Quantitative Visual Characterization of Contaminant Metals and Their Mobility in Fluid Catalytic Cracking Catalysts
Abstract
:1. Introduction
1.1. Fluid Catalytic Cracking
1.2. Imaging in FCC
1.3. Metals Mobility
2. Results and Discussion
2.1. Qualitative Metals Mobility
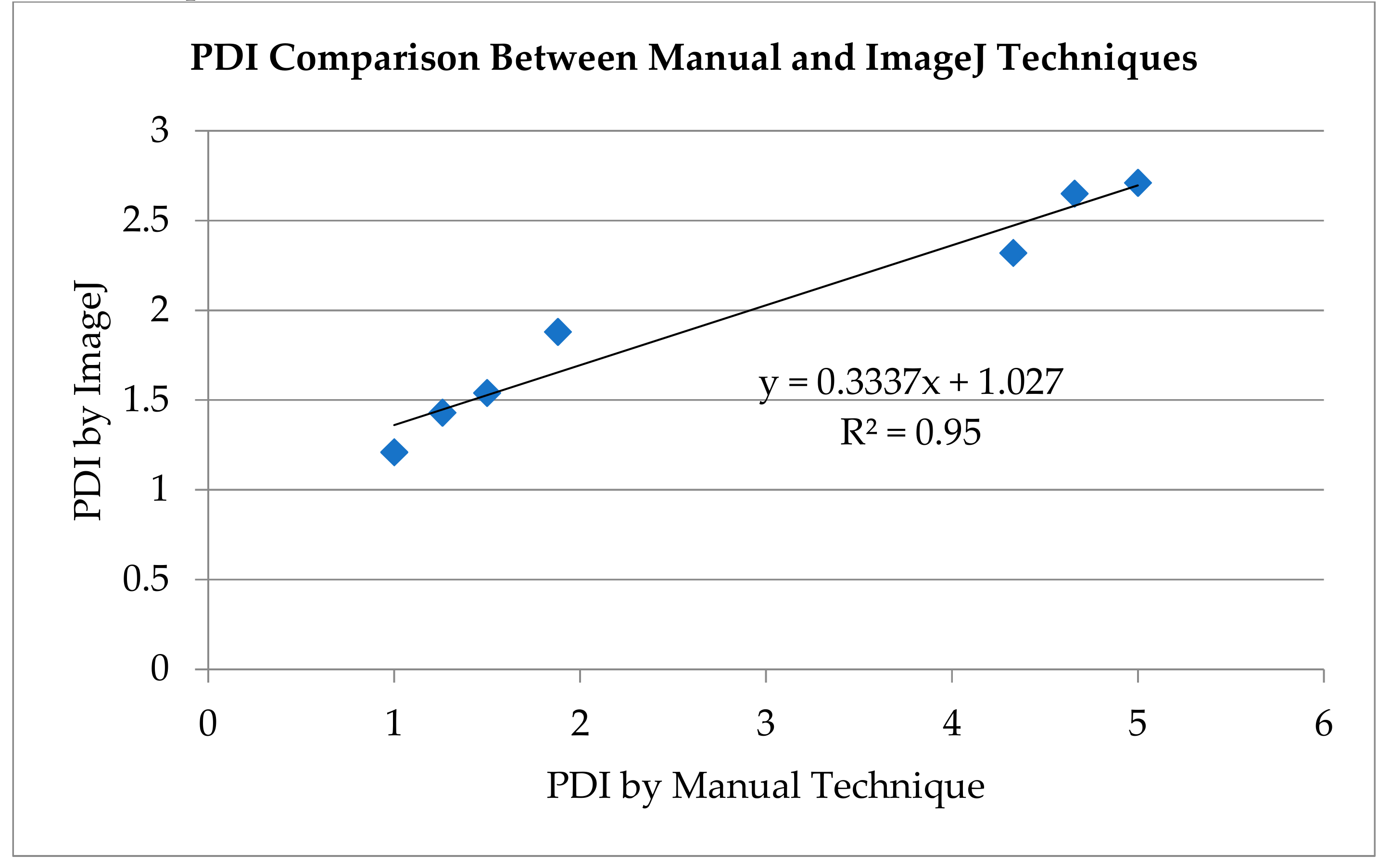
2.2. Intraparticle Mobility - Peripheral Deposition Index (PDI)
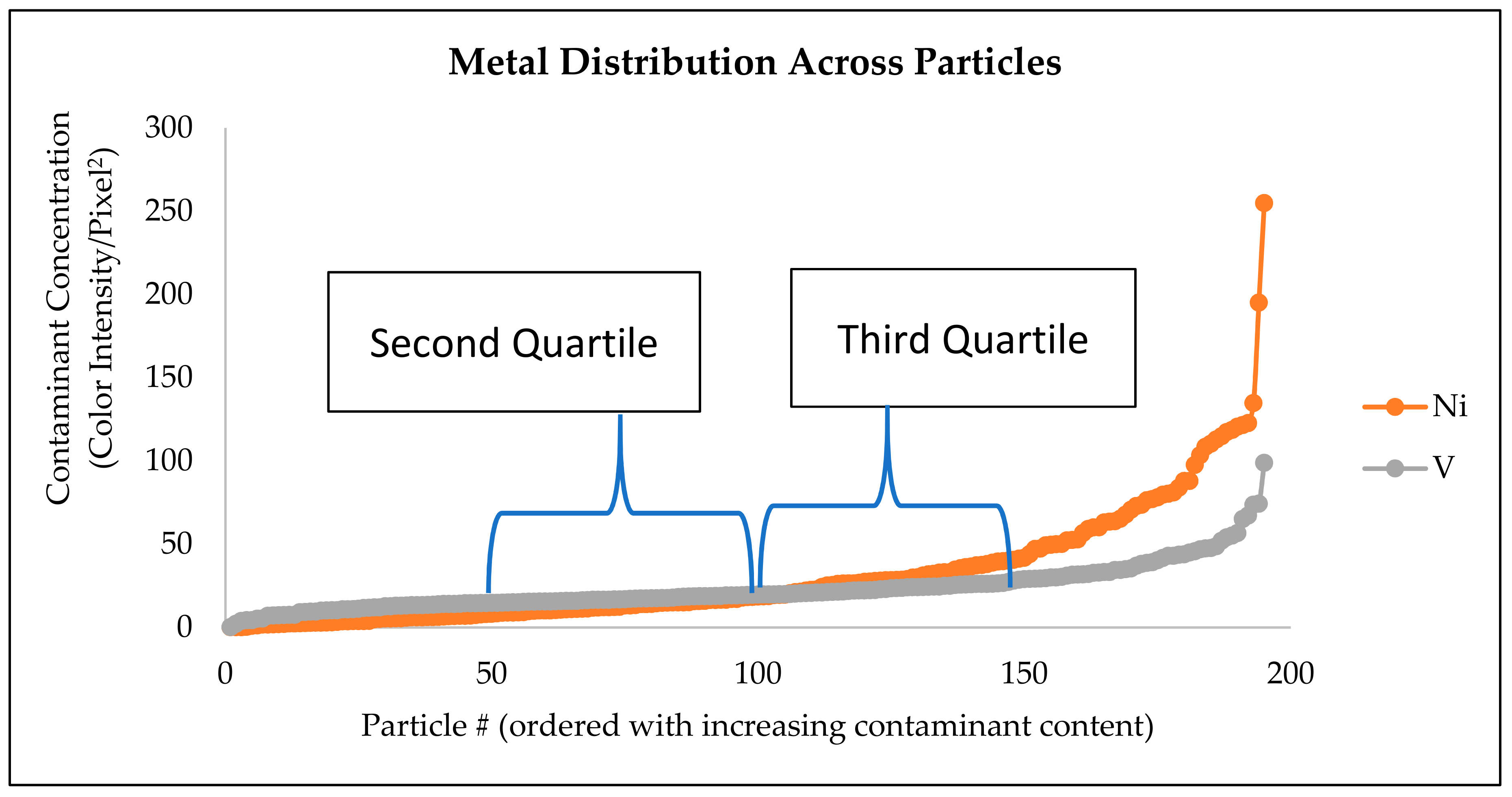
2.3. Interparticle Mobility Index (IMI)
3. Materials and Methods
3.1. Catalyst Samples
3.2. FE-SEM and EDX Analyses
3.3. Chemical Analysis
3.4. Metals Mobility Analyses
3.4.1. Intraparticle Mobility
3.4.2. Interparticle Mobility
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, S.S.; Lin, L.T.X.; Komvokis, V.; Spann, A.; Clough, M.; Yilmaz, B. Nanomaterials Fueling the World. In Nanomaterials for Sustainable Energy; American Chemical Society: Washington, DC, USA, 2015; Volume 1213, pp. 3–18. [Google Scholar]
- Clough, M.; Pope, J.C.; Lin, L.T.X.; Komvokis, V.; Pan, S.S.; Yilmaz, B. Nanoporous materials forge a path forward to enable sustainable growth: Technology advancements in fluid catalytic cracking. Microporous Mesoporous Mater. 2017, 254, 45–58. [Google Scholar] [CrossRef]
- Meirer, F.; Morris, D.T.; Kalirai, S.; Liu, Y.; Andrews, J.C.; Weckhuysen, B.M. Mapping Metals Incorporation of a Whole Single Catalyst Particle Using Element Specific X-ray Nanotomography. J. Am. Chem. Soc. 2015, 137, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Kugler, E.; Leta, D. Nickel and vanadium on equilibrium cracking catalysts by imaging secondary ion mass spectrometry. J. Catal. 1988, 109, 387–395. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feige, J.N.; Sage, D.; Wahli, W.; Desvergne, B.; Gelman, L. PixFRET, an ImageJ plug-in for FRET calculation that can accommodate variations in spectral bleed-throughs. Microsc. Res. Tech. 2005, 68, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalirai, S.; Boesenberg, U.; Falkenberg, G.; Meirer, F.; Weckhuysen, B.M. X-ray Fluorescence Tomography of Aged Fluid-Catalytic-Cracking Catalyst Particles Reveals Insight into Metal Deposition Processes. ChemCatChem 2015, 7, 3674–3682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Martínez, J.; Xiao, C.; Cychosz, K.A.; Li, K.; Wan, W.; Zou, X.; Thommes, M.; García-Martínez, J. Evidence of Intracrystalline Mesostructured Porosity in Zeolites by Advanced Gas Sorption, Electron Tomography and Rotation Electron Diffraction. ChemCatChem 2014, 6, 3110–3115. [Google Scholar] [CrossRef]
- Lappas, A.; Tsagrasouli, Z.; Vasalos, I.; Humphries, A. Effect of catalyst properties and feedstock composition on the evaluation of cracking catalysts. Stud. Surf. Sci. Catal. 2001, 134, 71–86. [Google Scholar]
- Lappas, A.; Nalbandian, L.; Iatridis, D.; Voutetakis, S.; Vasalos, I. Effect of metals poisoning on FCC products yields: studies in an FCC short contact time pilot plant unit. Catal. Today 2001, 65, 233–240. [Google Scholar] [CrossRef]
- Xu, M.; Liu, X.; Madon, R.J. Pathways for Y Zeolite Destruction: The Role of Sodium and Vanadium. J. Catal. 2002, 207, 237–246. [Google Scholar] [CrossRef]
- Etim, U.; Bai, P.; Liu, X.; Subhan, F.; Ullah, R.; Yan, Z. Vanadium and nickel deposition on FCC catalyst: Influence of residual catalyst acidity on catalytic products. Microporous Mesoporous Mater. 2019, 273, 276–285. [Google Scholar] [CrossRef]
- Fe´ron, B.; Gallezot, P.; Bourgogne, M. Hydrothermal aging of cracking catalysts V. Vanadium passivation by rare-earth compounds soluble in the feedstock. J. Catal. 1992, 134, 469–478. [Google Scholar] [CrossRef]
- Vincz, C.; Rath, R.; Smith, G.M.; Yilmaz, B.; McGuire, R., Jr. Dendritic nickel porphyrin for mimicking deposition of contaminant nickel on FCC catalysts. Appl. Catal. A 2015, 495, 39–44. [Google Scholar] [CrossRef]

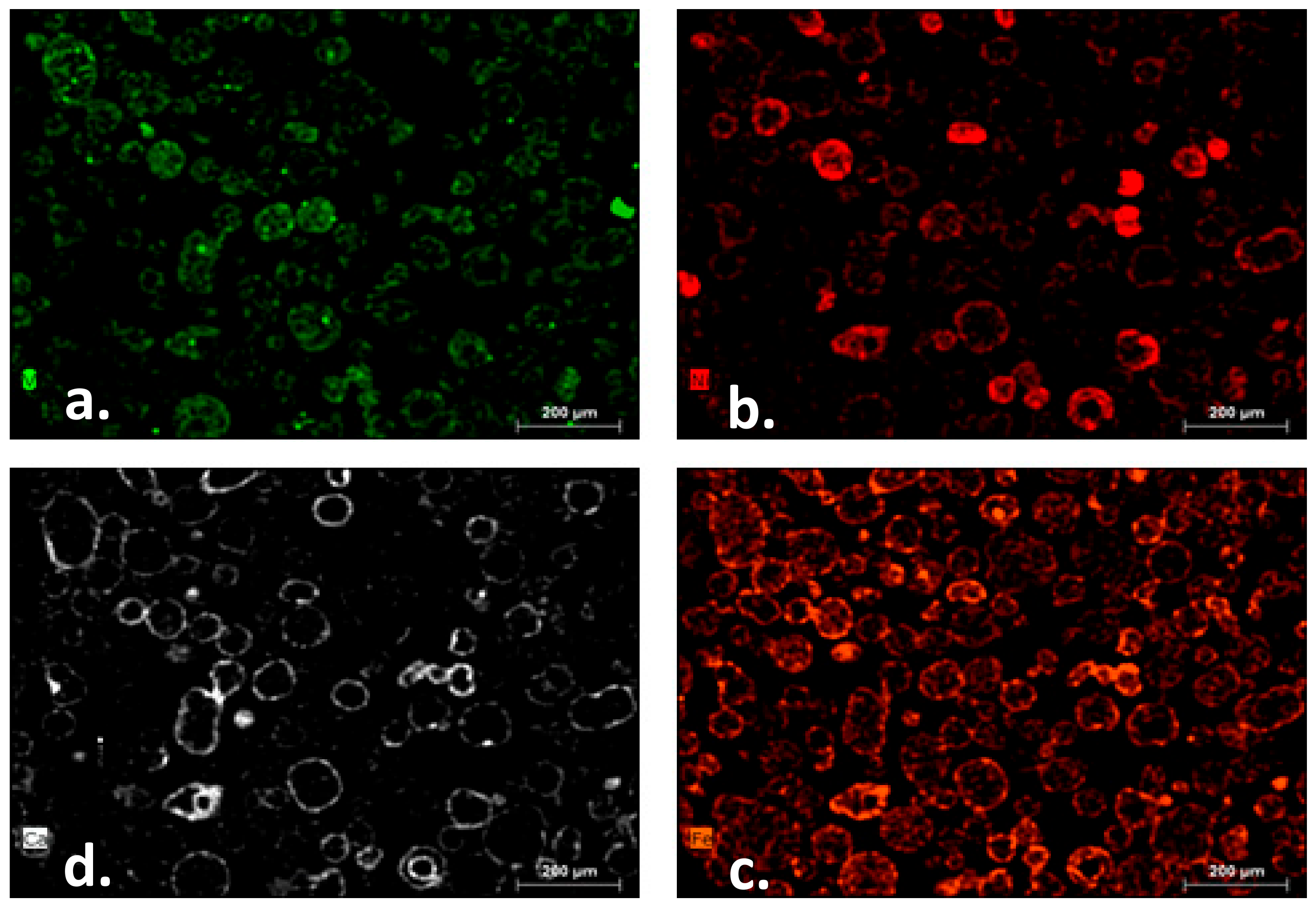


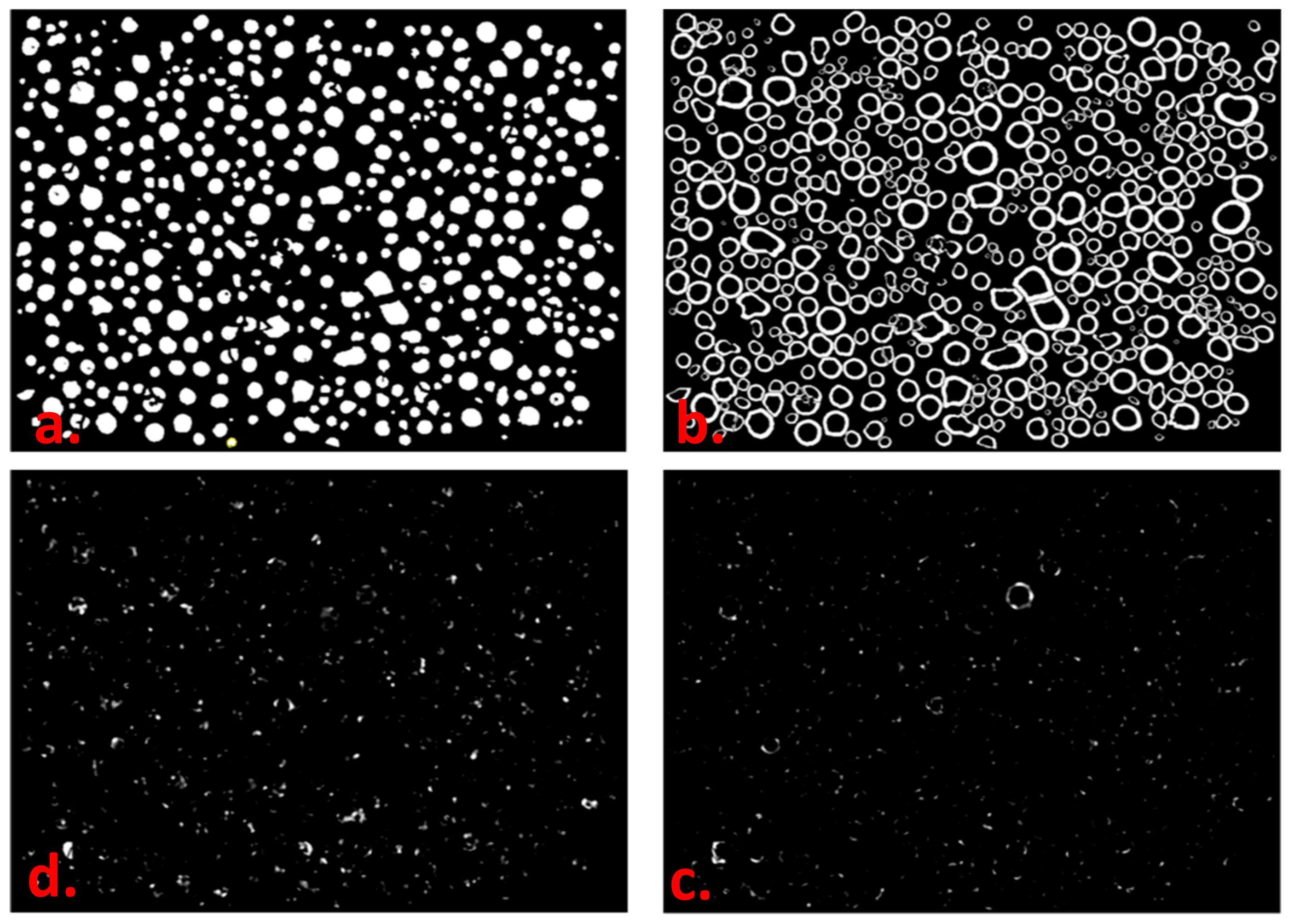
| Refinery | Al | V | Ni | Fe | Ca |
|---|---|---|---|---|---|
| A | 1.0 | 0.7 | 1.7 | 2.3 | 2.9 |
| B | 1.2 | 0.9 | 1.5 | 1.9 | 3.5 |
| C | 1.1 | 1.0 | 3.5 | 2.3 | n/a |
| Average | 1.1 | 0.9 | 2.2 | 2.2 | 3.2 |
| Refinery | Al | V | Ni | Fe | Added Fe | Ca |
|---|---|---|---|---|---|---|
| D | 1.1 | 1.4 | 2.2 | 1.6 | 2.2 | n/a |
| E | 1.2 | 1.6 | 1.9 | 1.9 | 2.1 | 3.0 |
| F | 1.0 | 1.6 | 2.5 | 1.4 | 2.7 | 3.3 |
| Average | 1.1 | 1.5 | 2.2 | 1.6 | 2.3 | 3.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senter, C.; Mastry, M.C.; Mannion, A.M.; McGuire, R., Jr.; Houtz, D.; Yilmaz, B. Quantitative Visual Characterization of Contaminant Metals and Their Mobility in Fluid Catalytic Cracking Catalysts. Catalysts 2019, 9, 831. https://doi.org/10.3390/catal9100831
Senter C, Mastry MC, Mannion AM, McGuire R Jr., Houtz D, Yilmaz B. Quantitative Visual Characterization of Contaminant Metals and Their Mobility in Fluid Catalytic Cracking Catalysts. Catalysts. 2019; 9(10):831. https://doi.org/10.3390/catal9100831
Chicago/Turabian StyleSenter, Corbett, Melissa Clough Mastry, Alexander M. Mannion, Robert McGuire, Jr., Daniel Houtz, and Bilge Yilmaz. 2019. "Quantitative Visual Characterization of Contaminant Metals and Their Mobility in Fluid Catalytic Cracking Catalysts" Catalysts 9, no. 10: 831. https://doi.org/10.3390/catal9100831




