Human Deoxycytidine Kinase Is a Valuable Biocatalyst for the Synthesis of Nucleotide Analogues
Abstract
:1. Introduction
2. Results
2.1. Production of Soluble and Immobilized HsdCK
2.2. Enzymatic Synthesis of NMP Analogues
2.3. Optimization of the Reaction Conditions
2.4. Reusability of Immobilized HsdCK
2.5. Impact of Increasing Substrate Concentrations on HsdCK-Catalyzed Reactions
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Expression of HsdCK
4.3. Immobilization of HsdCK
4.4. Standard Enzymatic Assay
4.5. Screening of the Substrate Spectrum of Soluble and Immobilized HsdCK
4.6. Determination of Specific Activities for NMP Analogues
4.7. Optimization of Reaction Conditions
4.8. Increasing the Substrate Concentration for Fludarabine-5´-Monophosphate Synthesis
4.9. Reusability of the Immobilized Enzyme
4.10. Photometric Assay
4.11. High Performance Liquid Chromatography (HPLC)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jordheim, L.P.; Durantel, D.; Zoulim, F.; Dumontet, C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013, 12, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Deville-Bonne, D.; El Amri, C.; Meyer, P.; Chen, Y.; Agrofoglio, L.A.; Janin, J. Human and viral nucleoside/nucleotide kinases involved in antiviral drug activation: Structural and catalytic properties. Antivir. Res. 2010, 86, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.R.; Iyer, V.V.; McIntee, E.J. Pronucleotides: Toward the in vivo delivery of antiviral and anticancer nucleotides. Med. Res. Rev. 2000, 20, 417–451. [Google Scholar] [CrossRef]
- Ray, A.S.; Hostetler, K.Y. Application of kinase bypass strategies to nucleoside antivirals. Antivir. Res. 2011, 92, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Pradere, U.; Garnier-Amblard, E.C.; Coats, S.J.; Amblard, F.; Schinazi, R.F. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 2014, 114, 9154–9218. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.J.; Bischofberger, N. Minireview: Nucleotide prodrugs. Antivir. Res. 1995, 27, 1–17. [Google Scholar] [CrossRef]
- Burgess, K.; Cook, D. Syntheses of nucleoside triphosphates. Chem. Rev. 2000, 100, 2047–2059. [Google Scholar] [CrossRef]
- Lee, T.T.; Momparler, R.L. Enzymatic Synthesis of 5-Azacytidine 5’-Triphosphate from 5-Azacytidine. Anal. Biochem. 1976, 71, 60–67. [Google Scholar] [CrossRef]
- Faber, K. Biotransformations in Organic Chemistry, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Serra, I.; Ubiali, D.; Piškur, J.; Munch-Petersen, B.; Bavaro, T.; Terreni, M. Immobilization of deoxyadenosine Kinase from Dictyostelium discoideum (DddAK) and its application in the 5’-phosphorylation of arabinosyladenine and arabinosyl-2-fluoroadenine. ChemistrySelect 2017, 2, 5403–5408. [Google Scholar] [CrossRef]
- Serra, I.; Conti, S.; Piškur, J.; Clausen, A.R.; Munch-Petersen, B.; Terreni, M.; Ubiali, D. Immobilized Drosophila melanogaster deoxyribonucleoside kinase (DmdNK) as a high performing biocatalyst for the synthesis of purine arabinonucleotides. Adv. Synth. Catal. 2014, 356, 563–570. [Google Scholar] [CrossRef]
- Krenitsky, T.A.; Tuttle, J.V.; Koszalka, G.W.; Chen, I.S.; Beacham, L.M.; Rideout, J.L.; Elion, G.B. Deoxycytidine kinase from calf thymus. Substrate and inhibitor specificity. J. Biol. Chem. 1976, 251, 4055–4061. [Google Scholar] [PubMed]
- Datta, N.S.; Shewach, D.S.; Hurley, M.C.; Mitchell, B.S.; Fox, I.H. Human T-lymphoblast deoxycytidine kinase: Purification and properties. Biochemistry 1989, 28, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Bohman, C.; Eriksson, S. Deoxycytidine kinase from human leukemic spleen: Preparation and characterization of the homogeneous enzyme. Biochemistry 1988, 27, 4258–4265. [Google Scholar] [CrossRef] [PubMed]
- Momparler, R.L.; Fischer, G.A. Mammalian deoxynucleoside kinase. I. Deoxycytidine kinase: Purification, properties, and kinetic studies with cytosine arabinoside. J. Biol. Chem. 1968, 243, 4298–4304. [Google Scholar] [PubMed]
- Sabini, E.; Hazra, S.; Konrad, M.; Burley, S.K.; Lavie, A. Structural basis for activation of the therapeutic L-nucleoside analogs 3TC and troxacitabine by human deoxycytidine kinase. Nucleic Acids Res. 2006, 35, 186–192. [Google Scholar] [CrossRef]
- Arnér, E.S.J. On the phosphorylation of 2-chlorodeoxy-adenosine (CdA) and its correlation with clinical response in leukemia treatment. Leuk. Lymphoma 1996, 21, 225–231. [Google Scholar] [CrossRef]
- Trelles, J.A.; Rivero, C.W.; Britos, C.N.; Lapponi, M.J. Enzymatic synthesis of nucleic Acid derivatives by immobilized cells. In Enzymatic and Chemical Synthesis of Nucleic Acid Derivatives; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 79–106. [Google Scholar]
- Fernández-Lucas, J.; Arroyo, M. Enzymatic synthesis of nucleic acid derivatives by immobilized enzymes. In Enzymatic and Chemical Synthesis of Nucleic Acid Derivatives; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 107–128. [Google Scholar]
- Eriksson, S.; Wang, L. Substrate specificities, expression and primary sequences of deoxynucleoside kinases; implications for chemotkerapy. Nucleosides Nucleotides 1997, 16, 653–659. [Google Scholar] [CrossRef]
- Eriksson, S.; Munch-Petersen, B.; Johansson, K.; Ecklund, H. Structure and function of cellular deoxyribonucleoside kinases. Cell. Mol. Life Sci. C MLS 2002, 59, 1327–1346. [Google Scholar] [CrossRef]
- Sitanggang, A.B.; Drews, A.; Kraume, M. Influences of operating conditions on continuous lactulose synthesis in an enzymatic membrane reactor system: A basis prior to long-term operation. J. Biotechnol. 2015, 203, 89–96. [Google Scholar] [CrossRef]
- AL-Muftah, A.E.; Abu-Reesh, I.M. Effects of external mass transfer and product inhibition on a simulated immobilized enzyme-catalyzed reactor for lactose hydrolysis. Eng. Life Sci. 2004, 4, 326–340. [Google Scholar] [CrossRef]
- Xiu, G.-H.; Jiang, L.; Li, P. Mass-transfer limitations for immobilized enzyme-catalyzed kinetic resolution of racemate in a fixed-bed reactor. Biotechnol. Bioeng. 2001, 74, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.K.; Gardner, C.R.; Colton, C.K. Basic concepts in the effects of mass transfer on immobilized enzyme kinetics. In Immobilized Enzymes in Food and Microbial Processes; Springer: Boston, MA, USA, 1974; pp. 205–224. [Google Scholar]
- Greco, G.; Alfani, F.; Cantarella, M.; Gianfreda, L.; Palescandolo, R.; Scardi, V. The effect of substrate mass transfer limitations in an immobilized enzyme membrane reactor. Chem. Eng. Commun. 1980, 7, 145–157. [Google Scholar] [CrossRef]
- Barros, R.J.; Wehtje, E.; Adlercreutz, P. Mass transfer studies on immobilized α-chymotrypsin biocatalysts prepared by deposition for use in organic medium. Biotechnol. Bioeng. 1998, 59, 364–373. [Google Scholar] [CrossRef]
- Hughes, T.L.; Hahn, T.M.; Reynolds, K.K.; Shewach, D.S. Kinetic analysis of human deoxycytidine kinase with the true phosphate donor uridine triphosphate. Biochemistry 1997, 36, 7540–7547. [Google Scholar] [CrossRef]
- Dickensheets, P.A.; Chen, L.F.; Tsao, G.T. Characteristics of yeast invertase immobilized on porous cellulose beads. Biotechnol. Bioeng. 1977, 19, 365–375. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- Rocchietti, S.; Ubiali, D.; Terreni, M.; Albertini, A.M.; Fernandez-Lafuente, R.; Guisan, J.M.; Pregnolato, M. Immobilization and stabilization of recombinant multimeric uridine and purine nucleoside phosphorylases from Bacillus subtilis. Biomacromolecules 2004, 5, 2195–2200. [Google Scholar] [CrossRef]
- Zhou, X.; Mikhailopulo, I.A.; Cruz Bournazou, M.N.; Neubauer, P. Immobilization of thermostable nucleoside phosphorylases on MagReSyn epoxide microspheres and their application for the synthesis of 2,6-dihalogenated purine nucleosides. J. Mol. Catal. B Enzym. 2015, 115, 119–127. [Google Scholar] [CrossRef]
- Serra, I.; Serra, C.D.; Rocchietti, S.; Ubiali, D.; Terreni, M. Stabilization of thymidine phosphorylase from Escherichia coli by immobilization and post immobilization techniques. Enzym. Microb. Technol. 2011, 49, 52–58. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; ISBN 0879695773. [Google Scholar]



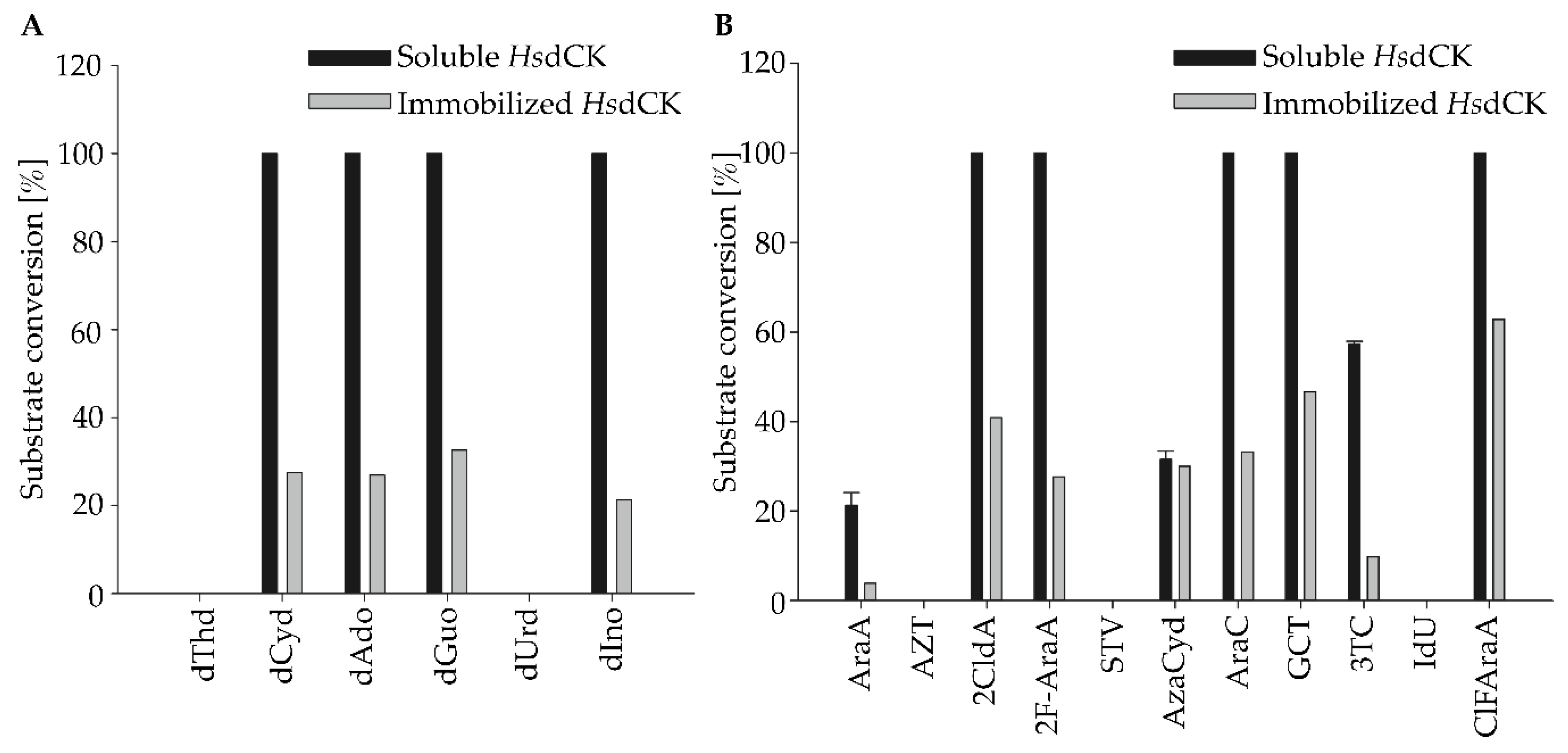
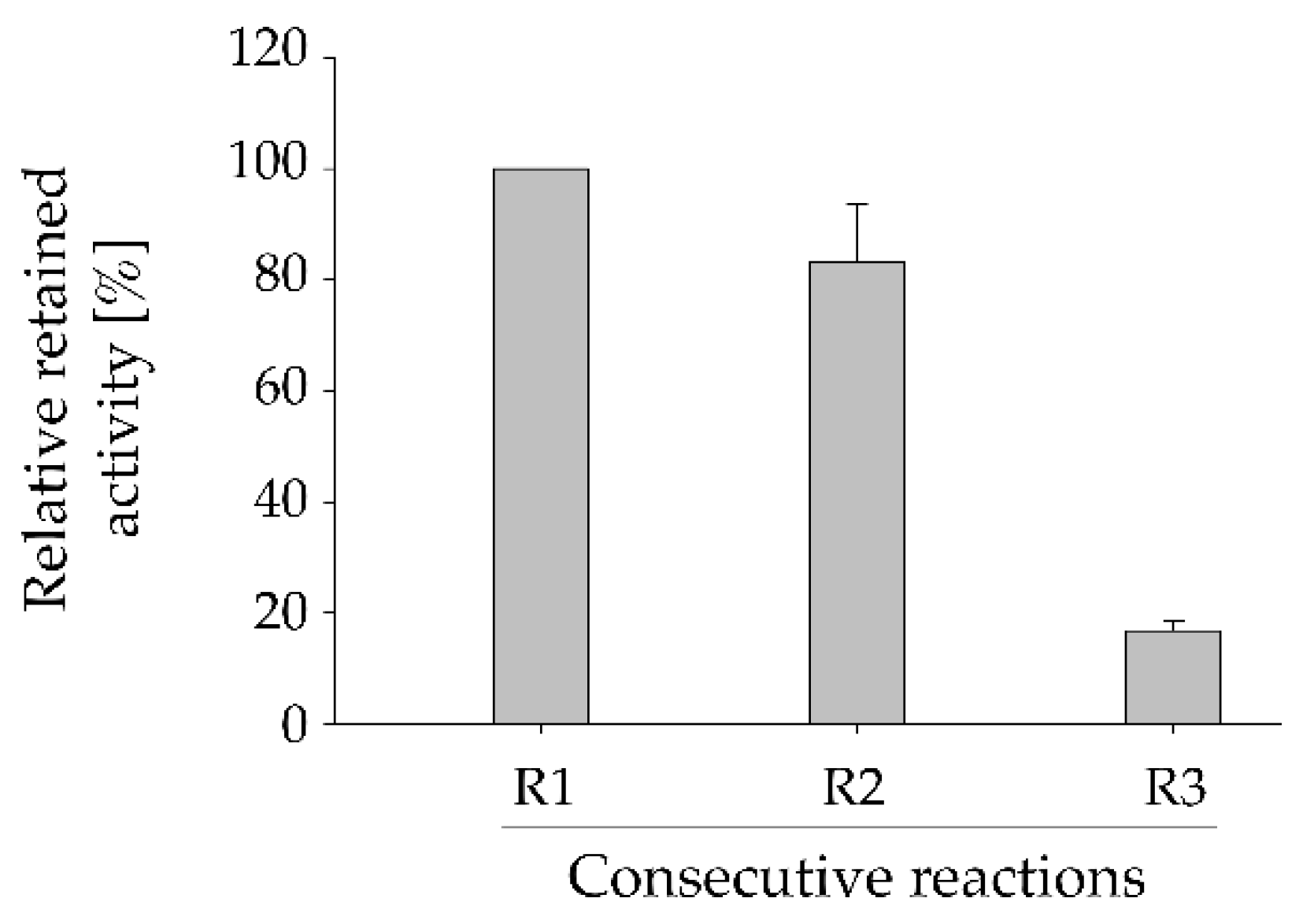
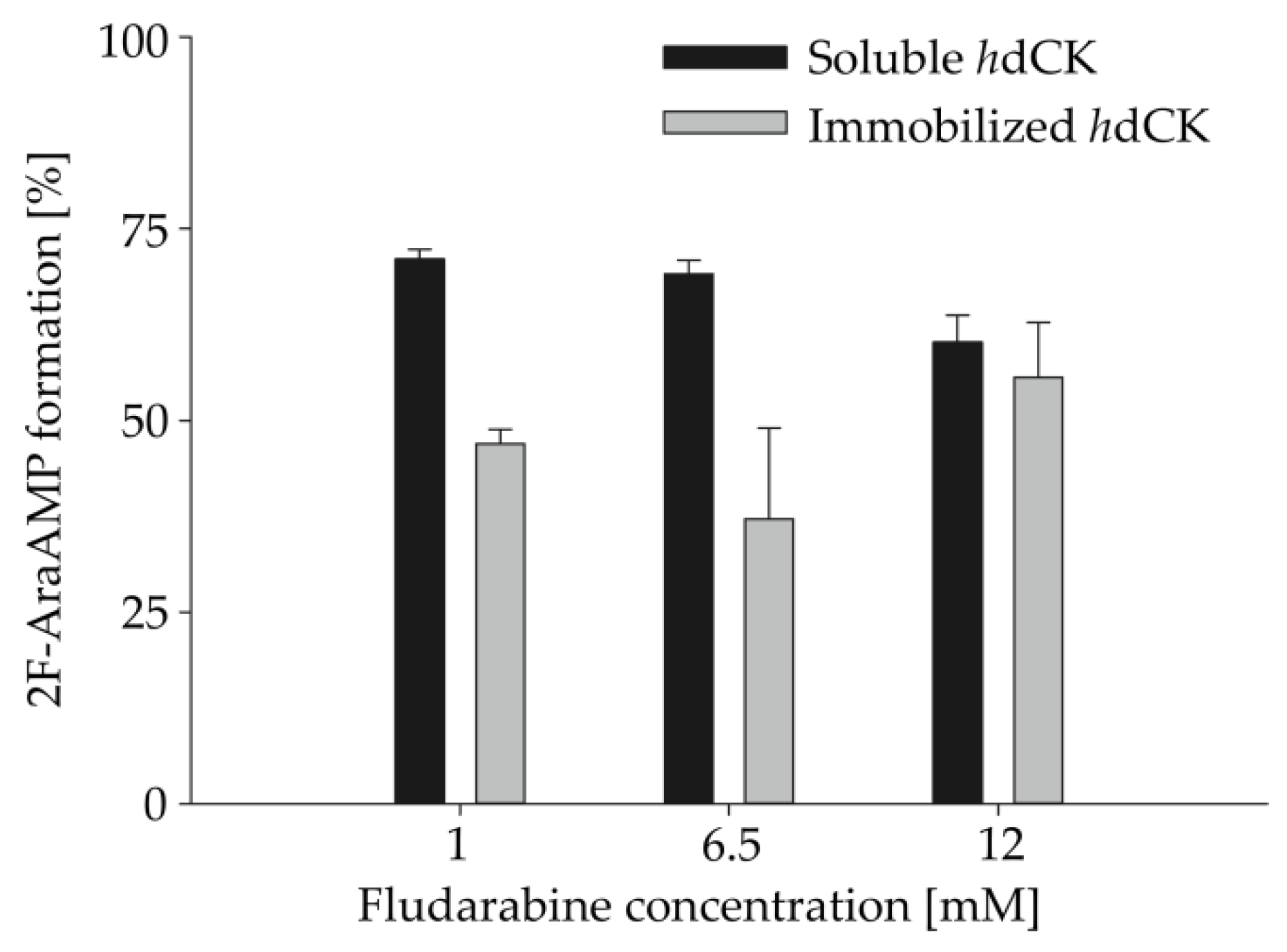
| Substrate | Enzyme | Specific Activity [U mg−1] | Retained Activity [%] | Conversion [%] |
|---|---|---|---|---|
| Cladribine | immobilized | 0.22 (±0.06) | 67 | 52 (±1) |
| soluble | 0.33 (±0.04) | 62 (±3) | ||
| Fludarabine | immobilized | 0.24 (±0.18) | 41 | 25 (±6) |
| soluble | 0.58 (±0.10) | 46 (±7) | ||
| Clofarabine | immobilized | 0.16 (±0.03) | 84 | 36 (±6) |
| soluble | 0.19 (±0.03) | 38 (±2) |
| Fludarabine (mM) | Phosphate Donor | MgCl2 (mM) | Enzyme | Conversion % (Standard Deviation) |
|---|---|---|---|---|
| Standard reaction condition | ||||
| 0.333 | GTP—0.4 mM | 10 mM | immobilized | 21 (0.6) |
| 0.333 | GTP—0.4 mM | 10 mM | soluble | 54 (0.4) |
| Impact of phosphate donor | ||||
| 0.333 | ATP—0.4 mM | 10 mM | immobilized | 35 (0.8) |
| 0.333 | ATP—0.4 mM | 10 mM | soluble | 53 (0.7) |
| 0.333 | UTP—0.4 mM | 10 mM | immobilized | 3 (0.8) |
| 0.333 | UTP—0.4 mM | 10 mM | soluble | 13 (0.9) |
| Impact of ratio of substrate to phosphate donor | ||||
| 0.4 | GTP—0.4 mM | 10 mM | immobilized | 21 (0.1) |
| 0.4 | GTP—0.4 mM | 10 mM | soluble | 49 (1.6) |
| 0.2 | GTP—0.4 mM | 10 mM | immobilized | 16 (1.4) |
| 0.2 | GTP—0.4 mM | 10 mM | soluble | 58 (2.1) |
| 0.1 | GTP—0.4 mM | 10 mM | immobilized | 12 (0.9) |
| 0.1 | GTP—0.4 mM | 10 mM | soluble | 75 (0.3) |
| Impact of MgCl2 concentration | ||||
| 0.333 | GTP—0.4 mM | 0.4 mM | immobilized | 18 (0.5) |
| 0.333 | GTP—0.4 mM | 0.4 mM | soluble | 51 (1) |
| 0.333 | GTP—0.4 mM | 1 mM | immobilized | 19 (0.7) |
| 0.333 | GTP—0.4 mM | 1 mM | soluble | 51 (0.1) |
| 0.333 | GTP—0.4 mM | 2 mM | immobilized | 22 (1.1) |
| 0.333 | GTP—0.4 mM | 2 mM | soluble | 53 (0.7) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellendahl, K.F.; Kamel, S.; Wetterwald, A.; Neubauer, P.; Wagner, A. Human Deoxycytidine Kinase Is a Valuable Biocatalyst for the Synthesis of Nucleotide Analogues. Catalysts 2019, 9, 997. https://doi.org/10.3390/catal9120997
Hellendahl KF, Kamel S, Wetterwald A, Neubauer P, Wagner A. Human Deoxycytidine Kinase Is a Valuable Biocatalyst for the Synthesis of Nucleotide Analogues. Catalysts. 2019; 9(12):997. https://doi.org/10.3390/catal9120997
Chicago/Turabian StyleHellendahl, Katja F., Sarah Kamel, Albane Wetterwald, Peter Neubauer, and Anke Wagner. 2019. "Human Deoxycytidine Kinase Is a Valuable Biocatalyst for the Synthesis of Nucleotide Analogues" Catalysts 9, no. 12: 997. https://doi.org/10.3390/catal9120997






