Unraveling Toluene Conversion during the Liquid Phase Hydrogenation of Cyclohexene (in Toluene) with Rh Hybrid Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Summary of the Supports Characterization Data
2.2. Cyclohexene Hydrogenation in Toluene with Homogeneous and Biphasic Systems
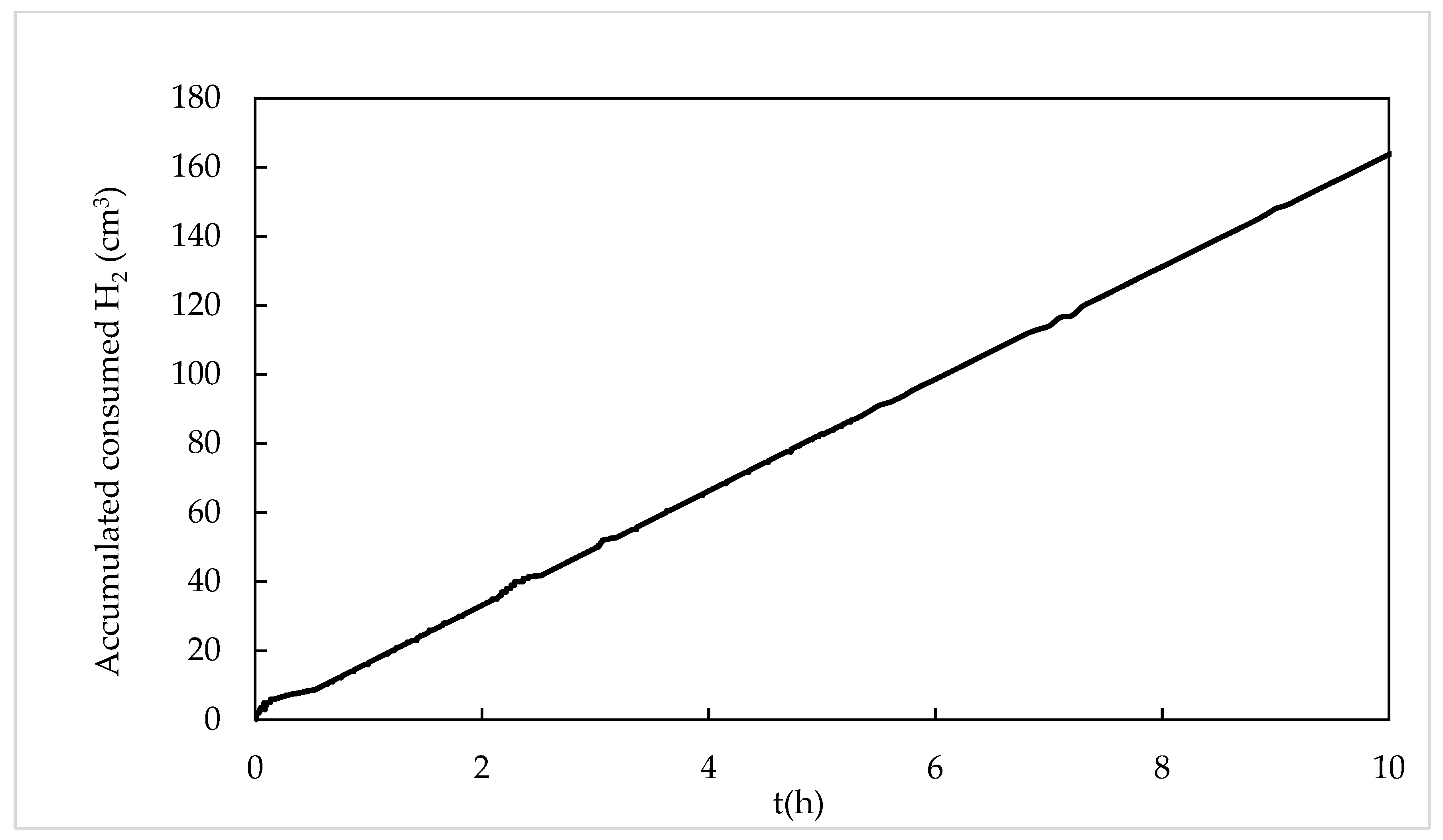
2.3. Cyclohexene Hydrogenation in Toluene with RhCOD SILP Catalysts
2.4. Kinetics of the Hydrogenation of Cyclohexene in Toluene
3. Materials and Methods
3.1. Supports
3.2. Catalysts’ Preparations and Characterizations
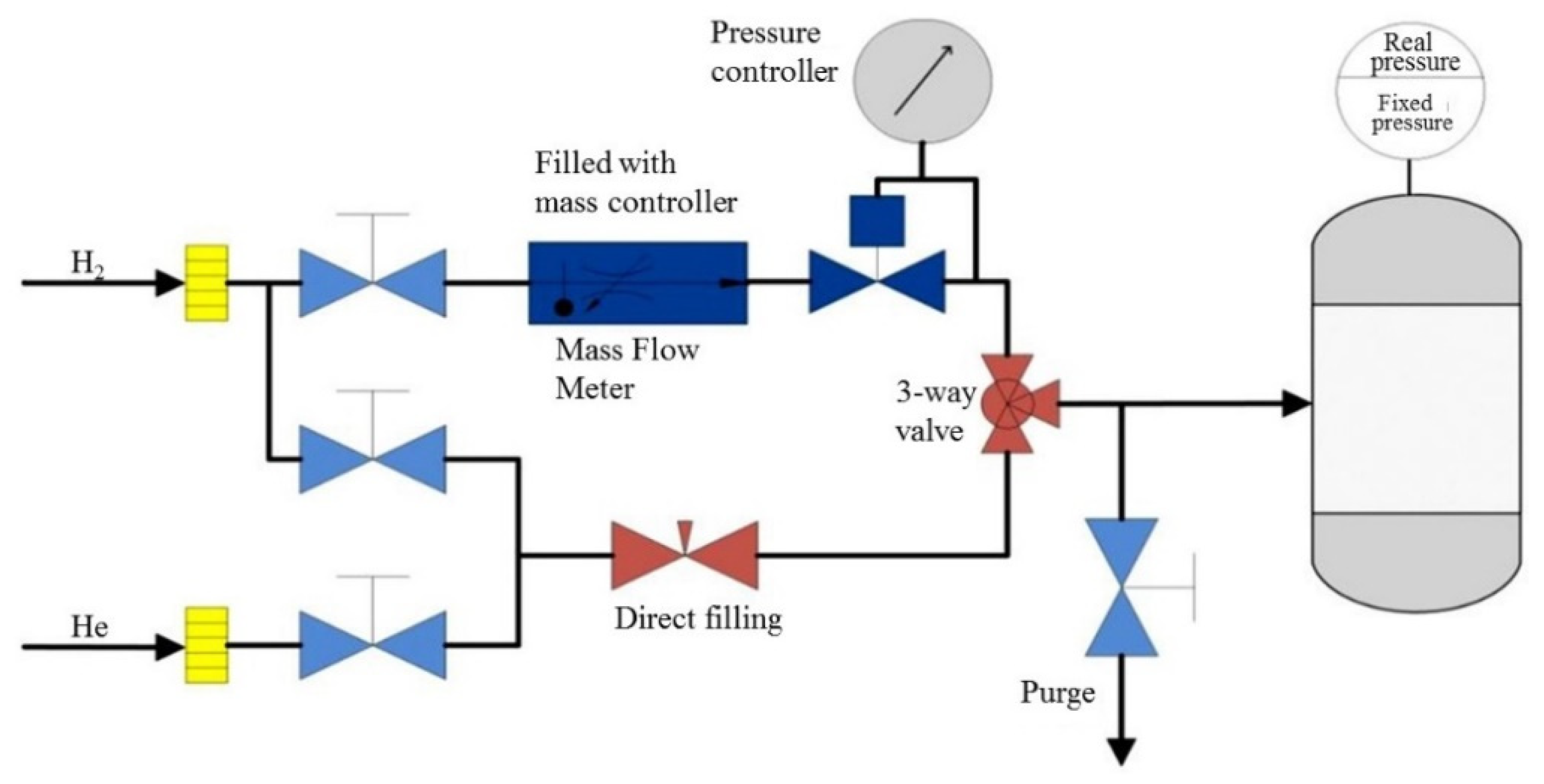
3.3. Liquid Phase Hydrogenation of Cyclohexene in Toluene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Standfest-Hauser, C.M.; Lummerstorfer, T.; Schmid, R.; Hoffmann, H.; Kirchner, K.; Puchberger, M.; Trzeciak, A.M.; Mieczyńska, E.; Tylus, W.; Ziółkowski, J.J. Rhodium Phosphine Complexes Immobilized on Silica as Active Catalysts for 1-Hexene Hydroformylation and Arene Hydrogenation. J. Mol. Catal. A Chem. 2004, 210, 179–187. [Google Scholar] [CrossRef]
- Lindner, E.; Mayer, H.A.; Warad, I.; Eichele, K. Supported Organometallic Complexes: Part XXXV. Synthesis, Characterization, and Catalytic Application of a New Family of Diamine(Diphosphine)Ruthenium Complexes. J. Organomet. Chem. 2003, 665, 176–185. [Google Scholar] [CrossRef]
- Cagnola, E.A.; Quiroga, M.E.; Liprandi, D.A.; L’Argentière, P.C. Immobilized Rh, Ru, Pd and Ni Complexes as Catalysts in the Hydrogenation of Cyclohexene. Appl. Catal. A Gen. 2004, 274, 205–212. [Google Scholar] [CrossRef]
- Sudheesh, N.; Sharma, V.K.; Shukla, R.S.; Jasra, R.V. Investigations on the Kinetics of Hydroformylation of 1-Hexene Using HRh(CO)(PPh3)3 Encapsulated Hexagonal Mesoporous Silica as Heterogeneous Catalyst. J. Mol. Catal. A Chem. 2009, 316, 23–29. [Google Scholar] [CrossRef]
- Sudheesh, N.; Chaturvedi, A.K.; Shukla, R.S. RhCl(TPPTS)3 Encapsulated into the Hexagonal Mesoporous Silica as an Efficient Heterogeneous Catalyst for Hydroformylation of Vinyl Esters. Appl. Catal. A Gen. 2011, 409, 99–105. [Google Scholar] [CrossRef]
- Wolfson, A.; Vankelecom, I.F.J.; Jacobs, P.A. Co-Immobilization of Transition-Metal Complexes and Ionic Liquids in a Polymeric Support for Liquid-Phase Hydrogenations. Tetrahedron Lett. 2003, 44, 1195–1198. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, C.; Yuan, Y. Characterization and Hydroformylation Performance of Mesoporous MCM-41-Supported Water-Soluble Rh Complex Dissolved in Ionic Liquids. J. Catal. 2005, 232, 108–116. [Google Scholar] [CrossRef]
- Pawelec, B.; Mariscal, R.; Navarro, R.M.; Van Bokhorst, S.; Rojas, S. Hydrogenation of Aromatics over Supported Pt-Pd Catalysts. Appl. Catal. 2005, 225, 223–237. [Google Scholar] [CrossRef]
- Rufete-Beneite, M.; Román-Martínez, M.C. Support Effects on SILP Hybrid Catalysts Prepared with Carbon Materials and the RhCOD Complex. RSC Adv. 2016, 6, 100976–100983. [Google Scholar] [CrossRef]
- Gu, Y.; Li, G. Ionic Liquids-Based Catalysis with Solids: State of the Art. Adv. Synth. Catal. 2009, 351, 817–847. [Google Scholar] [CrossRef]
- Riisager, A.; Fehrmann, R.; Haumann, M.; Wasserscheid, P. Supported Ionic Liquids: Versatile Reaction and Separation Media. Top. Catal. 2006, 40, 91–102. [Google Scholar] [CrossRef]
- Van Doorslaer, C.; Wahlen, J.; Mertens, P.; Binnemans, K.; De Vos, D. Immobilization of Molecular Catalysts in Supported Ionic Liquid Phases. Dalton Trans. 2010, 39, 8377–8390. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, Y.; Li, W.; Han, Y.; Zhang, J. Improvement of Imidazolium-Based Ionic Liquids on the Activity of Ruthenium Catalyst for Acetylene Hydrochlorination. Mol. Catal. 2017, 443, 220–227. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Deng, Y. Recent Advances in Ionic Liquid Catalysis. Green Chem. 2011, 13, 2619. [Google Scholar] [CrossRef]
- Selvam, T.; MacHoke, A.; Schwieger, W. Supported Ionic Liquids on Non-Porous and Porous Inorganic Materials - A Topical Review. Appl. Catal. A Gen. 2012, 445–446, 92–101. [Google Scholar] [CrossRef]
- Chacón, G.; Dupont, J. Arene Hydrogenation by Metal Nanoparticles in Ionic Liquids. ChemCatChem 2019, 11, 333–341. [Google Scholar] [CrossRef]
- Stanislaus, A.; Barry, H.C. Aromatic Hydrogenation Catalysis: A Review. Catal. Rev. 1994, 36, 75–123. [Google Scholar] [CrossRef]
- Rautanen, P.A.; Aittamaa, J.R.; Krause, A.O.I. Solvent Effect in Liquid-Phase Hydrogenation of Toluene. Ind. Eng. Chem. Res. 2000, 39, 4032–4039. [Google Scholar] [CrossRef]
- Chu, H.; Li, N.; Niu, L.; Liu, C.; Qiu, M.; Bai, G. Continuous Hydrogenation of Toluene over Sr- and PEG800-Modified Ni/g-AlO3 Catalysts. Chem. Eng. Technol. 2015, 38, 1585–1590. [Google Scholar] [CrossRef]
- Shuwa, S.M.; Jibril, B.Y.; Al-Hajri, R.S. Hydrogenation of Toluene on Ni-Co-Mo Supported Zeolite Catalysts. Niger. J. Technol. 2017, 36, 1114–1123. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders & Porous Solids—Principles, Methodology and Applications; Academic Press: London, UK, 1999. [Google Scholar]
- Antonels, N.C.; Benjamin Williams, M.; Meijboom, R.; Haumann, M. Well-Defined Dendrimer Encapsulated Ruthenium SCILL Catalysts for Partial Hydrogenation of Toluene in Liquid-Phase. J. Mol. Catal. A Chem. 2016, 421, 156–160. [Google Scholar] [CrossRef]
- Léger, B.; Denicourt-Nowicki, A.; Olivier-Bourbigou, H.; Roucoux, A. Imidazolium-Functionalized Bipyridine Derivatives: A Promising Family of Ligands for Catalytical Rh(0) Colloids. Tetrahedron Lett. 2009, 50, 6531–6533. [Google Scholar] [CrossRef]
- Suppino, R.S.; Landers, R.; Cobo, A.J.G. Influence of Noble Metals (Pd, Pt) on the Performance of Ru/Al2O3 Based Catalysts for Toluene Hydrogenation in Liquid Phase. Appl. Catal. A Gen. 2016, 525, 41–49. [Google Scholar] [CrossRef]
- Román-Martínez, M.C.; Díaz-Auñón, J.A.; L’Argentière, P.C.; Salinas-Martínez de Lecea, C. Long-Chain-Amine Metal Complexes as Hydrogenation Catalysts. Heterogenisation on Activated Carbon. Catal. Lett. 2001, 77, 41–46. [Google Scholar] [CrossRef]
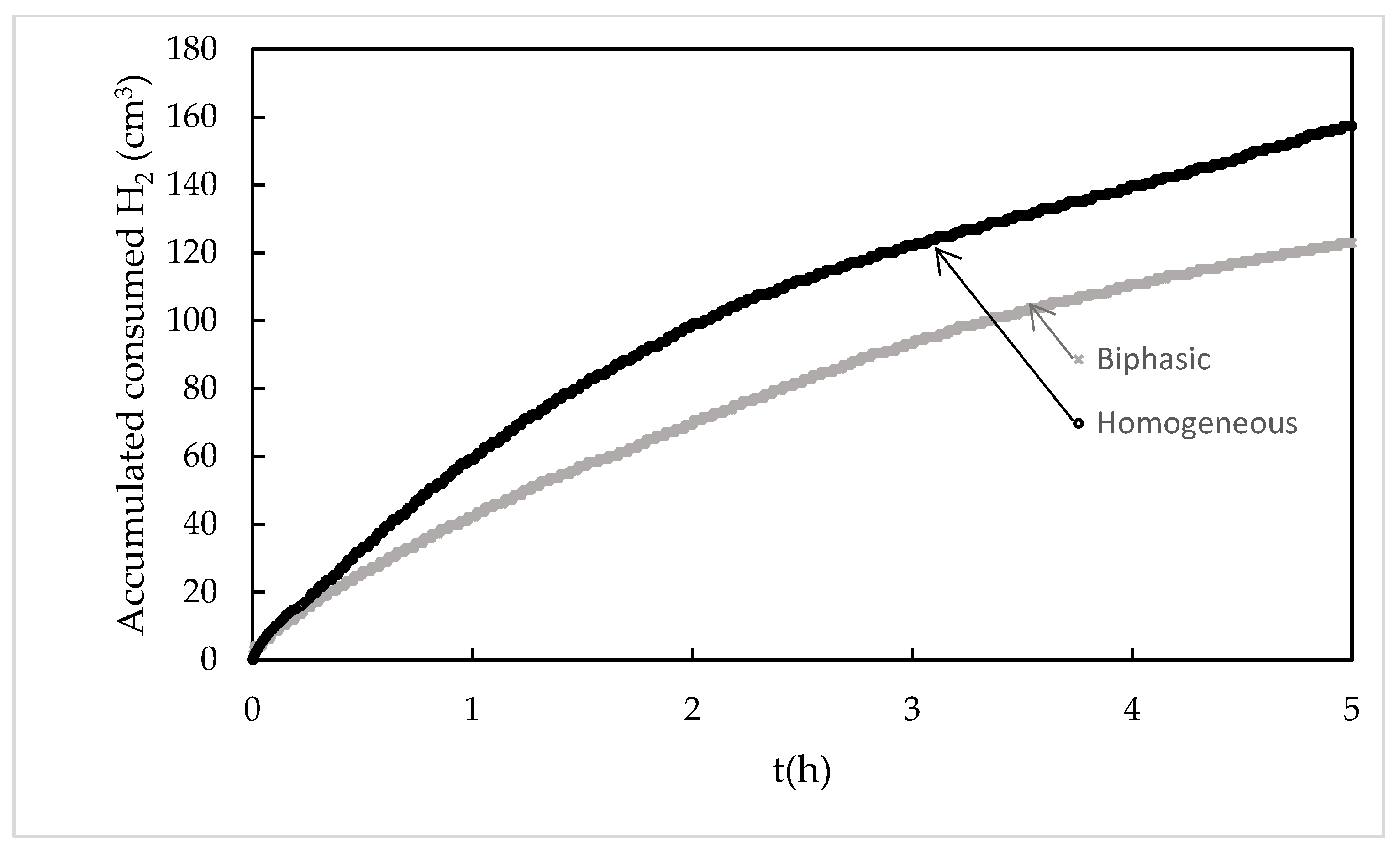


| Catalyst | H2 cons (cm3) a | CH conv (%) b | H2 for CH conv (cm3) c | r0(m3h−1) d |
|---|---|---|---|---|
| Homogeneous | 142.4 | 98.4 | 118.7 | 27.1 |
| Biphasic | 122.8 | 98.1 | 118.3 | 22.8 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| Catalyst | H2 cons (cm3) a | CHconv (GC) (%) b | H2 for CHconv (cm3) c | Tolconv from H2 excess (%) d | Tolconv (GC) (%) e | mol Tol /mol CH | r0 (m3h−1) f |
| KA20-RhCOD | 117.9 | 52.9 | 61.3 | 0.9 | 0.9 | 0.3 | 24.4 |
| GeA20-RhCOD | 193.7 | 92.8 | 111.9 | 1. | 1.4 | 0.3 | 42.6 |
| SA20-RhCOD | 225.9 | 100.0 | 120.6 | 1.6 | 1.7 | 0.3 | 109.2 |
| T20-RhCOD | 212.8 | 100.0 | 120.6 | 1.4 | 1.5 | 0.3 | 38.6 |
| KA-RhCOD | 63.5 | 16.3 | 19.6 | 0. 7 | 0.8 | 0.9 | 13.0 |
| CH Hydrogenation | Tol Hydrogenation | |||
|---|---|---|---|---|
| Catalyst | TOF (s−1) | TON | TOF (s−1) | TON |
| KA20-RhCOD | 0.050 | 900 | 0.016 | 288 |
| GeA20-RhCOD | 0.087 | 1566 | 0.023 | 414 |
| SA20-RhCOD | 0.094 | 1692 | 0.029 | 522 |
| T20-RhCOD | 0.094 | 1692 | 0.025 | 450 |
| KA-RhCOD | 0.015 | 270 | 0.013 | 234 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rufete-Beneite, M.; Román-Martínez, M.C. Unraveling Toluene Conversion during the Liquid Phase Hydrogenation of Cyclohexene (in Toluene) with Rh Hybrid Catalysts. Catalysts 2019, 9, 973. https://doi.org/10.3390/catal9120973
Rufete-Beneite M, Román-Martínez MC. Unraveling Toluene Conversion during the Liquid Phase Hydrogenation of Cyclohexene (in Toluene) with Rh Hybrid Catalysts. Catalysts. 2019; 9(12):973. https://doi.org/10.3390/catal9120973
Chicago/Turabian StyleRufete-Beneite, Mónica, and M. Carmen Román-Martínez. 2019. "Unraveling Toluene Conversion during the Liquid Phase Hydrogenation of Cyclohexene (in Toluene) with Rh Hybrid Catalysts" Catalysts 9, no. 12: 973. https://doi.org/10.3390/catal9120973





