 700 V was applied to the photomultiplier.
Time-averaged photoluminescence measurements were performed in an F-7100 fluorescence spectrophotometer (Hitachi, Tokyo, Japan). A wavelength of 355 nm was selected as the excitation source and the photoluminescence spectra were measured from 440 to 650 nm with a 240 nm min
−1 scan rate. Excitation and emission slit widths of 10 nm were used. The relative fluorescence quantum yield was determined by employing a reported standard method [
48,
49,
50]. Briefly, a 5 × 10
−3 mol L
−1 solution of quinine hemisulfate monohydrate (C
20H
24N
2O
2⋅0.5H
2SO
4⋅H
2O, Sigma Aldrich, Taufkirchen, Germany, ≥ 98.0%) in 1 N H
2SO
4, with an absolute quantum yield efficiency of 0.51 at 25 °C [
48], was used to determine the relative photoluminescence quantum yield of the ZFO pellets. For the time-averaged and the transient photoluminescence measurements, suspensions of the ZFO pellets were prepared. ZFO pellets were ground in an agate mortar and dispersed in deionized water by a one-hour ultrasound treatment (340 W L
−1). A concentration of 2.5 g L
−1 was used for the measurements.
Work function measurements were performed with a scanning Kelvin probe system SKP5050 (KP Technology, Wick, Scotland) versus a gold reference probe electrode (probe area of 2 mm
2). The probe oscillation frequency was 74 Hz, and the backing potential was 7000 mV. Work function values were obtained by averaging 1000 data points for two different sites of each pellet. Prior to the measurements, the pellets were heated at 673 K for 12 h to remove adsorbed water. The cation diffusion process in ZFO is kinetically hindered at temperatures lower than 773 K [
12]. Thus, the degree of inversion of the pellets did not change during the heat treatment.