The Use of Power Ultrasound for the Production of PEMFC and PEMWE Catalysts and Low-Pt Loading and High-Performing Electrodes
Abstract
:1. Introduction
2. Power Ultrasound, Sonochemistry, and Sonoelectrochemistry
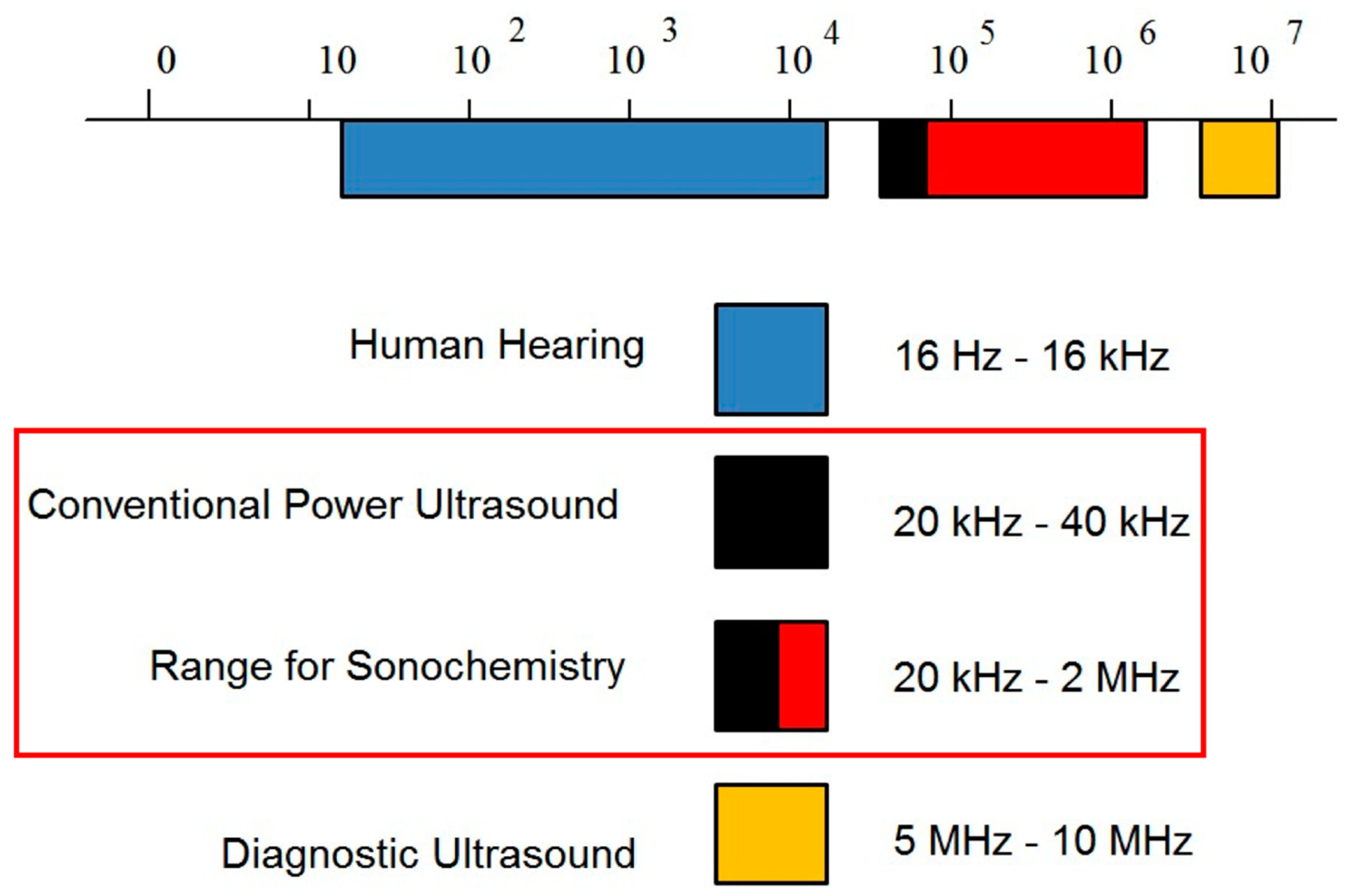
2.1. Power Ultrasound
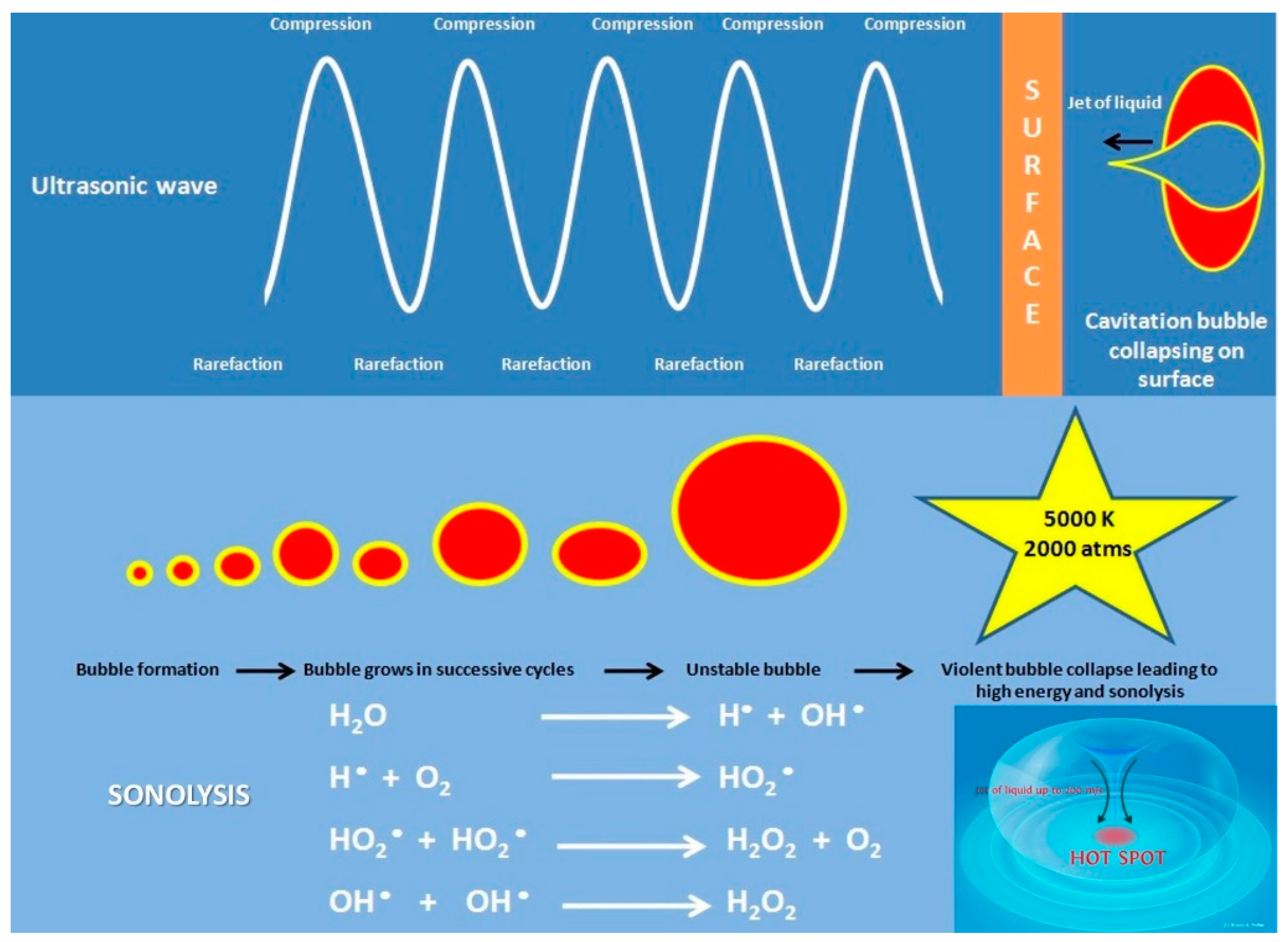
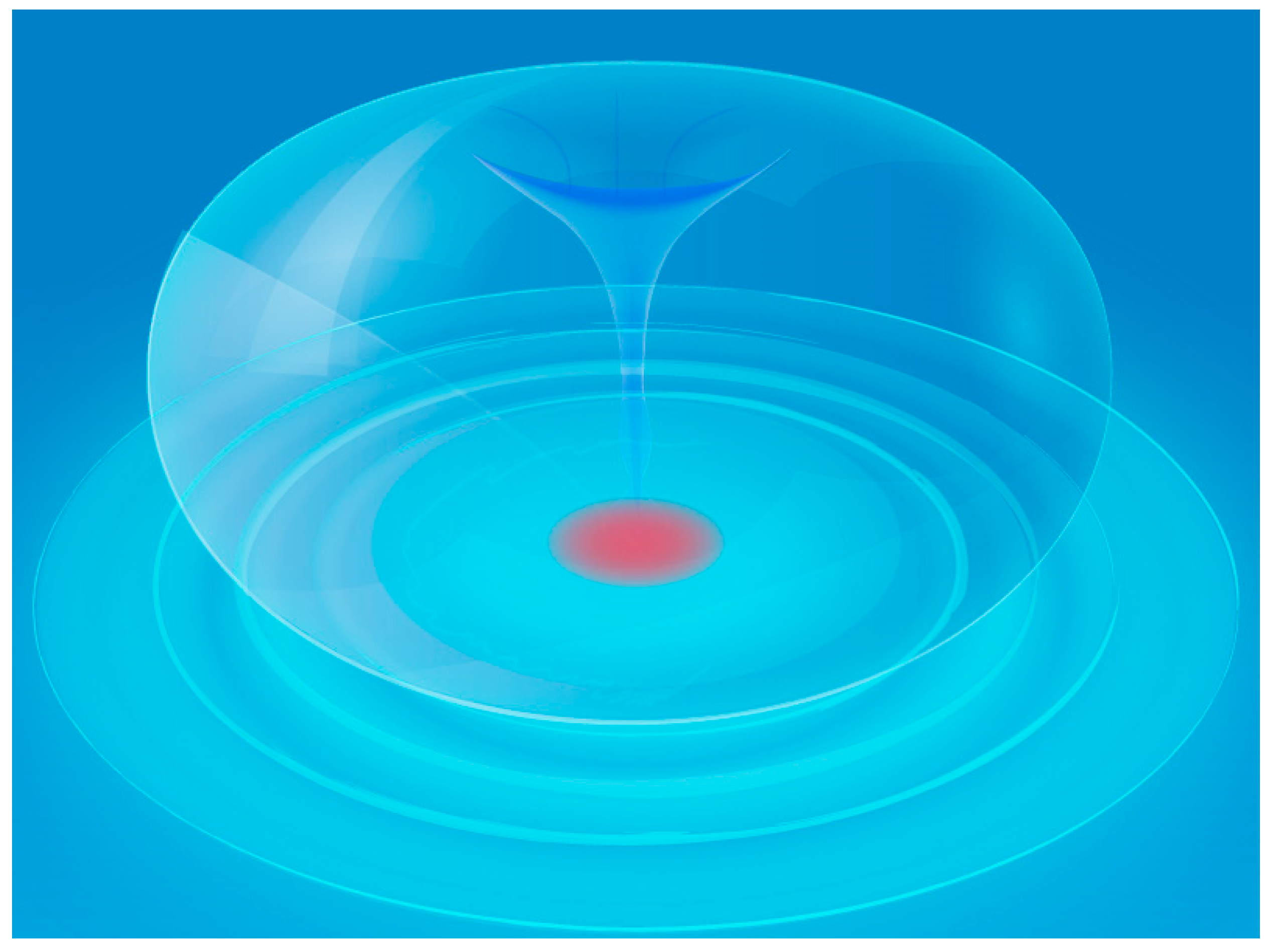
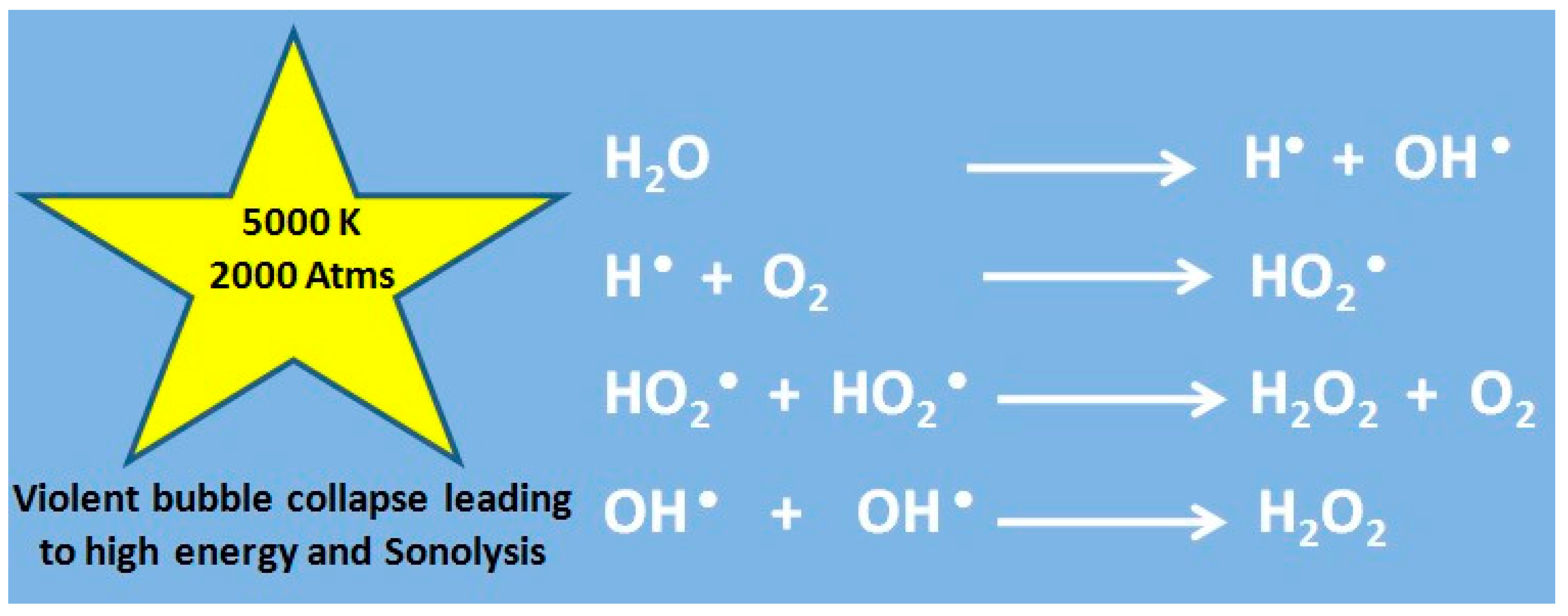
2.2. Sonochemistry
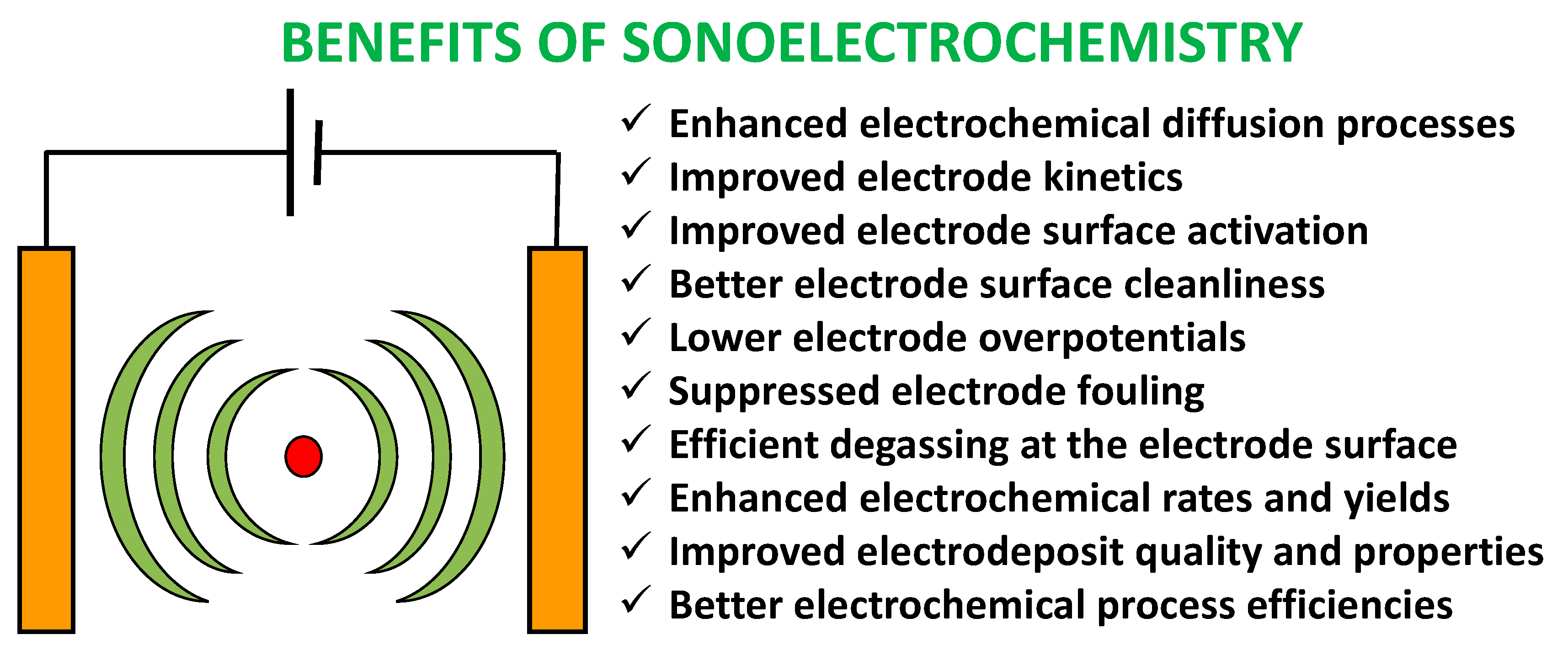
2.3. Sonoelectrochemistry
3. Sonochemical and Sonoelectrochemical Fabrication of PEMFC and PEMWE Catalysts
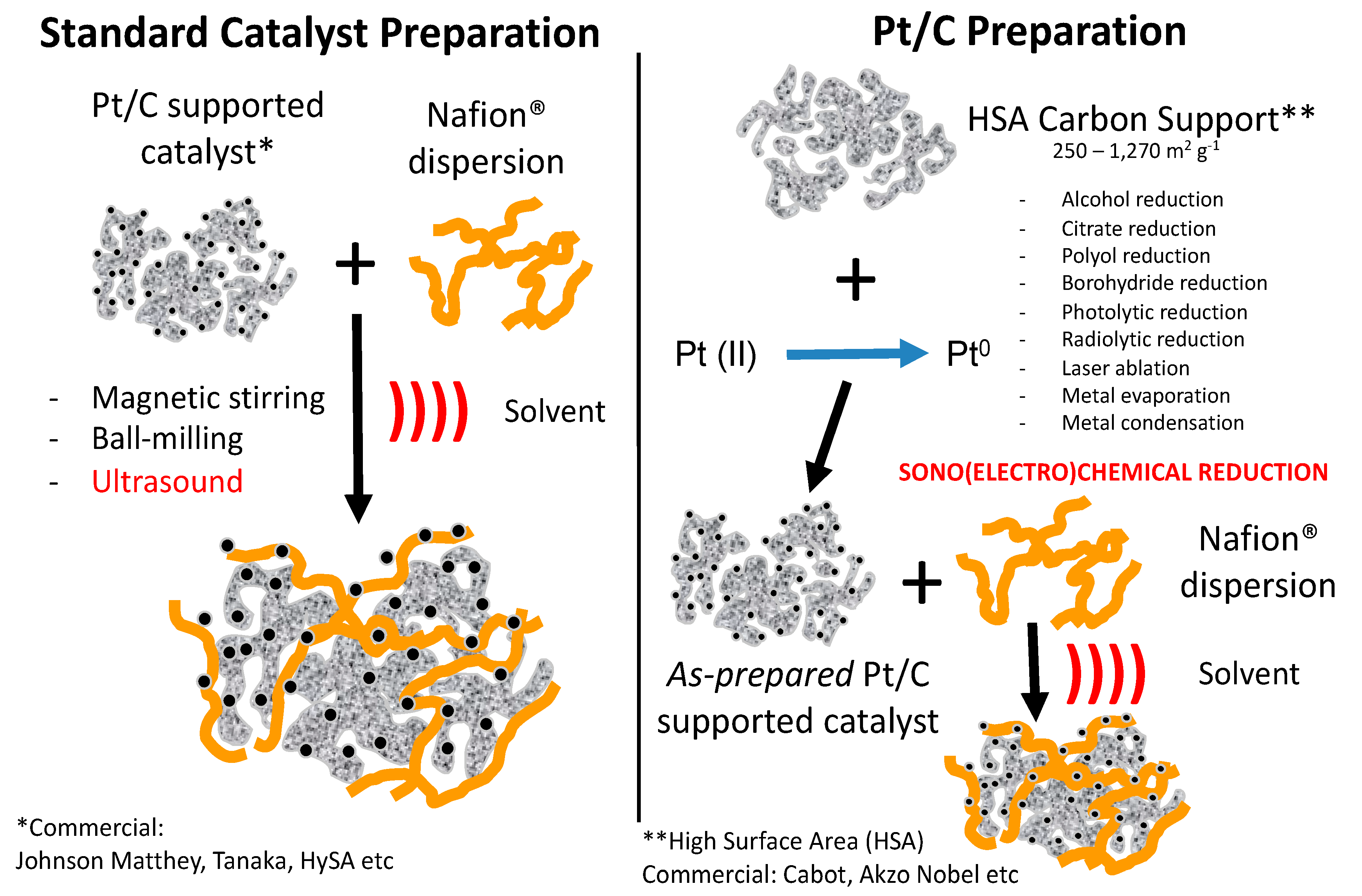
3.1. Sonochemical Production of PEMFC and PEMWE Catalysts
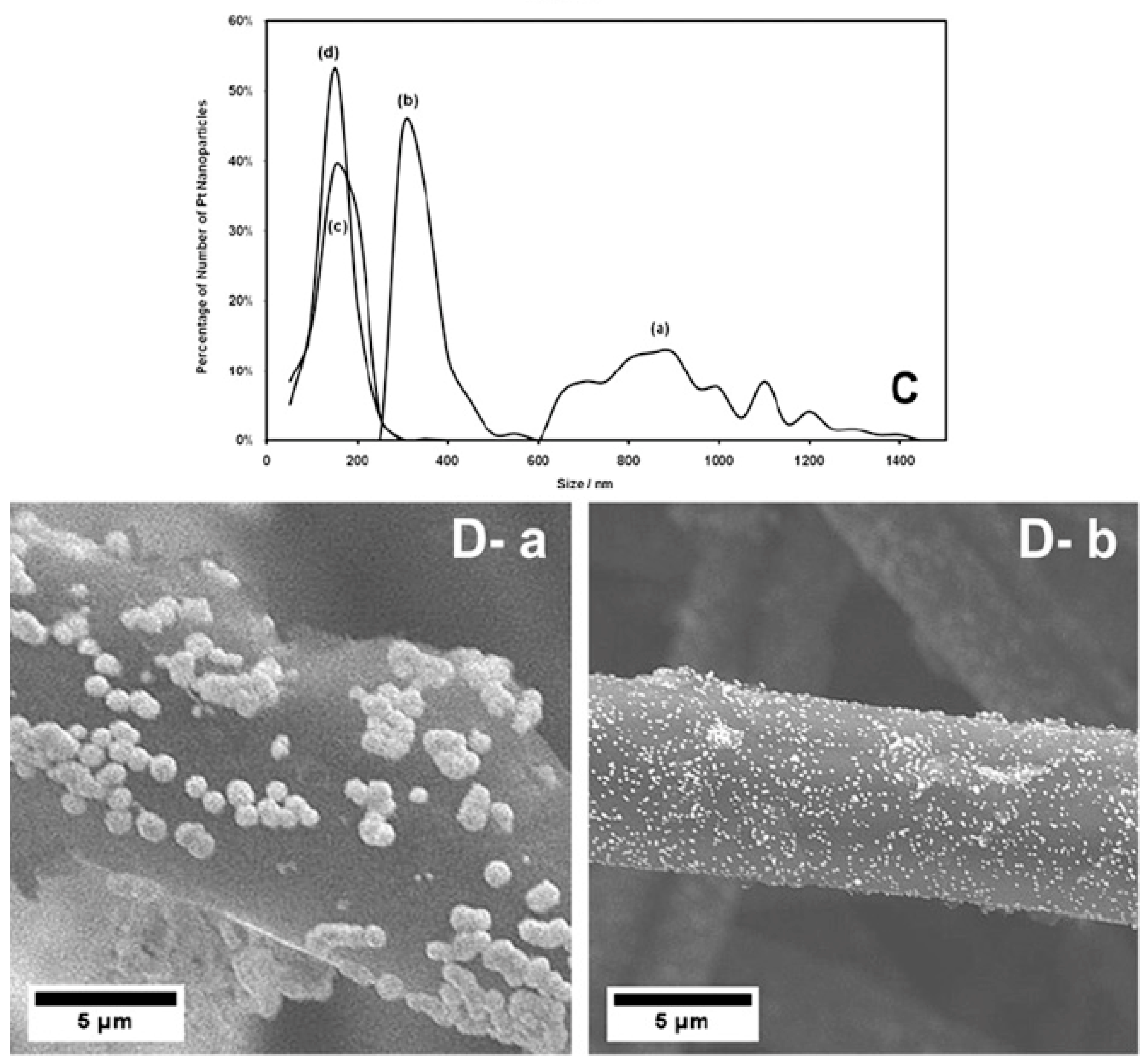
3.2. Sonoelectrochemical Production of PEMFC and PEMWE Catalysts
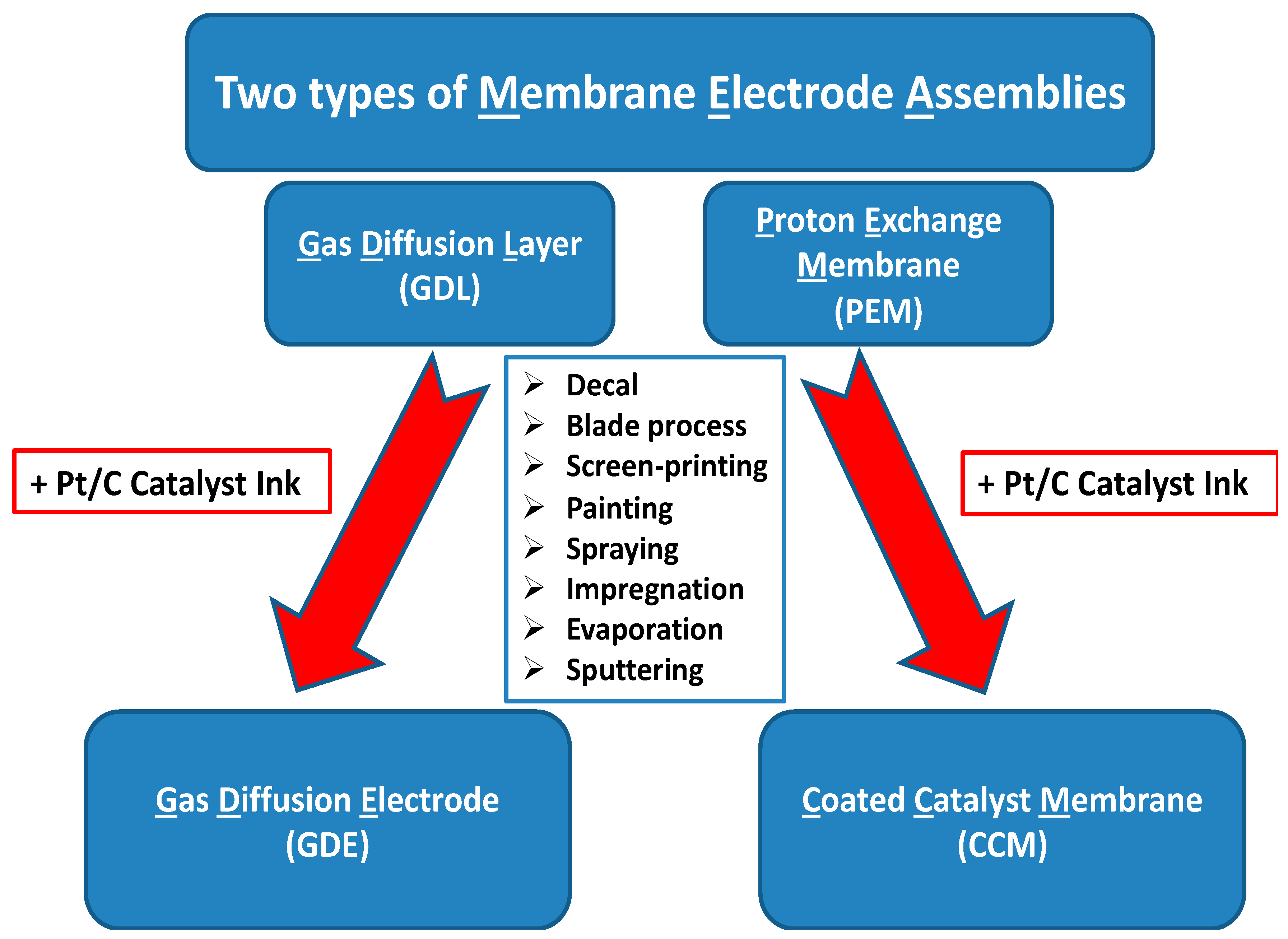
4. Ultrasonic, Sonochemical, and Sonoelectrochemical Production of PEMWE and PEMWE Electrodes
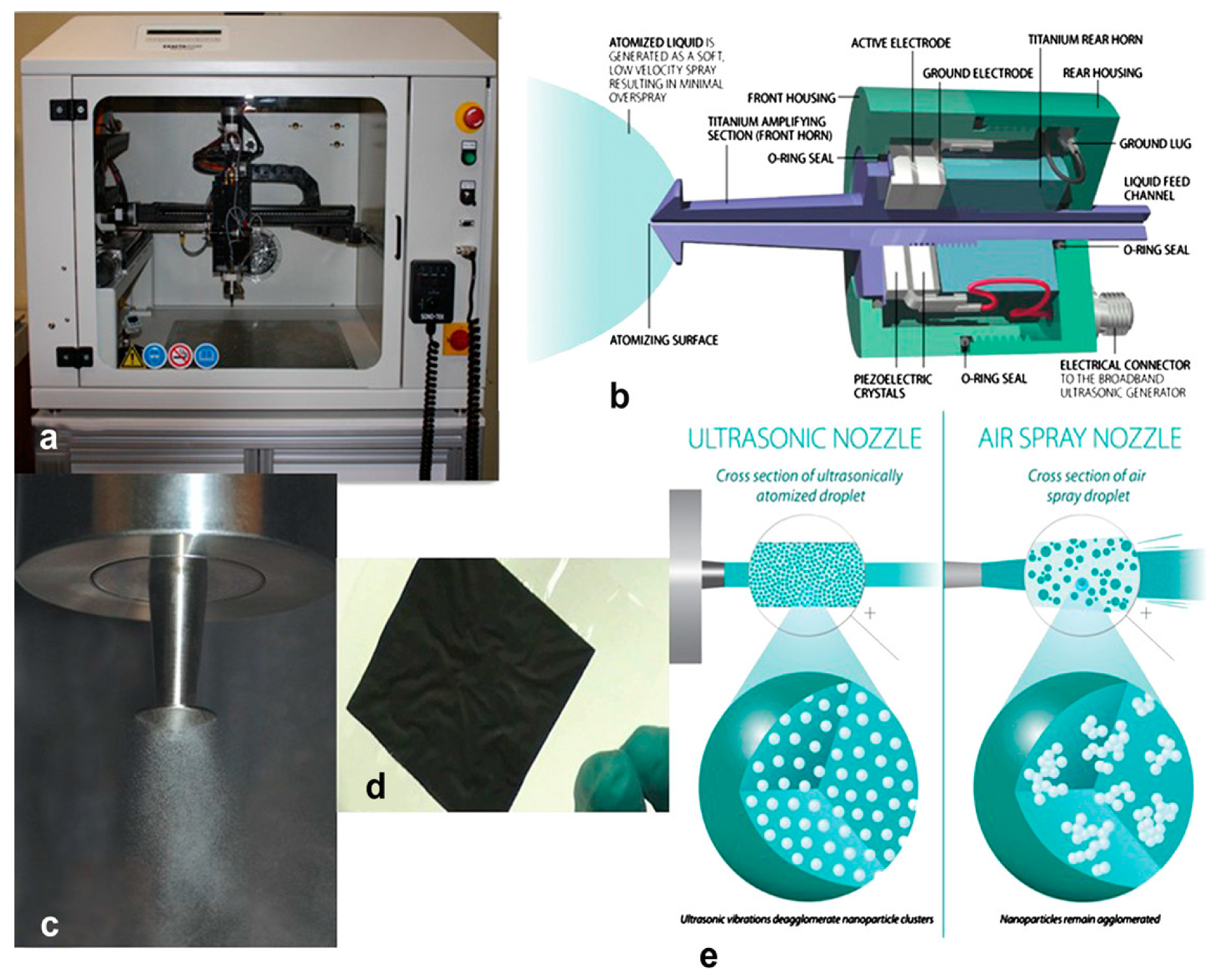
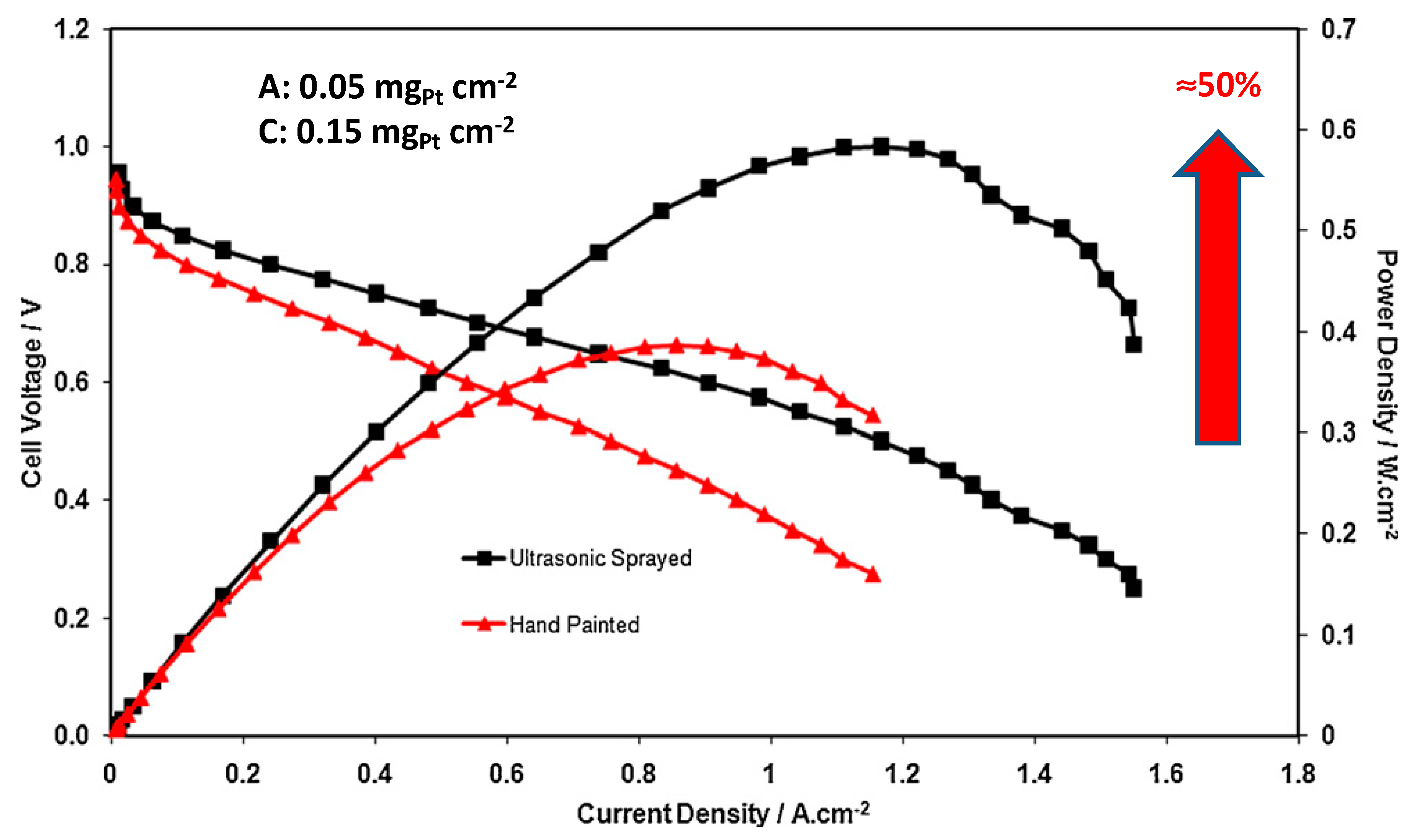
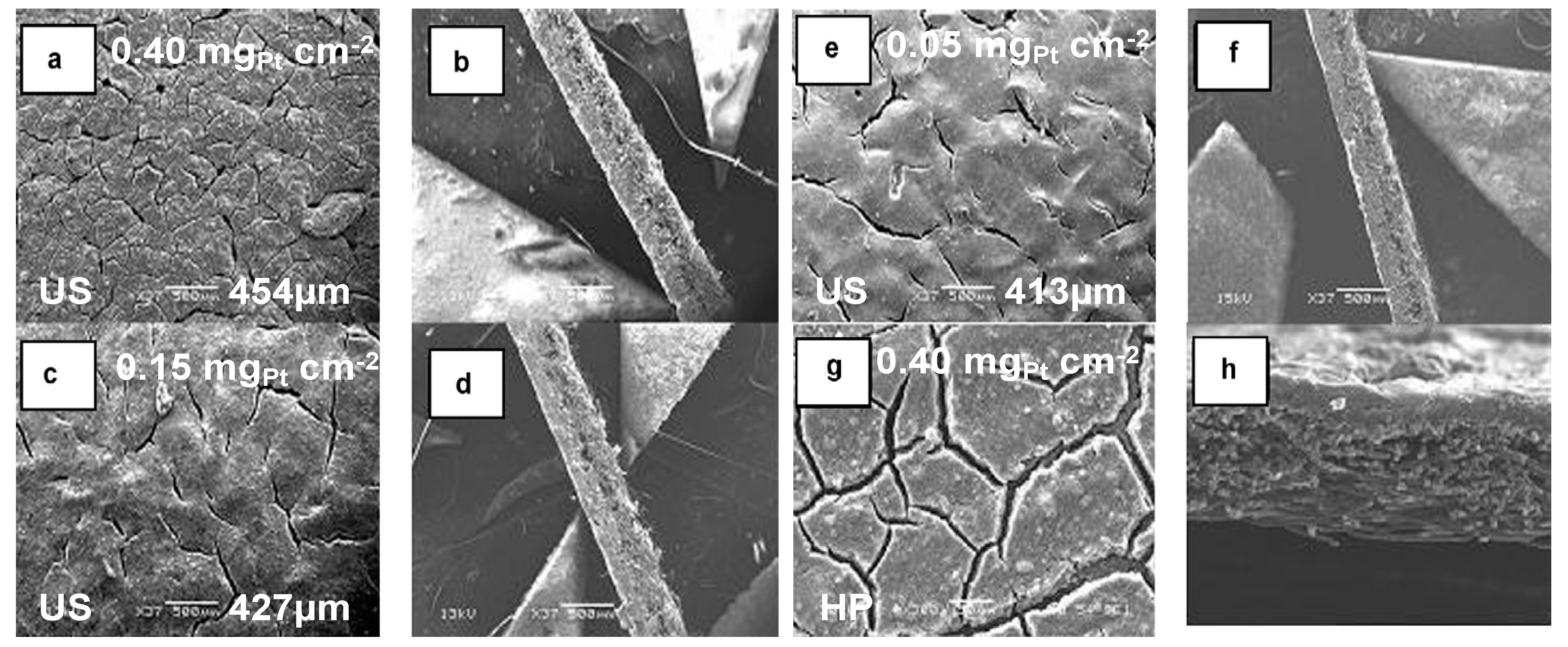
4.1. Ultrasonic Inclusion of Nano-Electrocatalytic Materials on Substrates
4.2. Sonochemical Production of PEMFC and PEMWE Electrodes
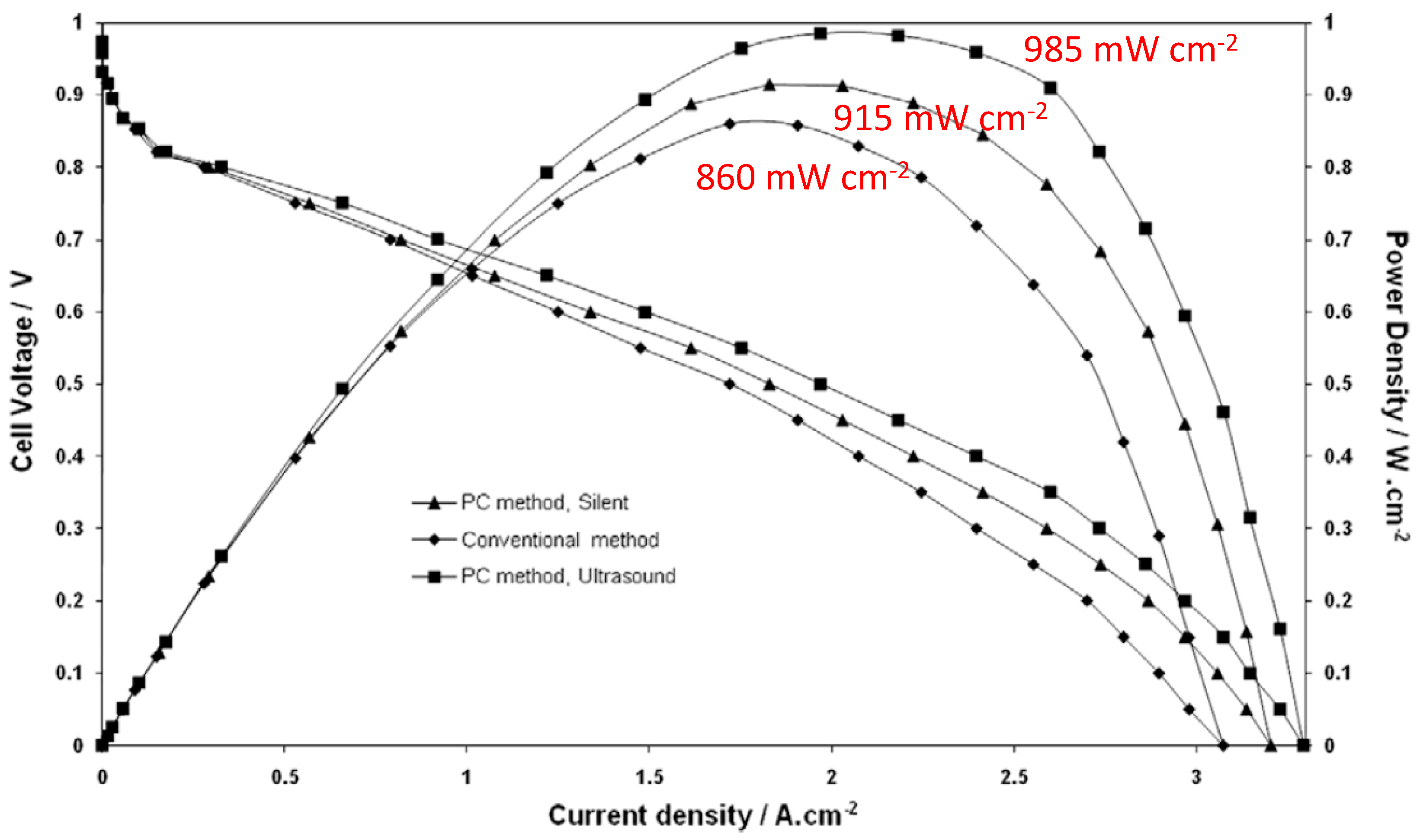
4.3. Sonoelectrochemical Production of PEMFC and PEMWE Electrodes
5. Conclusions
Funding
Acknowledgements
Conflicts of Interest
References
- Pollet, B.G.; Staffell, I.; Shang, J.L. Current status of hybrid, battery and fuel cell electric vehicles: From electrochemistry to market prospects. Electrochim. Acta 2016, 84, 235–249. [Google Scholar] [CrossRef]
- Pollet, B.G. Polymer Electrolyte Membrane and Direct Methanol Fuel Cell Technology—A review. Platinum Metals Rev. 2013, 57, 137–142. [Google Scholar] [CrossRef]
- Litster, S.; McLean, G. Review: PEM fuel cell electrode. J. Power Sour. 2004, 130, 61–76. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Jorge, A.B.; Dedigama, I.; Miller, T.S.; Shearing, P.; Brett, D.J.L.; McMillan, P.F. Carbon Nitride Materials as Efficient Catalyst Supports for Proton Exchange Membrane Water Electrolyzers. Nanomaterials 2018, 8, 432. [Google Scholar] [CrossRef] [PubMed]
- Felix, C.; Bladergroen, B.; Linkov, V.; Pollet, B.G.; Pasupathi, S. Ex-situ electrochemical characterization of IrO2 synthesized by a modified Adams fusion method for the oxygen evolution reaction. Catalysts 2019. under review. [Google Scholar]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts—A review. J. Power Sour. 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Du, S.; Pollet, B.G. Catalyst loading for Pt-nanowire thin film electrodes in PEFCs. Int. J. Hydrog. Energy 2012, 37, 17892–17898. [Google Scholar] [CrossRef]
- Du, S.; Koenigsmann, C.; Sun, S. One-dimensional Nanostructures for PEM Fuel Cell Applications. In Hydrogen and Fuel Cells Primers Series; Pollet, B.G., Ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Bessarabov, D.; Millet, P. PEM Water Electrolysis, 1st ed. In Hydrogen and Fuel Cells Primers Series; Pollet, B.G., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 1. [Google Scholar]
- Bessarabov, D.; Millet, P. PEM Water Electrolysis, 2nd ed. In Hydrogen and Fuel Cells Primers Series; Pollet, B.G., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 2. [Google Scholar]
- Felix, C.; Jao, T.-C.; Pasupathi, S.; Linkov, V.; Pollet, B.G. Fabrication of gas diffusion electrodes via electrophoretic deposition for high temperature polymer electrolyte membrane fuel cells. J. Power Sour. 2014, 258, 238–245. [Google Scholar] [CrossRef]
- Curnick, O.J.; Mendes, P.M.; Pollet, B.G. Enhanced durability of a Pt/C electrocatalyst derived from Nafion®-stabilised colloidal platinum nanoparticles. Electrochem. Comm. 2010, 12, 1017–1020. [Google Scholar] [CrossRef]
- Wee, J.-H.; Lee, K.-Y.; Kim, S.H. Review: Fabrication methods for low-Pt-loading electrocatalysts in proton exchange membrane fuel cell systems. J. Power Sour. 2007, 165, 667–677. [Google Scholar] [CrossRef]
- Pollet, B.G. The use of ultrasound for the fabrication of fuel cell materials. Int. J. Hydrog. Energy 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Pollet, B.G.; Ashokkumar, M. Introduction to Ultrasound, Sonochemistry and Sonoelectrochemistry; Pollet, B.G., Ashokkumar, M., Eds.; SpringerBriefs: Berlin, Germany, 2019; in press. [Google Scholar]
- Pollet, B.G. (Ed.) Power Ultrasound in Electrochemistry: From Versatile Laboratory Tool to Engineering Solution; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Islam, Md.H.; Burheim, O.S.; Pollet, B.G. Review: Sonochemical and sonoelectrochemical production of hydrogen. Ultrason. Sonochem. 2019, 51, 533–555. [Google Scholar] [CrossRef] [PubMed]
- Pollet, B.G. A short introduction to Sonoelectrochemistry. Electrochem. Soc. Interface 2018, 27, 41–42. [Google Scholar] [CrossRef]
- Bock, C.; Halvorsen, H.; MacDougall, B. Catalyst synthesis techniques. In PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications; Zhang, J., Ed.; Springer: London, UK; Guildford, UK, 2008; 1137p. [Google Scholar]
- Dabalá, M.; Pollet, B.G.; Zin, V.; Campadello, E.; Mason, T.J. Sonoelectrochemical (20 kHz) production of Co65Fe35 alloy nanoparticles from Aotani solutions. J. Appl. Electrochem. 2008, 38, 395–402. [Google Scholar] [CrossRef]
- Zin, V.; Pollet, B.G.; Dabalà, M. Sonoelectrochemical (20 kHz) production of platinum nanoparticles from aqueous solutions. Electrochim. Acta 2009, 54, 7201–7206. [Google Scholar] [CrossRef]
- Karousos, D.S.; Desdenakis, K.I.; Sakkas, P.M.; Sourkouni, G.; Pollet, B.G. Argirusis. Sonoelectrochemical one-pot synthesis of Pt–Carbon black nanocomposite PEMFC electrocatalyst. Ultrason. Sonochem. 2017, 35, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Gedanken, A. Doping nanoparticles into polymers and ceramics using ultrasound radiation. Ultrason. Sonochem. 2007, 14, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Pollet, B.G. A novel method for preparing PEMFC electrodes by the ultrasonic and sonoelectrochemical techniques. Electrochem. Comm. 2009, 11, 1445–1448. [Google Scholar] [CrossRef]
- Mehta, V.; Cooper, J.S. Review and analysis of PEM fuel cell design and manufacturing. J. Power Sour. 2003, 114, 32–53. [Google Scholar] [CrossRef]
- Millington, B.; Whipple, V.; Pollet, B.G. A novel method for preparing proton exchange membrane fuel cell electrodes by the ultrasonic-spray technique. J. Power Sour. 2011, 196, 8500–8508. [Google Scholar] [CrossRef]
- Pollet, B.G.; Valzer, E.F.; Curnick, O.J. Platinum sonoelectrodeposition on glassy carbon and gas diffusion layer electrodes. Int. J. Hydrog. Energy 2011, 36, 6248–6258. [Google Scholar] [CrossRef]







| Noble Metals | Ultrasonic Frequency/kHz | Ultrasonic Power | Surfactant | Solvent | Particle Size/nm |
|---|---|---|---|---|---|
| Ag | 200 | 200 W | SDS, PEG40-MS, Tween20 | Propan-2-ol | 13 ± 3 |
| Pd | 200 | 6.0 W cm−2 | SDS, Tween20, PEG40-MS, PVP | - | 6–110 |
| Pd | 50 | - | PVP, EG | - | 3–6 |
| Ag, Au, Pt, Rh | 200 | 6.0 W cm−2 | Tween20, PEG-MS, SDS, PVP | - | 5 |
| Pt | 200 | 6.0 W cm−2 | SDS | - | 2.6 ± 0.9 |
| Pt | 200 | 200 W | SDS, DBS, PEG-MS, DTAC, DTAB | - | 1 (PEG-MS) 3 (SDS) 3 (DBS) |
| Pt | 20 | 10.0 W cm−2 | - | Ethanol, Propan-1-ol, Pentan-1-ol | 2.6 |
| Pt, Pd | 20 | 100 W | PVP | Propan-2-ol | 2 (N2)–Pd 3.6 (Ar)–Pd 3.2 (N2)–Pt 2.9 (Ar)–Pt 1.5(Xe)–Pt |
| Ag | 20 | 100 W cm−2 | - | Ethanol | 20 (Ar–H2) |
| Pt | 20 | 22 & 27 W cm−2 | PVP, EG | Citrate | < 1 |
| Pt, Au | 42 | - | PVP | - | 6 (Pt) 10 (Au) |
| Au | 20 | 23–47 W cm−3 | - | Methanol, Propan-2-ol, n-pentanol | 7 ± 2 (methanol) 6 ± 4 (propan-2-ol) 5 ± 3 (n-pentanol) |
| Au | 20 | 9 W cm−2 | - | Methanol, 1-Propanol, Butanol | 9–25 (depending on alcohol concentrations) |
| Au | 20,213,358,647&1062 | 0.1 ± 0.01 W cm−3 | - | 1-Propanol | 15–30 |
| Ru | 20,213,355,647&1056 | - | SDS | 1-Propanol | 10–20 |
| Pt–Ru | 20 | 17 W cm−2 | - | Methanol, THF | 2.0 ± 0.1 |
| Pt–Ru | 213 | 110–125 W cm−3 | SDS, PVP | - | 5–10 |
| Pd–Sn | 20 | 42 W cm−2 | - | Citric acid, ethanol | 3–5 |
| Au–Ru | 355 | - | PEG | - | 15 |
| Au (core)–Pd (shell) | 200 | 200 W | SDS | - | 8 [6 nm Au core & 1 nm Pd shell] |
| Au (core)–Pd (shell) | 40 | 100 W | PVP, EG | - | 8 [4 nm Au core & 4 nm Pd shell] |
| Au (core)–Pt (shell) | 200 | 4.2 W cm−2 | SDS, PFG | - | 6.5 ± 1.5 to 10.1 ± 3.6 |
| Au (core)–Ag (shell) | 20 | 23–47 W cm−2 | PEG, EG | - | 20 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollet, B.G. The Use of Power Ultrasound for the Production of PEMFC and PEMWE Catalysts and Low-Pt Loading and High-Performing Electrodes. Catalysts 2019, 9, 246. https://doi.org/10.3390/catal9030246
Pollet BG. The Use of Power Ultrasound for the Production of PEMFC and PEMWE Catalysts and Low-Pt Loading and High-Performing Electrodes. Catalysts. 2019; 9(3):246. https://doi.org/10.3390/catal9030246
Chicago/Turabian StylePollet, Bruno G. 2019. "The Use of Power Ultrasound for the Production of PEMFC and PEMWE Catalysts and Low-Pt Loading and High-Performing Electrodes" Catalysts 9, no. 3: 246. https://doi.org/10.3390/catal9030246





