Bioinspired ZnO-Based Solar Photocatalysts for the Efficient Decontamination of Persistent Organic Pollutants and Hexavalent Chromium in Wastewater
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrosynthesis and Characterization
2.2. Adsorption Experiments
2.3. Photooxidation of POPs
2.4. Photoreduction of Cr(VI)
2.5. Synergetic Effects of Organic-Inorganic Pollutants on Photooxidation and Photoreduction Processes
3. Materials and Methods
3.1. Electrosynthesis and Characterization
3.2. Adsorption Experiments
3.3. Photocatalysis experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Serrà, A.; Zhang, Y.; Sepúlveda, B.; Gómez, E.; Nogués, J.; Michler, J.; Philippe, L. Highly active ZnO-based biomimetic fern-like microleaves for photocatalytic water decontamination using sunlight. Appl. Catal. B Environ. 2019, 248, 129–146. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on Ti02 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Saravanan, R.; Karthikeyan, S.; Gupta, V.K.; Sekaran, G.; Narayanan, V.; Stephen, A. Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater. Sci. Eng. C 2013, 33, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yuan, Y.; Qiu, L.; Jiang, X.; Xie, A.; Shen, Y.; Zhu, J. Fabrication of composite photocatalyst g-C3N4 – ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans. 2012, 41, 6756. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yu, X.; Lei, B.; Chen, H.; Kuang, D.; Su, C. Reduced graphene oxide-hierarchical ZnO hollow sphere composites with enhanced photocurrent and photocatalytic activity. J. Phys. Chem. C 2012, 116, 8111–8117. [Google Scholar] [CrossRef]
- Boon, C.; Yong, L.; Wahab, A. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.; Cao, R. Solar-to-hydrogen energy conversion based on water splitting. Adv. Energy Mater. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Wang, H.; Lin, Q.; Yin, L.; Yang, Y.; Qiu, Y.; Lu, C.; Yang, H. Biomimetic design of hollow flower-like g-C3N4@PDA organic framework nanospheres for realizing an efficient photoreactivity. Small 2019, 15, 1–8. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Fabrication of Bi2MoO6/ZnO hierarchical heterostructures with enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2019, 250, 313–324. [Google Scholar] [CrossRef]
- Sapkota, K.P.; Lee, I.; Hanif, A.; Islam, A. Carbon nanotube photocatalyst for the degradation of a persistent water pollutant organic dye. Catalysts 2019, 9, 498. [Google Scholar] [CrossRef]
- Violet, C.; Franco, P.; Sacco, O.; Marco, I. Zinc oxide nanoparticles obtained by supercritical antisolvent precipitation for the photocatalytic degradation of crystal violet dye. Catalysts 2019, 9, 346. [Google Scholar]
- Fei, W.; Song, Y.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. Fabrication of visible-light-active ZnO/ZnFe-LDH heterojunction on Ni foam for pollutants removal with enhanced photoelectrocatalytic performance. Sol. Energy 2019, 188, 593–602. [Google Scholar] [CrossRef]
- Bao, C.; Chen, M.; Jin, X.; Hu, D.; Huang, Q. Efficient and stable photocatalytic reduction of aqueous hexavalent chromium ions by polyaniline surface-hybridized ZnO nanosheets. J. Mol. Liq. 2019, 279, 133–145. [Google Scholar] [CrossRef]
- Yuan, X.; Cheng, X.; Jing, Q.; Niu, J.; Peng, D.; Feng, Z.; Wu, X. ZnO/ZnAl2O4 nanocomposite with 3D sphere-like hierarchical structure for photocatalytic reduction of aqueous Cr(VI). Materials 2018, 11, 1624. [Google Scholar] [CrossRef]
- Shraavan, S.; Challagulla, S.; Banerjee, S.; Roy, S. Unusual photoluminescence of Cu–ZnO and its correlation with photocatalytic reduction of Cr (VI). Bull. Mater. Sci. 2017, 40, 1415–1420. [Google Scholar] [CrossRef]
- Skompska, M.; Zar, K. Electrodeposition of ZnO nanorod arrays on transparent conducting substrates—A Review. Electrochim. Acta 2014, 127, 467–488. [Google Scholar] [CrossRef]
- Environ, E.; Gu, V. From nanowires to hierarchical structures of template-free electrodeposited ZnO for efficient dye-sensitized solar cells. Energy Environ. Sci. 2011, 2971–2979. [Google Scholar]
- Serrà, A.; Zhang, Y.; Sepúlveda, B.; Gómez, E.; Nogués, J.; Michler, J.; Philippe, L. Highly reduced ecotoxicity of ZnO-based micro/nanostructures on aquatic biota: Influence of architecture, chemical composition, fixation, and photocatalytic efficiency. Water Res. 2019, 169, 115210. [Google Scholar] [CrossRef]
- Vaiano, V.; Matarangolo, M.; Murcia, J.J.; Rojas, H.; Navío, J.A.; Hidalgo, M.C. Enhanced photocatalytic removal of phenol from aqueous solutions using ZnO modified with Ag. Appl. Catal. B Environ. 2018, 225, 197–206. [Google Scholar] [CrossRef]
- Yu, X.X.; Wu, Y.; Dong, B.; Dong, Z.F.; Yang, X. Enhanced solar light photocatalytic properties of ZnO nanocrystals by Mg-doping via polyacrylamide polymer method. J. Photochem. Photobiol. 2018, 356, 681–688. [Google Scholar] [CrossRef]
- Sekar, A.D.; Muthukumar, H.; Chandrasekaran, N.I.; Matheswaran, M. Photocatalytic degradation of naphthalene using calcined Fe-ZnO/PVA nanofibers. Chemosphere 2018, 205, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wu, X.; Feng, Z.; Jia, W.; Zheng, X.; Li, C. Facile synthesis of heterojunctioned ZnO/Bi2S3 nanocomposites for enhanced photocatalytic reduction of aqueous Cr(VI) under visible-light irradiation. Catalysts 2019, 9, 624. [Google Scholar] [CrossRef]
- Liu, N.; Li, Z. Materials science in semiconductor processing bimetal-organic frameworks derived carbon doped ZnO/Co3O4 heterojunction as visible-light stabilized photocatalysts. Mater. Sci. Semicond. Process. 2018, 79, 24–31. [Google Scholar] [CrossRef]
- Fathi, M.; Esmail, A. Pollutant degradation of different organic dyes using the photocatalytic activity of ZnO@ZnS nanocomposite materials. J. Environ. Chem. Eng. 2018, 6, 3981–3990. [Google Scholar]
- Yousefi, R.; Jamali-sheini, F.; Cheraghizade, M. Enhanced visible-light photocatalytic activity of strontium-doped zinc oxide nanoparticles. Mater. Sci. Semicond. Process. 2015, 32, 152–159. [Google Scholar] [CrossRef]
- Qamar, M.T.; Aslam, M.; Ismail, I.M.I.; Salah, N.; Hameed, A. Synthesis, Characterization, and Sunlight Mediated Photocatalytic Activity of CuO Coated ZnO for the Removal of Nitrophenols. ACS Appl. Mater. Interfaces 2015, 7, 8757–8769. [Google Scholar] [CrossRef]
- Servant, L. Nanoparticulate heterostructures due to inhomogeneous space charge effects. Phys. Chem. Chem. Phys. 2015, 17, 5090–5102. [Google Scholar]
- Verma, S.; Dutta, R.K. Enhanced ROS generation by ZnO-ammonia modified graphene oxide nanocomposites for photocatalytic degradation of trypan blue dye and 4- nitrophenol. J. Environ. Chem. Eng. 2017, 5, 4776–4787. [Google Scholar] [CrossRef]
- Jadhav, J.; Biswas, S. Hybrid ZnO: Ag core-shell nanoparticles for wastewater treatment: Growth mechanism and plasmonically enhanced photocatalytic activity. Appl. Surf. Sci. 2018, 456, 49–58. [Google Scholar] [CrossRef]
- Jin, Y.; Long, J.; Ma, X.; Zhou, T.; Zhang, Z.; Lin, H.; Long, J.; Wang, X. Synthesis of caged iodine-modified ZnO nanomaterials and study on their visible light photocatalytic antibacterial properties. Appl. Catal. B Environ. 2019, 256, 117873. [Google Scholar] [CrossRef]
- Gadisa, B.T.; Appiah-Ntiamoah, R.; Kim, H. In-situ derived hierarchical ZnO/Zn-C nano fiber with high photocatalytic activity and recyclability under solar light. Appl. Surf. Sci. 2019, 491, 350–359. [Google Scholar] [CrossRef]
- Alkaim, A.F.; Aljeboree, A.M.; Alrazaq, N.A.; Baqir, S.J.; Hussein, F.H.; Lilo, A.J. Effect of pH on adsorption and photocatalytic degradation efficiency of different catalysts on removal of methylene blue. Asian J. Chem. 2014, 26, 6097–6100. [Google Scholar] [CrossRef]
- Kong, J.Z.; Li, A.D.; Li, X.Y.; Zhai, H.F.; Zhang, W.Q.; Gong, Y.P.; Li, H.; Wu, D. Photo-degradation of methylene blue using Ta-doped ZnO nanoparticle. J. Solid State Chem. 2010, 183, 1359–1364. [Google Scholar] [CrossRef]
- Talebian, N.; Nilforoushan, M.R. Comparative study of the structural, optical and photocatalytic properties of semiconductor metal oxides toward degradation of methylene blue. Thin Solid Films 2010, 518, 2210–2215. [Google Scholar] [CrossRef]
- Chakraborty, T.; Chakraborty, A.; Shukla, M. ZnO—Bentonite nanocomposite: An efficient catalyst for discharge of dyes, phenol and Cr(VI) from water. J. Coord. Chem. 2019, 72, 53–68. [Google Scholar] [CrossRef]
- Yuan, X.; Feng, Z.; Zhao, J.; Niu, J.; Liu, J.; Peng, D.; Cheng, X. Significantly enhanced aqueous Cr(VI) removal performance of Bi/ZnO nanocomposites via synergistic effect of adsorption and SPR-promoted visible light photoreduction. Catalysts 2019, 8, 426. [Google Scholar] [CrossRef] [Green Version]
- Kabra, K.; Chaudhary, R.; Sawhney, R.L. Treatment of hazardous organic and inorganic compounds through aqueous-phase photocatalysis: A Review. Ind. Eng. Chem. Res. 2004, 43, 7683–7696. [Google Scholar] [CrossRef]
- Sasikala, S.P.; Nibila, T.A.; Babitha, K.B.; Mohamed, A.A.P.; Solaiappan, A. Competitive photo-degradation performance of ZnO modified bentonite clay in water containing both organic and inorganic contaminants. Sustain. Environ. Res. 2019, 29, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Egerton, T.A.; Mattinson, J.A. Comparison of photooxidation and photoreduction reactions on TiO2 nanoparticles. J. Photochem. Photobiol. A Chem. 2007, 186, 115–120. [Google Scholar] [CrossRef]
- Mara, D. The all-inclusive sustainable development goals: The WASH professional′s guide (or should that be ‘nightmare’?). J. Water Sanit. Hyg. Dev. 2016, 6, 349–352. [Google Scholar] [CrossRef]
- Assembly, T.G.; Rights, C.; Rights, P. Presented at General Assembly, Denver, CO, USA, September 2016.
- Shanmugam, K.; Burgos, R.; Sillanpaa, M. Effective shell wall thickness of vertically aligned ZnO-ZnS core-shell nanorod arrays on visible photocatalytic and photo sensing properties. Appl. Catal. B Environ. 2018, 237, 128–139. [Google Scholar]
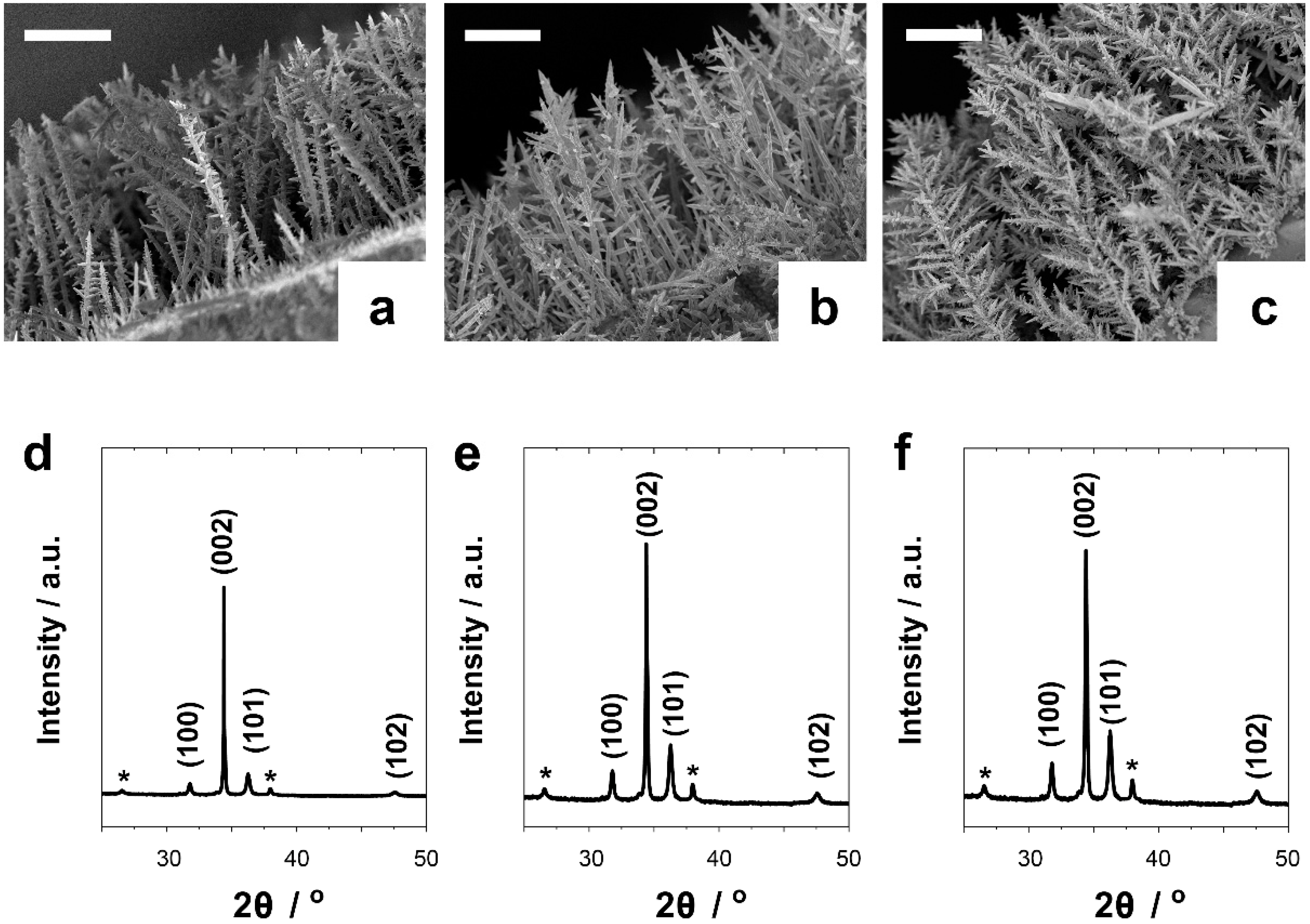
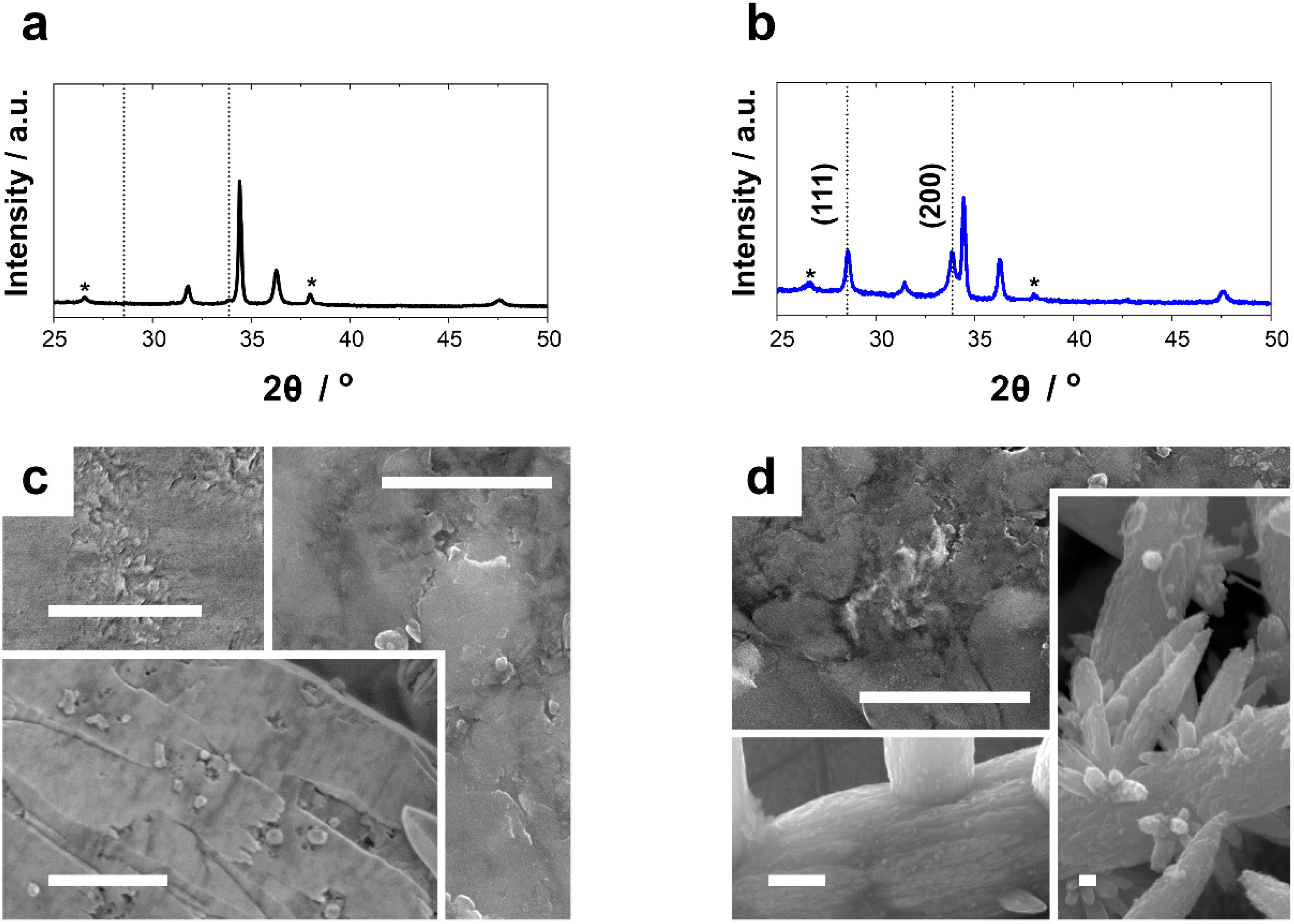
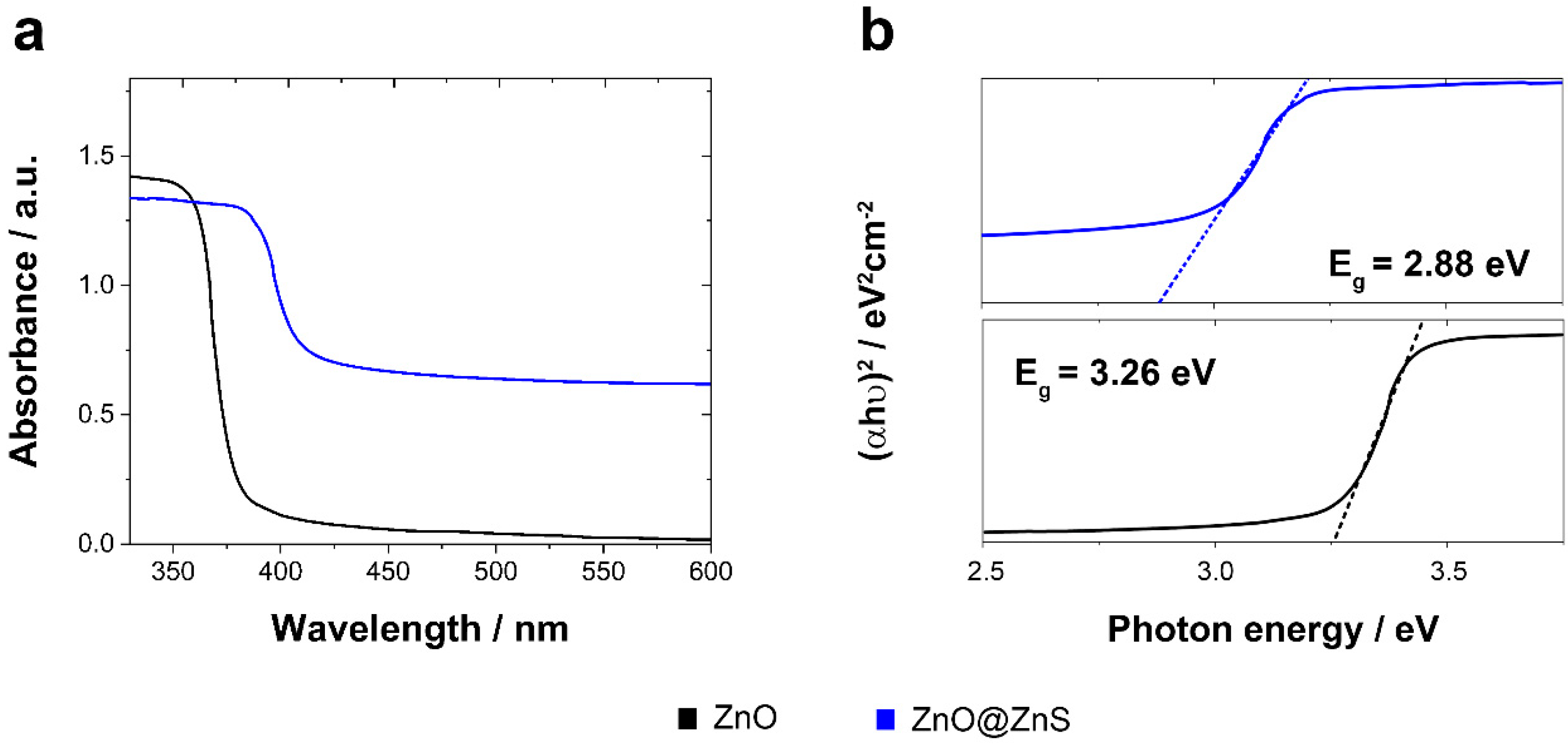
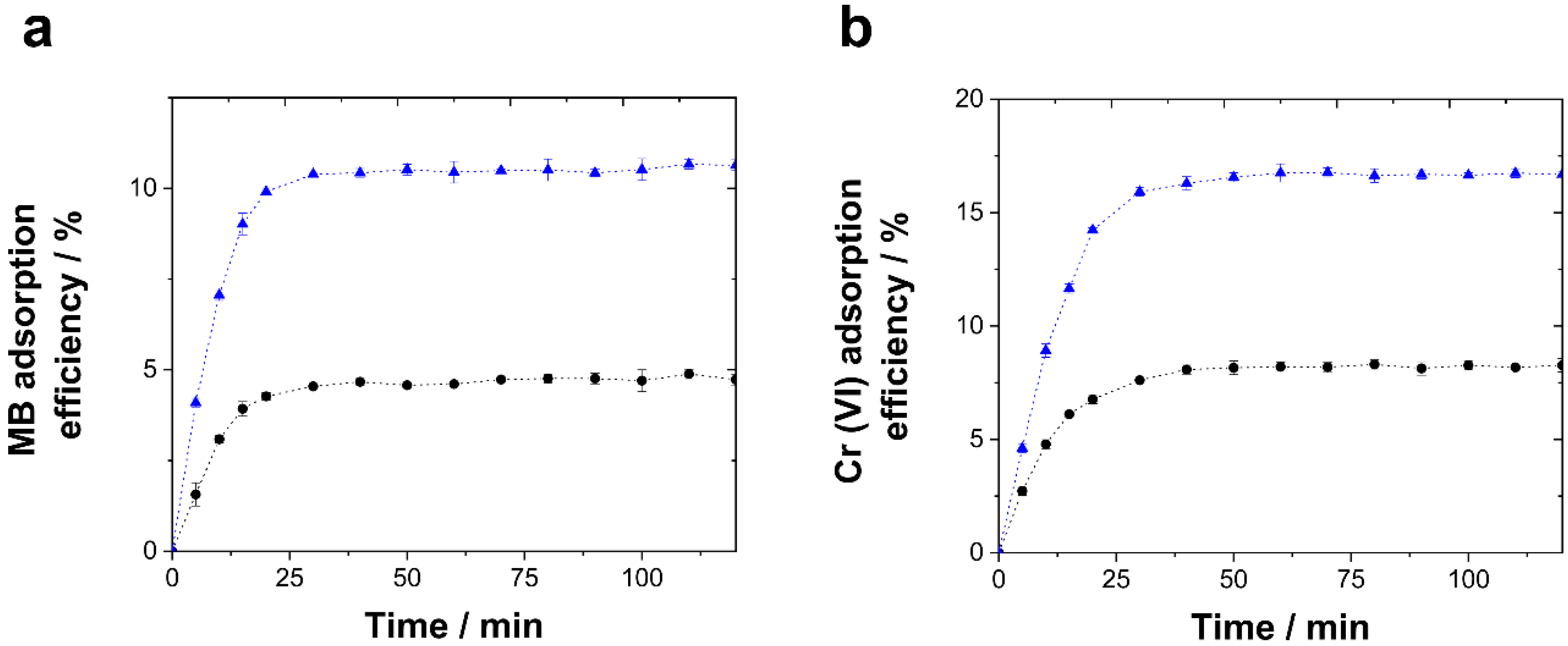
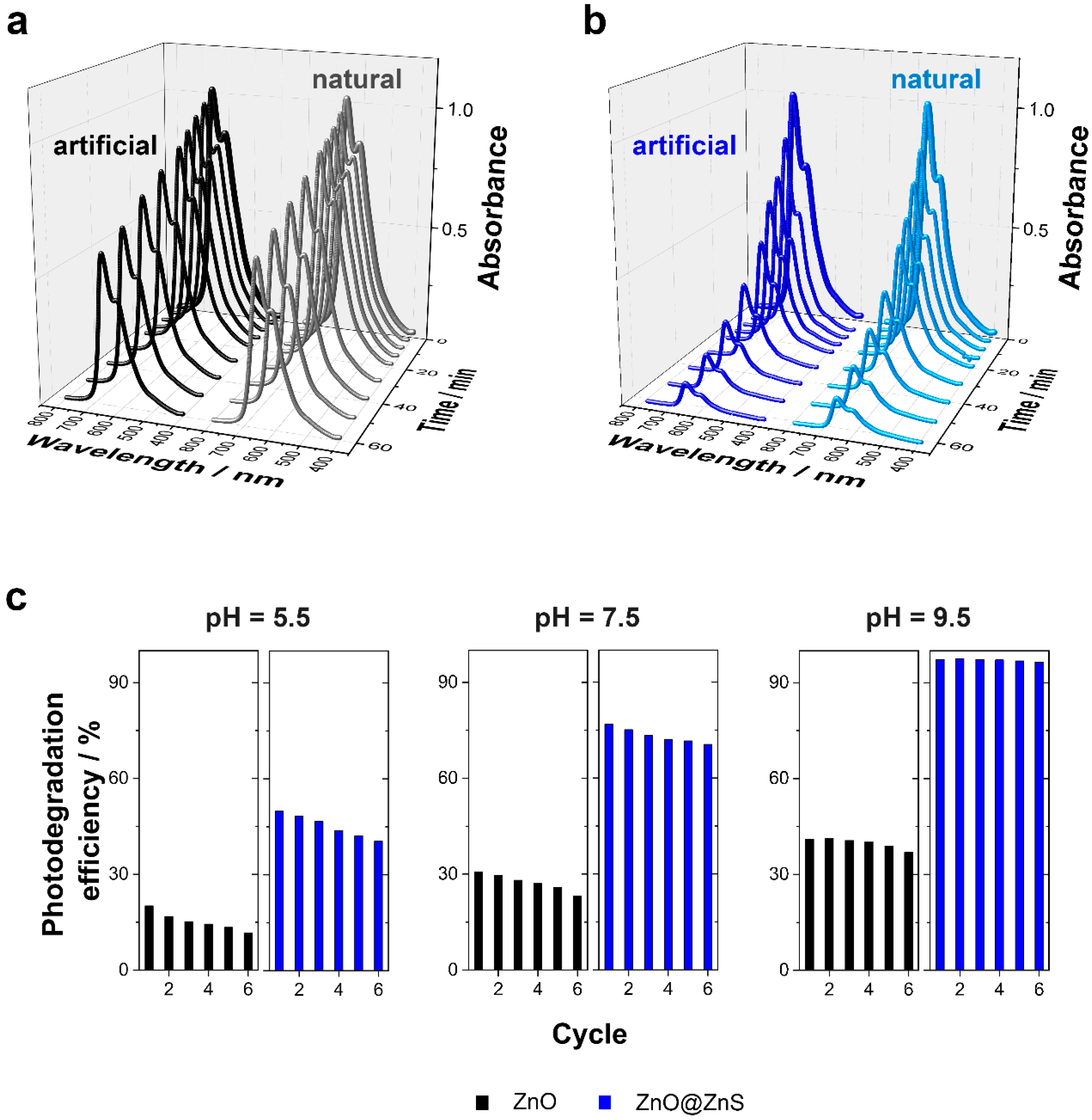


| Oxygen Flux/L min−1 | Magnetic Stirring/rpm | Potential/V vs Ag/AgCl/KCl (3 M) | Morphology | Structure (Preferred Growth) | BET Surface Area/m2g−1 |
|---|---|---|---|---|---|
| 1 | 400 | −1 | Low ramification | ZnO wurtzite (002) | 38.2 ± 1.8 |
| 3 | 400 | −1 | Moderate ramification | ZnO wurtzite (002) | 42.1 ± 1.2 |
| 12 | 400 | −1 | High ramification | ZnO wurtzite (002) | 67.4 ± 1.2 |
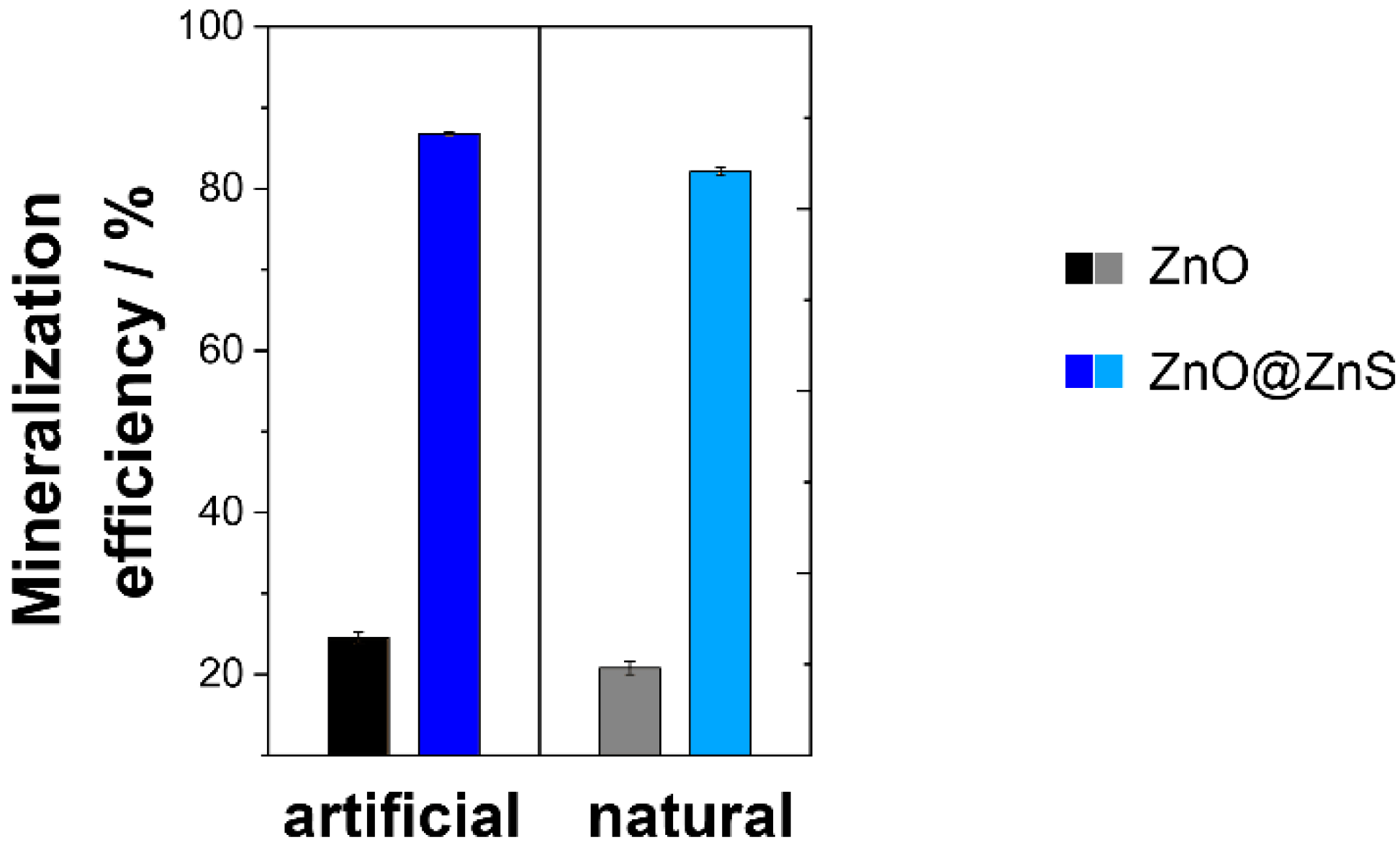
| Photocatalyst | Artificial UV-Filtered Sunlight Irradiation (60 min λ > 400 nm) | Natural UV-Filtered Sunlight Irradiation (60 min λ > 400 nm) | ||||
|---|---|---|---|---|---|---|
| Degradation Efficiency/% | Mass Normalized Kinetic/min−1 g−1 | Mineralization Efficiency/% | Degradation Efficiency/% | Mass Normalized Kinetic/min−1 g−1 | Mineralization Efficiency/% | |
| ZnO micro/nanoferns | 41 ± 2 | 2.21 | 21.5 ± 1.1 | 36 ± 2 | 1.83 | 18.2 ± 0.6 |
| ZnO@ZnS micro/nanoferns | 97 ± 1 | 9.74 | 73.1 ± 1.6 | 88 ± 2 | 8.65 | 64.9 ± 0.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrà, A.; Gómez, E.; Philippe, L. Bioinspired ZnO-Based Solar Photocatalysts for the Efficient Decontamination of Persistent Organic Pollutants and Hexavalent Chromium in Wastewater. Catalysts 2019, 9, 974. https://doi.org/10.3390/catal9120974
Serrà A, Gómez E, Philippe L. Bioinspired ZnO-Based Solar Photocatalysts for the Efficient Decontamination of Persistent Organic Pollutants and Hexavalent Chromium in Wastewater. Catalysts. 2019; 9(12):974. https://doi.org/10.3390/catal9120974
Chicago/Turabian StyleSerrà, Albert, Elvira Gómez, and Laetitia Philippe. 2019. "Bioinspired ZnO-Based Solar Photocatalysts for the Efficient Decontamination of Persistent Organic Pollutants and Hexavalent Chromium in Wastewater" Catalysts 9, no. 12: 974. https://doi.org/10.3390/catal9120974





