Accelerated H2 Evolution during Microbial Electrosynthesis with Sporomusa ovata
Abstract
:1. Introduction
2. Results and Discussion
2.1. H2 Evolution in an Abiotic MES Reactor
2.2. H2 Evolution in the Presence of a S. ovata Cell Suspension
2.3. H2 Evolution Shifting in the Presence of a Cell-Free Filtrate from S. ovata Culture
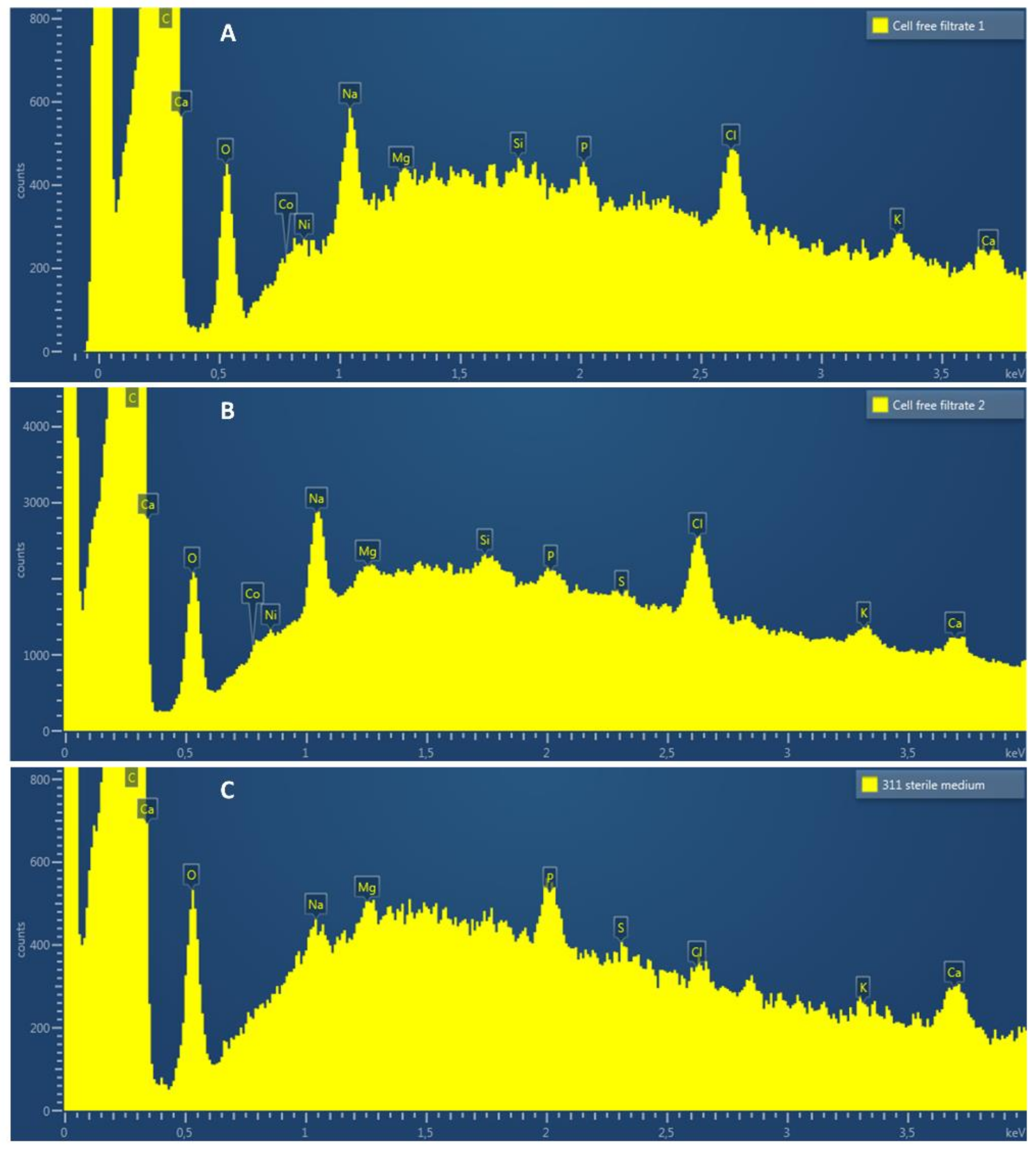
2.4. Metals at the Surface of the Cathode after Exposure to Cell-Free Spent Medium
3. Materials and Methods
3.1. Bacterium and Growth Conditions
3.2. Preparation of Cell Suspension
3.3. Cell-Free Spent Medium of S. ovata
3.4. MES Reactor and H2 Evolution
3.5. High-Performance Liquid Chromatography (HPLC)
3.6. SDS-PAGE and Mass Spectrometry
3.7. Hydrogenase Activity Assay
3.8. Energy-Dispersive X-ray Spectroscopy (EDS)
3.9. Equations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tremblay, P.-L.; Zhang, T. Electrifying microbes for the production of chemicals. Front. Microbiol. 2015, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Woodard, T.L.; Franks, A.E.; Summers, Z.M.; Lovley, D.R. Microbial electrosynthesis: Feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. mBio 2010, 1, e00103–e00110. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Electromicrobiology. Annu. Rev. Microbiol. 2012, 66, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef] [PubMed]
- Ganigué, R.; Puig, S.; Batlle-Vilanova, P.; Balaguer, M.D.; Colprim, J. Microbial electrosynthesis of butyrate from carbon dioxide. Chem. Commun. 2015, 51, 3235–3238. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, S.; Vanbroekhoven, K.; Buisman, C.J.N.; Pant, D.; Strik, D.P.B.T.B. Application of gas diffusion biocathode in microbial electrosynthesis from carbon dioxide. Environ. Sci. Pollut. Res. Int. 2016, 23, 22292–22308. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, S.; Yuliasni, R.; Vanbroekhoven, K.; Buisman, C.J.N.; Strik, D.P.B.T.B.; Pant, D. Long-term operation of microbial electrosynthesis cell reducing CO2 to multi-carbon chemicals with a mixed culture avoiding methanogenesis. Bioelectrochemistry 2017, 113, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tremblay, P.-L. Hybrid photosynthesis-powering biocatalysts with solar energy captured by inorganic devices. Biotechnol. Biofuels 2017, 10, 249. [Google Scholar] [PubMed]
- Jourdin, L.; Raes, S.M.T.; Buisman, C.J.N.; Strik, D.P.B.T.B. Critical biofilm growth throughout unmodified carbon felts allows continuous bioelectrochemical chain elongation from CO2 up to caproate at high current density. Front. Energy Res. 2018, 6, 7. [Google Scholar] [CrossRef]
- Harnisch, F.; Rosa, L.F.M.; Kracke, F.; Virdis, B.; Krömer, J.O. Electrifying white biotechnology: Engineering and economic potential of electricity-driven bio-production. ChemSusChem 2015, 8, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Kracke, F.; Vassilev, I.; Krömer, J.O. Microbial electron transport and energy conservation—The foundation for optimizing bioelectrochemical systems. Front. Microbiol. 2015, 6, 575. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.B.; Bond, D.R.; Lovley, D.R. Graphite electrodes as electron donors for anaerobic respiration. Environ. Microbiol. 2004, 6, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.-L.; Angenent, L.T.; Zhang, T. Extracellular electron uptake: Among autotrophs and mediated by surfaces. Trends Biotechnol. 2017, 35, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Nevin, K.P. Electrobiocommodities: Powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity. Curr. Opin. Biotechnol. 2013, 24, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.D.; Eddie, B.J.; Kotloski, N.J.; Lebedev, N.; Malanoski, A.P.; Lin, B.; Strycharz-Glaven, S.M.; Tender, L.M. Toward understanding long-distance extracellular electron transport in an electroautotrophic microbial community. Energy Environ. Sci. 2016, 9, 3544–3558. [Google Scholar] [CrossRef]
- Aulenta, F.; Reale, P.; Catervi, A.; Panero, S.; Majone, M. Kinetics of trichloroethene dechlorination and methane formation by a mixed anaerobic culture in a bio-electrochemical system. Electrochim. Acta 2008, 53, 5300–5305. [Google Scholar] [CrossRef]
- Summers, Z.M.; Gralnick, J.A.; Bond, D.R. Cultivation of an obligate Fe(II)-oxidizing lithoautotrophic bacterium using electrodes. mBio 2013, 4, e00420. [Google Scholar] [CrossRef]
- Deutzmann, J.S.; Sahin, M.; Spormann, A.M. Extracellular enzymes facilitate electron uptake in biocorrosion and bioelectrosynthesis. mBio 2015, 6, e00496. [Google Scholar] [CrossRef]
- Jourdin, L.; Lu, Y.; Flexer, V.; Keller, J.; Freguia, S. Biologically induced hydrogen production drives high rate/high efficiency microbial electrosynthesis of acetate from carbon dioxide. ChemElectroChem 2016, 3, 581–591. [Google Scholar] [CrossRef]
- LaBelle, E.V.; Marshall, C.W.; Gilbert, J.A.; May, H.D. Influence of acidic pH on hydrogen and acetate production by an electrosynthetic microbiome. PLoS ONE 2014, 9, e109935. [Google Scholar] [CrossRef] [PubMed]
- May, H.D.; Evans, P.J.; LaBelle, E.V. The bioelectrosynthesis of acetate. Curr. Opin. Biotechnol. 2016, 42, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Sahu, J.N.; Redzwan, G.; Hashim, M.A. An overview of cathode material and catalysts suitable for generating hydrogen in microbial electrolysis cell. Int. J. Hydrogen Energy 2013, 38, 1745–1757. [Google Scholar] [CrossRef]
- Call, D.; Logan, B.E. Hydrogen production in a single chamber microbial electrolysis cell lacking a membrane. Environ. Sci. Technol. 2008, 42, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Logan, B.E. High hydrogen production rate of microbial electrolysis cell (MEC) with reduced electrode spacing. Bioresour. Technol. 2011, 102, 3571–3574. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Fan, Y.; Liu, H. Hydrogen production using single-chamber membrane-free microbial electrolysis cells. Water Res. 2008, 42, 4172–4178. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Salerno, M.B.; Rittmann, B.E. Thermodynamic evaluation on H2 production in glucose fermentation. Environ. Sci. Technol. 2008, 42, 2401–2407. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Rittmann, B.E. Characterization of energy losses in an upflow single-chamber microbial electrolysis cell. Int. J. Hydrogen Energy 2010, 35, 920–927. [Google Scholar] [CrossRef]
- Selembo, P.A.; Merrill, M.D.; Logan, B.E. The use of stainless steel and nickel alloys as low-cost cathodes in microbial electrolysis cells. J. Power Sources 2009, 190, 271–278. [Google Scholar] [CrossRef]
- Vincent, K.A.; Parkin, A.; Armstrong, F.A. Investigating and exploiting the electrocatalytic properties of hydrogenases. Chem. Rev. 2007, 107, 4366–4413. [Google Scholar] [CrossRef] [PubMed]
- Jeremiasse, A.W.; Hamelers, H.V.M.; Kleijn, J.M.; Buisman, C.J.N. Use of biocompatible buffers to reduce the concentration overpotential for hydrogen evolution. Environ. Sci. Technol. 2009, 43, 6882–6887. [Google Scholar] [CrossRef]
- Liang, D.; Liu, Y.; Peng, S.; Lan, F.; Lu, S.; Xiang, Y. Effects of bicarbonate and cathode potential on hydrogen production in a biocathode electrolysis cell. Front. Environ. Sci. Eng. 2014, 8, 624–630. [Google Scholar] [CrossRef]
- De Silva Muñoz, L.; Bergel, A.; Féron, D.; Basséguy, R. Hydrogen production by electrolysis of a phosphate solution on a stainless steel cathode. Int. J. Hydrogen Energy 2010, 35, 8561–8568. [Google Scholar] [CrossRef]
- Merrill, M.D.; Logan, B.E. Electrolyte effects on hydrogen evolution and solution resistance in microbial electrolysis cells. J. Power Sources 2009, 191, 203–208. [Google Scholar] [CrossRef]
- Daniele, S.; Lavagnini, I.; Baldo, M.A.; Magno, F. Steady state voltammetry at microelectrodes for the hydrogen evolution from strong and weak acids under pseudo-first and second order kinetic conditions. J. Electroanal. Chem. 1996, 404, 105–111. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Aulenta, F.; Villano, M.; Angenent, L.T. Cathodes as electron donors for microbial metabolism: Which extracellular electron transfer mechanisms are involved? Bioresour. Technol. 2011, 102, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.D.; Siegert, M.; Logan, B.E. Hydrogen evolution catalyzed by viable and non-viable cells on biocathodes. Int. J. Hydrogen Energy 2014, 39, 16841–16851. [Google Scholar] [CrossRef]
- Tremblay, P.-L.; Höglund, D.; Koza, A.; Bonde, I.; Zhang, T. Adaptation of the autotrophic acetogen Sporomusa ovata to methanol accelerates the conversion of CO2 to organic products. Sci. Rep. 2015, 5, 16168. [Google Scholar] [CrossRef] [PubMed]
- Aryal, N.; Tremblay, P.-L.; Xu, M.; Daugaard, A.E.; Zhang, T. Highly conductive poly(3,4-ethylenedioxythiophene) polystyrene sulfonate polymer coated cathode for the microbial electrosynthesis of acetate from carbon dioxide. Front. Energy Res. 2018, 6, 72. [Google Scholar] [CrossRef]
- Aryal, N.; Tremblay, P.-L.; Lizak, D.M.; Zhang, T. Performance of different Sporomusa species for the microbial electrosynthesis of acetate from carbon dioxide. Bioresour. Technol. 2017, 233, 184–190. [Google Scholar] [CrossRef]
- Zhang, T.; Nie, H.; Bain, T.S.; Lu, H.; Cui, M.; Snoeyenbos-West, O.L.; Franks, A.E.; Nevin, K.P.; Russell, T.P.; Lovley, D.R. Improved cathode materials for microbial electrosynthesis. Energy Environ. Sci. 2013, 6, 217–224. [Google Scholar] [CrossRef]
- Nie, H.; Zhang, T.; Cui, M.; Lu, H.; Lovley, D.R.; Russell, T.P. Improved cathode for high efficient microbial-catalyzed reduction in microbial electrosynthesis cells. Phys. Chem. Chem. Phys. 2013, 15, 14290–14294. [Google Scholar] [CrossRef] [PubMed]
- Ammam, F.; Tremblay, P.-L.; Lizak, D.M.; Zhang, T. Effect of tungstate on acetate and ethanol production by the electrosynthetic bacterium Sporomusa ovata. Biotechnol. Biofuels 2016, 9, 163. [Google Scholar] [PubMed]
- Aryal, N.; Halder, A.; Tremblay, P.-L.; Chi, Q.; Zhang, T. Enhanced microbial electrosynthesis with three-dimensional graphene functionalized cathodes fabricated via solvothermal synthesis. Electrochim. Acta 2016, 217, 117–122. [Google Scholar] [CrossRef]
- Aryal, N.; Halder, A.; Zhang, M.; Whelan, P.R.; Tremblay, P.-L.; Chi, Q.; Zhang, T. Freestanding and flexible graphene papers as bioelectrochemical cathode for selective and efficient CO2 conversion. Sci. Rep. 2017, 7, 9107. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tremblay, P.-L.; Mohanty, S.; Xu, K.; Zhang, T. Electrosynthesis of acetate from CO2 by a highly structured biofilm assembled with reduced graphene oxide–tetraethylene pentamine. J. Mater. Chem. A 2016, 4, 8395–8401. [Google Scholar] [CrossRef]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood–Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta 2008, 1784, 1873–1898. [Google Scholar] [CrossRef]
- Ragsdale, S.W. Nickel-based enzyme systems. J. Biol. Chem. 2009, 284, 18571–18575. [Google Scholar] [CrossRef]
- Ragsdale, S.W. Enzymology of the Wood–Ljungdahl pathway of acetogenesis. Ann. N. Y. Acad. Sci. 2008, 1125, 129–136. [Google Scholar] [CrossRef]
- Yu, N.Y.; Wagner, J.R.; Laird, M.R.; Melli, G.; Rey, S.; Lo, R.; Dao, P.; Sahinalp, S.C.; Ester, M.; Foster, L.J.; et al. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010, 26, 1608–1615. [Google Scholar] [CrossRef]
- Möller, B.; Oßmer, R.; Howard, B.H.; Gottschalk, G.; Hippe, H. Sporomusa, a new genus of gram-negative anaerobic bacteria including Sporomusa sphaeroides spec. nov. and Sporomusa ovata spec. nov. Arch. Microbiol. 1984, 139, 388–396. [Google Scholar] [CrossRef]
- Nakashimada, Y.; Rachman, M.A.; Kakizono, T.; Nishio, N. Hydrogen production of Enterobacter aerogenes altered by extracellular and intracellular redox states. Int. J. Hydrogen Energy 2002, 27, 1399–1405. [Google Scholar] [CrossRef]
- Fernández, V.M.; Gutiérrez, C.; Ballesteros, A. Determination of hydrogenase activity using an anaerobic spectrophotometric device. Anal. Biochem. 1982, 120, 85–90. [Google Scholar] [CrossRef]



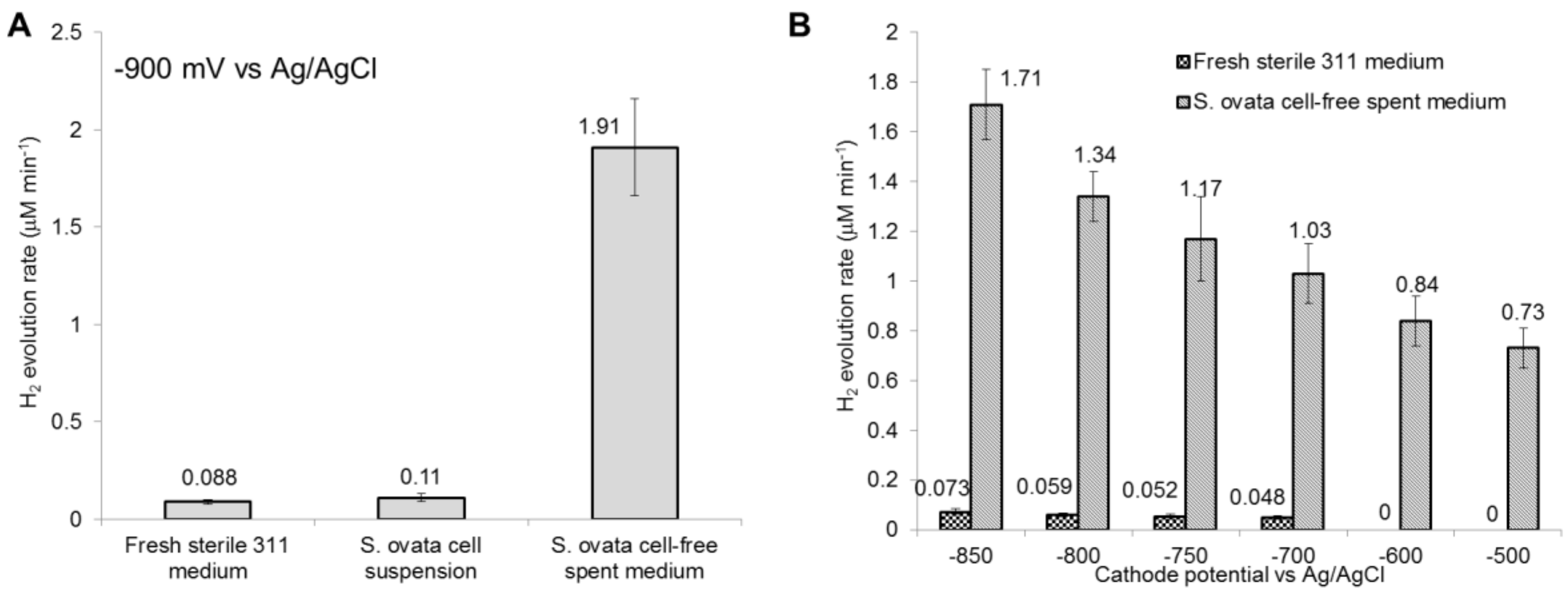
| Potential vs. Ag/AgCl (mV) | H2 Evolution Rates (µM min−1) | Current Density (mA m−2) | Electrons Recovery in H2 (%) |
|---|---|---|---|
| −900 | 0.088 ± 0.012 | −19.8 ± 2.1 | 87 ± 1.8 |
| −850 | 0.073 ± 0.010 | −16.3 ± 1.7 | 85 ± 3.7 |
| −800 | 0.059 ± 0.008 | −14.9 ± 1.8 | 80 ± 2.1 |
| −750 | 0.052 ± 0.011 | −13.7 ± 1.2 | 79 ± 9.0 |
| −700 | 0.048 ± 0.007 | −12.8 ± 1.0 | 78 ± 7.0 |
| −600 | n.d. b | n/a c | n/a |
| Potential vs. Ag/AgCl (mV) | H2 Evolution Rates (µM min−1) | Current Density (mA m−2) | Electrons Recovery in H2 (%) |
|---|---|---|---|
| −900 | 0.11 ± 0.02 | −317 ± 32 | 8.5 ± 1.5 |
| −850 | n.d. b | n/a c | n/a |
| −800 | n.d. | n/a | n/a |
| −750 | n.d. | n/a | n/a |
| −700 | n.d. | n/a | n/a |
| −600 | n.d. | n/a | n/a |
| Potential vs. Ag/AgCl (mV) | H2 eVolution Rates (µM min−1) | Current Density (mA m−2) | Electrons Recovery in H2 (%) |
|---|---|---|---|
| −900 | 1.91 ± 0.25 | −398 ± 90 | 87 ± 8.4 |
| −850 | 1.71 ± 0.14 | −320 ± 86 | 89 ± 8.0 |
| −800 | 1.34 ± 0.10 | −289 ± 38 | 85 ± 7.9 |
| −750 | 1.17 ± 0.17 | −276 ± 51 | 80 ± 3.2 |
| −700 | 1.03 ± 0.12 | −254 ± 60 | 75 ± 3.0 |
| −600 | 0.84 ± 0.10 | −225 ± 47 | 74 ± 5.9 |
| −500 | 0.73 ± 0.08 | −179 ± 42 | 77 ± 9.0 |
| −400 | n.d. b | n/a c | n/a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tremblay, P.-L.; Faraghiparapari, N.; Zhang, T. Accelerated H2 Evolution during Microbial Electrosynthesis with Sporomusa ovata. Catalysts 2019, 9, 166. https://doi.org/10.3390/catal9020166
Tremblay P-L, Faraghiparapari N, Zhang T. Accelerated H2 Evolution during Microbial Electrosynthesis with Sporomusa ovata. Catalysts. 2019; 9(2):166. https://doi.org/10.3390/catal9020166
Chicago/Turabian StyleTremblay, Pier-Luc, Neda Faraghiparapari, and Tian Zhang. 2019. "Accelerated H2 Evolution during Microbial Electrosynthesis with Sporomusa ovata" Catalysts 9, no. 2: 166. https://doi.org/10.3390/catal9020166





