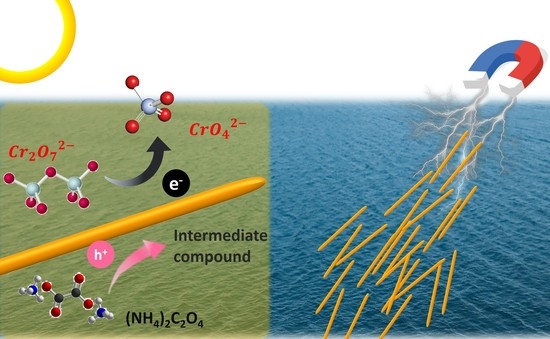
Enhanced Photocatalytic Reduction of Cr(VI) by Combined Magnetic TiO2-Based NFs and Ammonium Oxalate Hole Scavengers
Abstract
:1. Introduction
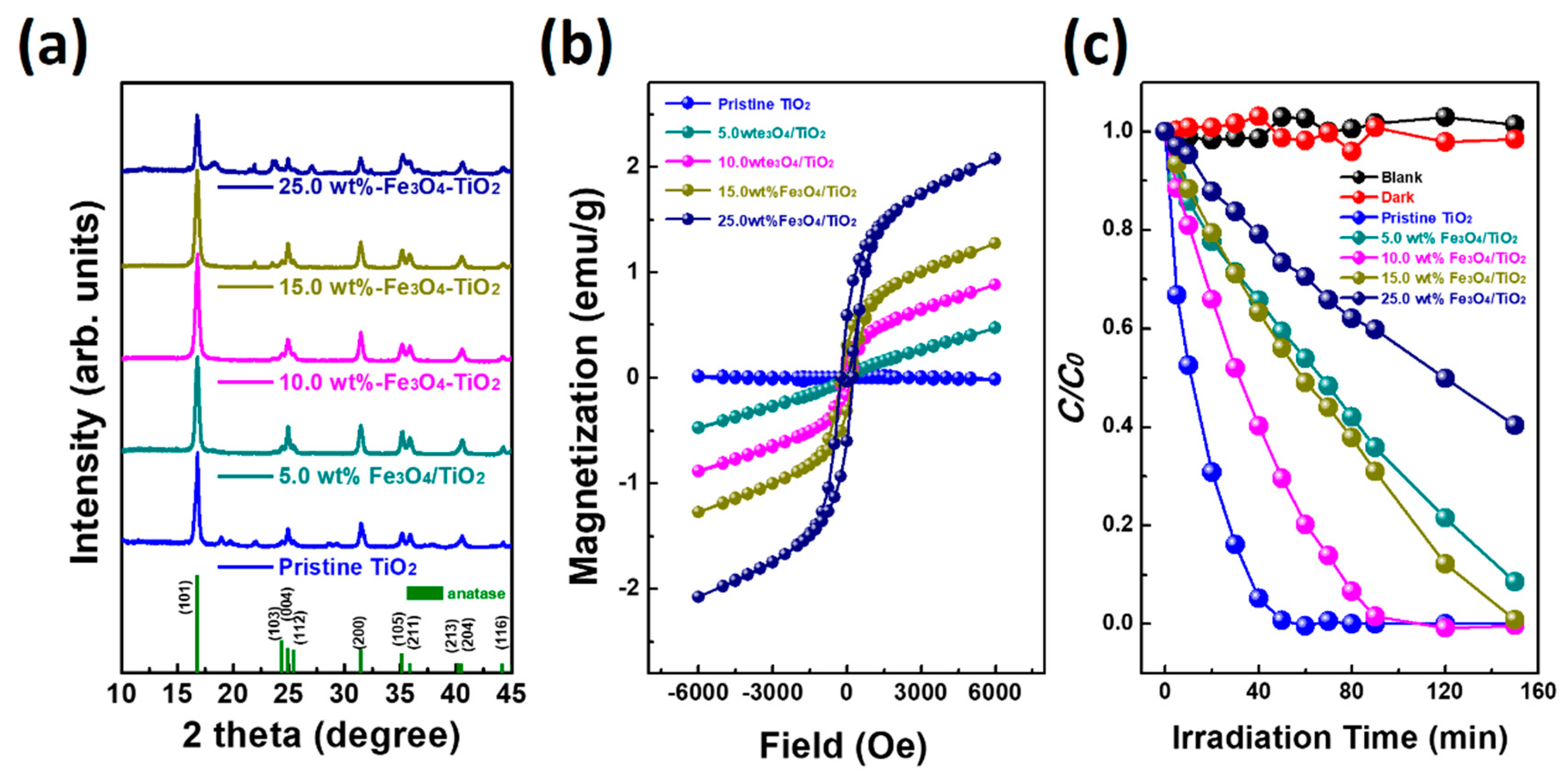
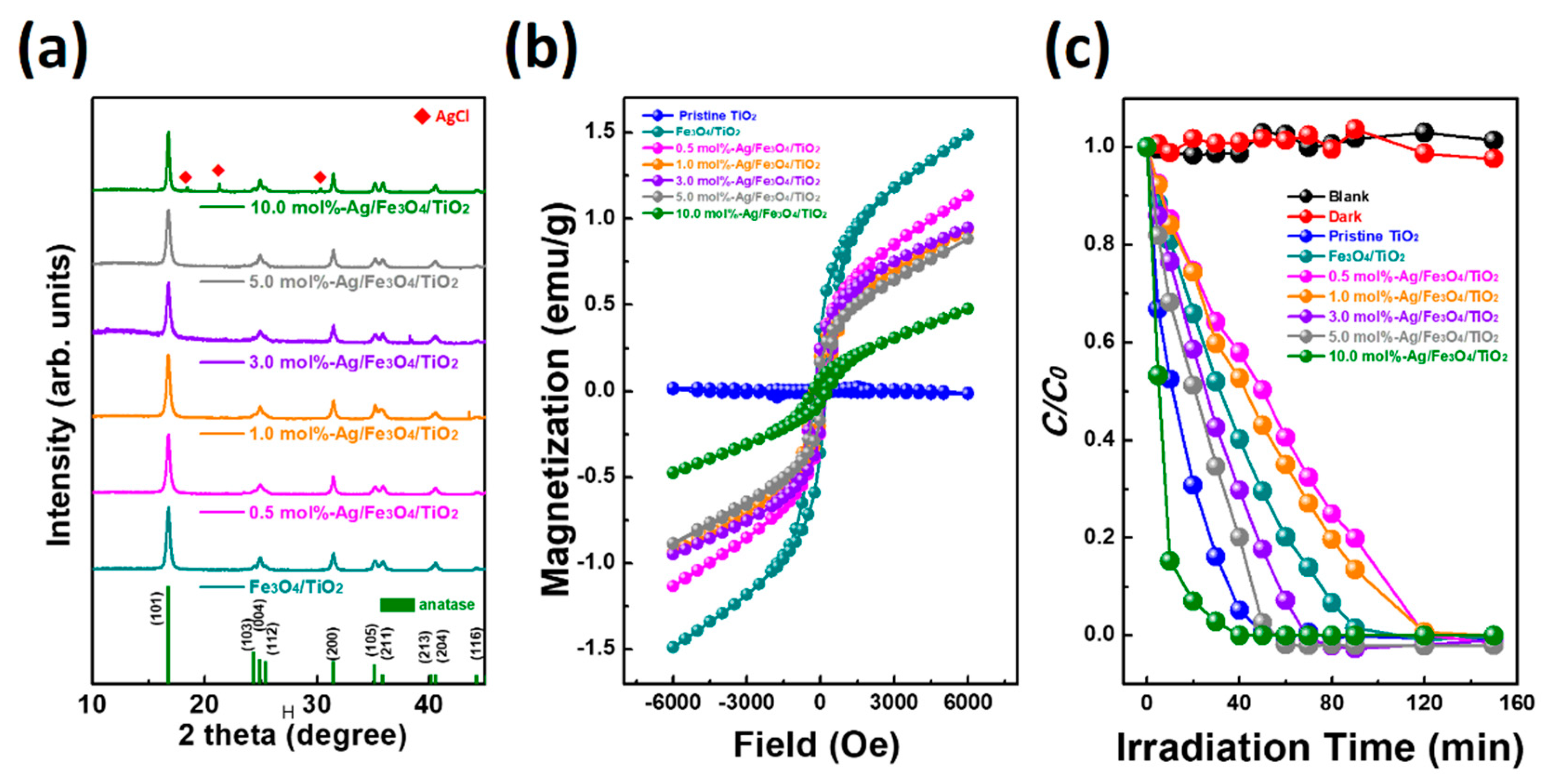
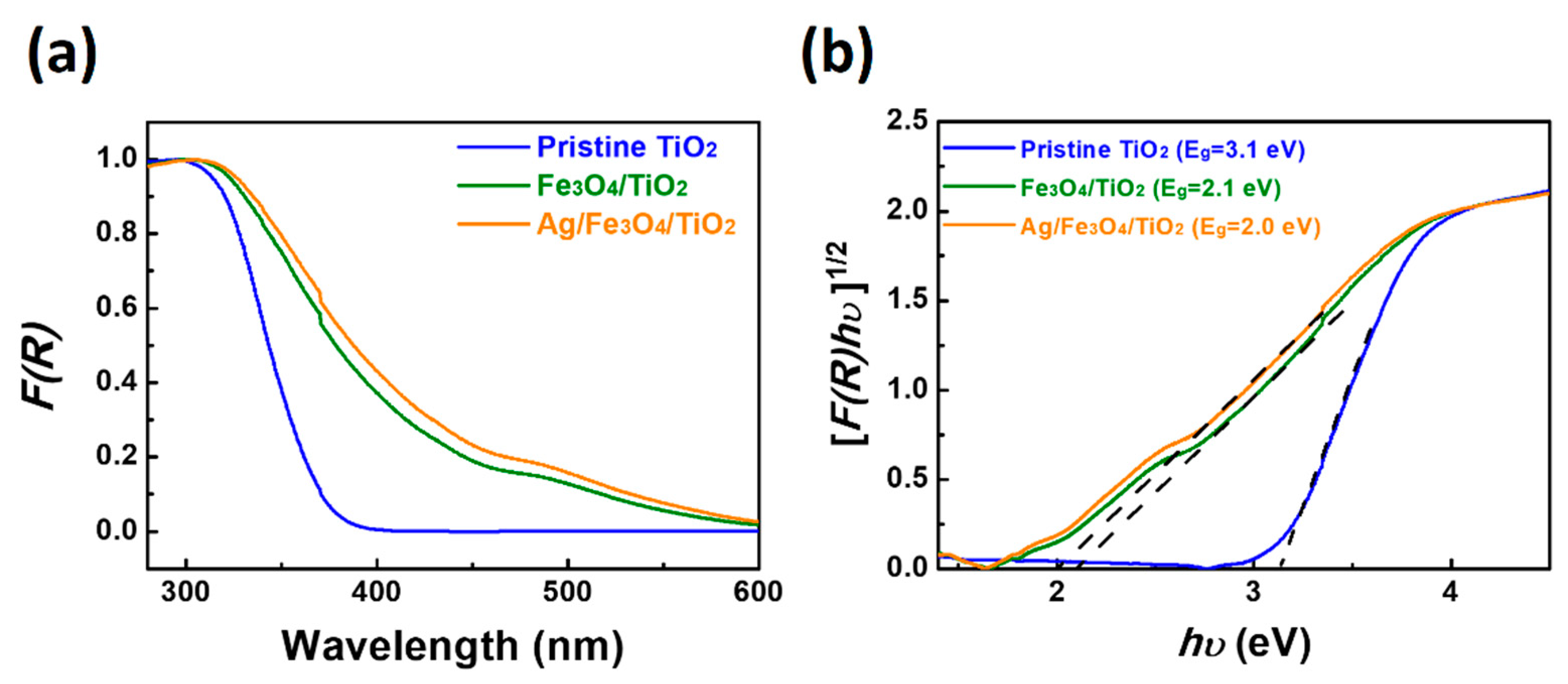
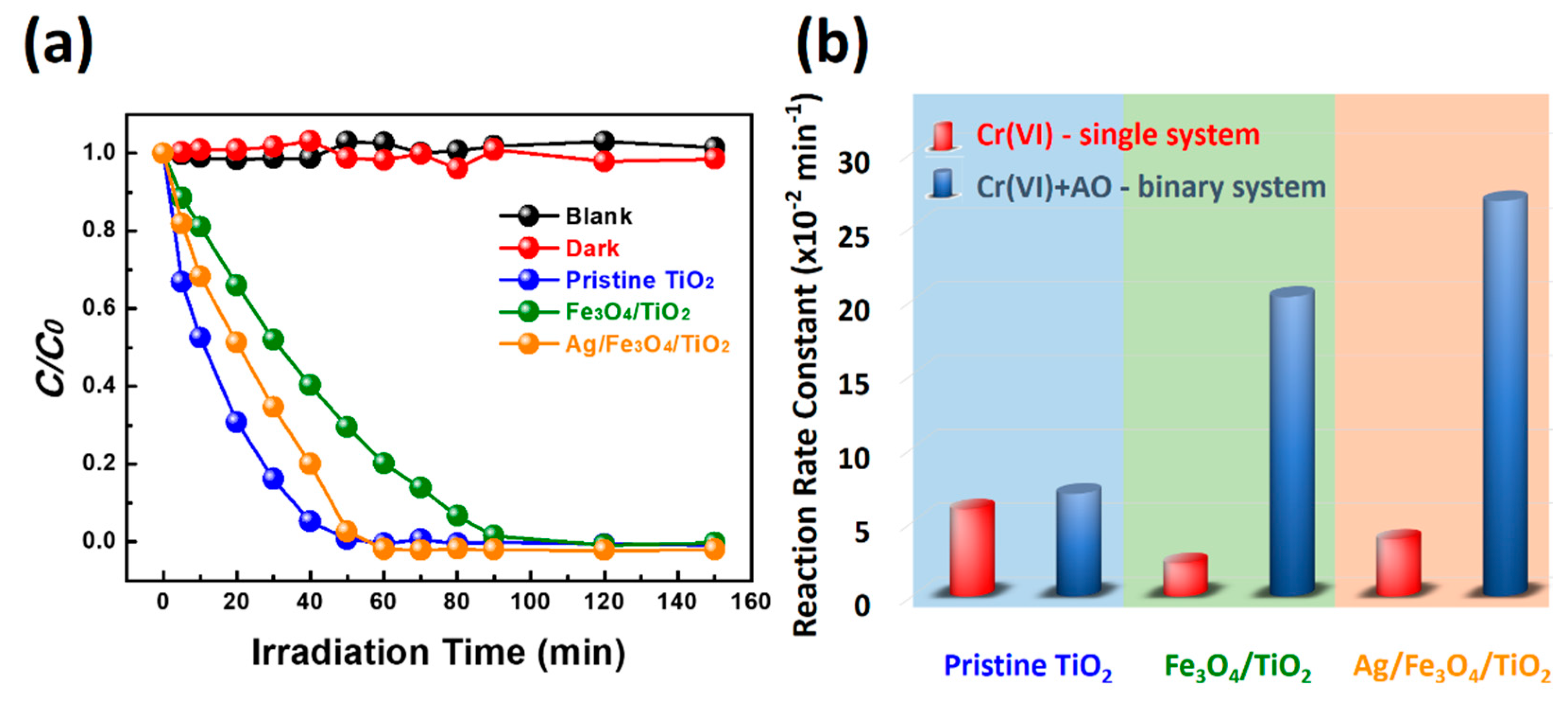
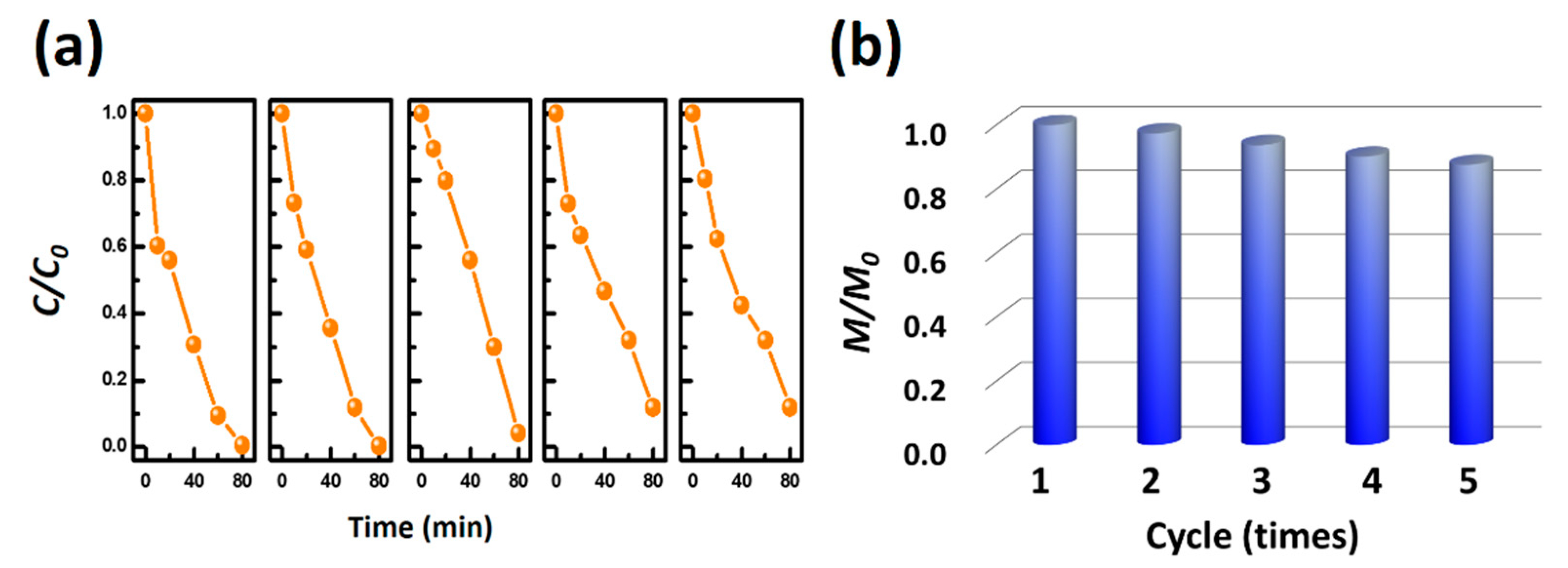
2. Results
3. Materials and Methods
3.1. Synthesis of Fe3O4 Magnetic NPs
3.2. Synthesis of Ag/Fe3O4/TiO2 NFs
3.3. Characterization
3.4. Photocatalytic Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Costa, M. Toxicity and Carcinogenicity of Cr(VI) in Animal Models and Humans. Crit. Rev. Toxicol. 1997, 27, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Rowbotham, A.L.; Levy, L.S.; Shuker, L.K. Chromium in the Environment: An Evaluation of Exposure of the UK General Population and Possible Adverse Health Effects. J. Toxicol. Environ. Health 2000, 3, 145–178. [Google Scholar] [CrossRef]
- Kongsricharoern, N.; Polprasert, C. Electrochemical Precipitation of Chromium (Cr6+) from an Electroplating Wastewater. Water Res. 1995, 31, 109–117. [Google Scholar] [CrossRef]
- Hunsom, M.; Pruksathorn, K.; Damronglerd, S.; Vergnes, H.; Duverneuil, P. Electrochemical Treatment of Heavy Metals (Cu2+, Cr6+, Ni2+) from Industrial Effluent and Modeling of Copper Reduction. Water Res. 2005, 39, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, M.; Samadi, S.; Yazd, S.S.; Jafari, P.; Yousefi, N.; Aliabadi, M. Selective Adsorption of Cr(VI) Ions from Aqueous Solutions Using Cr6+-Imprinted Pebax/Chitosan/GO/Aptes Nanofibrous Adsorbent. Int. J. Biol. Macromol. 2017, 95, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Yogeshwaran, V.; Priya, A.K. Removal of Hexavalent Chromium (Cr6+) Using Different Natural Adsorbents—A Review. J. Chromatogr. Sep. Tech. 2017, 8, 1000392. [Google Scholar] [CrossRef]
- Ohtake, H. New Biological Method for Detoxification and Removal of Hexavalent Chromium. Water Res. 1992, 25, 395–402. [Google Scholar] [CrossRef]
- Bennett, R.M.; Cordero, P.R.F.; Bautista, G.S.; Dedeles, G.R. Reduction of Hexavalent Chromium Using Fungi and Bacteria Isolated from Contaminated Soil and Water Samples. Chem. Ecol. 2013, 29, 320–328. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, X.; Wang, D. Electrically Regenerated Ion Exchange for Removal and Recovery of Cr(VI) from Wastewater. Environ. Sci. Technol. 2007, 41, 1439–1443. [Google Scholar] [CrossRef]
- Mekatel, H.; Amokrane, S.; Benturki, A.; Nibou, D. Treatment of Polluted Aqueous Solutions by Ni2+, Pb2+, Zn2+, Cr+6,Cd+2 and Co+2 Ions by Ion Exchange Process Using Faujasite Zeolite. Procedia Eng. 2012, 33, 52–57. [Google Scholar] [CrossRef]
- Cai, J.; Wu, X.; Zheng, F.; Li, S.; Wu, Y.; Lin, Y.; Lin, L.; Liu, B.; Chen, Q.; Lin, L. Influence of TiO2 Hollow Sphere Size on Its Photo-Reduction Activity for Toxic Cr(VI) Removal. J. Colloid Interface Sci. 2017, 490, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Yang, M.; Fang, P.; Li, C.; Jiang, L. Enhanced Photocatalytic Degradation of Aqueous Phenol and Cr(VI) over Visible-Light-Driven Tbxoy Loaded TiO2-Oriented Nanosheets. Appl. Surf. Sci. 2017, 399, 167–184. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Gao, F.; Mailhot, G.; Pan, G. Algae Decorated TiO2/Ag Hybrid Nanofiber Membrane with Enhanced Photocatalytic Activity for Cr(VI) Removal under Visible Light. Chem. Eng. J. 2017, 314, 622–630. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Wu, Y.; Chen, J.; Zhao, J.; Jin, F.; Na, P. Carbon Dots-TiO2 Nanosheets Composites for Photoreduction of Cr(VI) under Sunlight Illumination: Favorable Role of Carbon Dots. Appl. Catal. B 2018, 224, 508–517. [Google Scholar] [CrossRef]
- Wang, W.; Lai, M.; Fang, J.; Lu, C. Au and Pt Selectively Deposited on {001}-Faceted TiO2 toward SPR Enhanced Photocatalytic Cr(VI) Reduction: The Influence of Excitation Wavelength. Appl. Surf. Sci. 2018, 439, 430–438. [Google Scholar] [CrossRef]
- Ngo, A.; Nguyen, H.; Hollmann, D. Criticial Assessment of the Photocatalytic Reduction of Cr(VI) over Au/TiO2. Catalysts 2018, 8, 606. [Google Scholar] [CrossRef]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Visible-Light-Activated Oxygen-Rich TiO2 as Next Generation Photocatalyst: Importance of Annealing Temperature on the Photoactivity toward Reduction of Carbon Dioxide. Chem. Eng. J. 2016, 283, 1254–1263. [Google Scholar] [CrossRef]
- Busiakiewicz, A.; Kisielewska, A.; Piwoński, I.; Batory, D. The Effect of Fe Segregation on the Photocatalytic Growth of Ag Nanoparticles on Rutile TiO2 (001). Appl. Surf. Sci. 2017, 401, 378–384. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface Modification and Enhanced Photocatalytic CO2 Reduction Performance of TiO2: A Review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Photocatalytic Reduction of CO2 with H2O over Graphene Oxide-Supported Oxygen-Rich TiO2 Hybrid Photocatalyst under Visible Light Irradiation: Process and Kinetic Studies. Chem. Eng. J. 2017, 308, 248–255. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, D.; Zhu, L.; Wang, B. Highly Ordered Fe3+/TiO2 Nanotube Arrays for Efficient Photocataltyic Degradation of Nitrobenzene. Appl. Surf. Sci. 2017, 420, 896–904. [Google Scholar] [CrossRef]
- Duan, Y.; Liang, L.; Lv, K.; Li, Q.; Li, M. TiO2 Faceted Nanocrystals on the Nanofibers: Homojunction TiO2 Based Z-Scheme Photocatalyst for Air Purification. Appl. Surf. Sci. 2018, 456, 817–826. [Google Scholar] [CrossRef]
- Makal, P.; Das, D. Self-Doped TiO2 Nanowires in TiO2-B Single Phase, TiO2-B/Anatase and TiO2-Anatase/Rutile Heterojunctions Demonstrating Individual Superiority in Photocatalytic Activity under Visible and UV Light. Appl. Surf. Sci. 2018, 455, 1106–1115. [Google Scholar] [CrossRef]
- Wu, M.-C.; Hsiao, K.-C.; Chang, Y.-H.; Chan, S.-H. Photocatalytic Hydrogen Evolution of Palladium Nanoparticles Decorated Black TiO2 Calcined in Argon Atmosphere. Appl. Surf. Sci. 2018, 430, 407–414. [Google Scholar] [CrossRef]
- Jing, J.; Li, J.; Feng, J.; Li, W.; Yu, W.W. Photodegradation of Quinoline in Water over Magnetically Separable Fe3O4/TiO2 Composite Photocatalysts. Chem. Eng. J. 2013, 219, 355–360. [Google Scholar] [CrossRef]
- Li, Z.-J.; Huang, Z.-W.; Guo, W.-L.; Wang, L.; Zheng, L.-R.; Chai, Z.-F.; Shi, W.-Q. Enhanced Photocatalytic Removal of Uranium(VI) from Aqueous Solution by Magnetic TiO2/Fe3O4 and Its Graphene Composite. Environ. Sci. Technol. 2017, 51, 5666–5674. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, L.; Cheng, J.; Jing, C. Mechanistic Insights into TiO2 Thickness in Fe3O4@TiO2-GO Composites for Enrofloxacin Photodegradation. Chem. Eng. J. 2017, 325, 647–654. [Google Scholar] [CrossRef]
- Liu, M.-C.; Liu, B.; Sun, X.-Y.; Lin, H.-C.; Lu, J.-Z.; Jin, S.-F.; Yan, S.-Q.; Li, Y.-Y.; Zhao, P. Core/Shell Structured Fe3O4@TiO2-DNM Nanospheres as Multifunctional Anticancer Platform: Chemotherapy and Photodynamic Therapy Research. J. Nanosci. Nanotechnol. 2018, 18, 4445–4456. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xiao, X.; Zhang, S.; Ren, F.; Jiang, C. Facile Method to Synthesize Magnetic Iron Oxides/TiO2 Hybrid Nanoparticles and Their Photodegradation Application of Methylene Blue. Nanoscale Res. Lett. 2011, 6, 533. [Google Scholar] [CrossRef]
- Shojaei, A.F.; Shams-Nateri, A.; Ghomashpasand, M. Magnetically Recyclable Fe3+/TiO2@Fe3O4Nanocomposites Towards Degradation of Direct Blue 71 under Visible-Light Irradiation. IET Micro Nano Lett. 2017, 12, 161–165. [Google Scholar] [CrossRef]
- Jia, X.; Dai, R.; Lian, D.; Han, S.; Wu, X.; Song, H. Facile Synthesis and Enhanced Magnetic, Photocatalytic Properties of One-Dimensional Ag@Fe3O4-TiO2. Appl. Surf. Sci. 2017, 392, 268–276. [Google Scholar] [CrossRef]
- Wang, H.; Fei, X.; Wang, L.; Li, Y.; Xu, S.; Sun, M.; Sun, L.; Zhang, C.; Li, Y.; Yang, Q.; et al. Magnetically Separable Iron Oxide Nanostructures-TiO2 Nanofibers Hierarchical Heterostructures: Controlled Fabrication and Photocatalytic Activity. New J. Chem. 2011, 35, 1795–1802. [Google Scholar] [CrossRef]
- Jafari, A.; Shayesteh, S.F.; Salouti, M.; Boustani, K. Effect of Annealing Temperature on Magnetic Phase Transition in Fe3O4 Nanoparticles. J. Magn. Magn. Mater. 2015, 379, 305–312. [Google Scholar] [CrossRef]
- Wang, W.-K.; Chen, J.-J.; Gao, M.; Huang, Y.-X.; Zhang, X.; Yu, H.-Q. Photocatalytic Degradation of Atrazine by Boron-Doped TiO2 with a Tunable Rutile/Anatase Ratio. Appl. Catal. B 2016, 195, 69–76. [Google Scholar] [CrossRef]
- Rossi, G.; Pasquini, L.; Catone, D.; Piccioni, A.; Patelli, N.; Paladini, A.; Molinari, A.; Caramori, S.; O’Keeffe, P.; Boscherini, F. Charge Carrier Dynamics and Visible Light Photocatalysis in Vanadium-Doped TiO2 Nanoparticles. Appl. Catal. B 2018, 237, 603–612. [Google Scholar] [CrossRef]
- Wu, M.-C.; Wu, P.-Y.; Lin, T.-H.; Lin, T.-F. Photocatalytic Performance of Cu-Doped TiO2 Nanofibers Treated by the Hydrothermal Synthesis and Air-Thermal Treatment. Appl. Surf. Sci. 2018, 430, 390–398. [Google Scholar] [CrossRef]
- Avilés-García, O.; Espino-Valencia, J.; Romero-Romero, R.; Rico-Cerda, J.; Arroyo-Albiter, M.; Solís-Casados, D.; Natividad-Rangel, R. Enhanced Photocatalytic Activity of Titania by Co-Doping with Mo and W. Catalysts 2018, 8, 631. [Google Scholar] [CrossRef]
- Ramírez-Sánchez, I.; Bandala, E. Photocatalytic Degradation of Estriol Using Iron-Doped TiO2 under High and Low UV Irradiation. Catalysts 2018, 8, 625. [Google Scholar] [CrossRef]
- Ali, T.; Ahmed, A.; Alam, U.; Uddin, I.; Tripathi, P.; Muneer, M. Enhanced Photocatalytic and Antibacterial Activities of Ag-Doped TiO2 Nanoparticles under Visible Light. Mater. Chem. Phys 2018, 212, 325–335. [Google Scholar] [CrossRef]
- Mandari, K.K.; Do, J.Y.; Police, A.K.R.; Kang, M. Natural Solar Light-Driven Preparation of Plasmonic Resonance-Based Alloy and Core-Shell Catalyst for Sustainable Enhanced Hydrogen Production: Green Approach and Characterization. Appl. Catal. B 2018, 231, 137–150. [Google Scholar] [CrossRef]
- Chen, S.-H.; Chan, S.-H.; Lin, Y.-T.; Wu, M.-C. Enhanced Power Conversion Efficiency of Perovskite Solar Cells Based on Mesoscopic Ag-Doped TiO2 Electron Transport Layer. Appl. Surf. Sci. 2019, 469, 18–26. [Google Scholar] [CrossRef]
- Wu, M.-C.; Liao, Y.-H.; Chan, S.-H.; Lu, C.-F.; Su, W.-F. Enhancing Organolead Halide Perovskite Solar Cells Performance through Interfacial Engineering Using Ag-Doped TiO2 Hole Blocking Layer. Sol. RRL 2018, 2, 1800072. [Google Scholar] [CrossRef]
- Akel, S.; Dillert, R.; Balayeva, N.; Boughaled, R.; Koch, J.; El Azzouzi, M.; Bahnemann, D. Ag/Ag2O as a Co-Catalyst in TiO2 Photocatalysis: Effect of the Co-Catalyst/Photocatalyst Mass Ratio. Catalysts 2018, 8, 647. [Google Scholar] [CrossRef]
- Wen, L.; Liu, B.; Zhao, X.; Nakata, K.; Murakami, T.; Fujishima, A. Synthesis, Characterization, and Photocatalysis of Fe-Doped TiO2: A Combined Experimental and Theoretical Study. Int. J. Photoenergy 2012, 2012, 368750. [Google Scholar] [CrossRef]
- Zhan, J.; Zhang, H.; Zhu, G. Magnetic Photocatalysts of Cenospheres Coated with Fe3O4/TiO2 Core/Shell Nanoparticles Decorated with Ag Nanopartilces. Ceram. Int. 2014, 40, 8547–8559. [Google Scholar] [CrossRef]
- Mogal, S.; Gandhi, V.G.; Mishra, M.; Tripathi, S.; Shripathi, T.; Joshi, P.; Shah, D. Single-Step Synthesis of Silver-Doped Titanium Dioxide: Influence of Silver on Structural, Textural, and Photocatalytic Properties. Ind. Eng. Chem. Res. 2014, 53, 5749–5758. [Google Scholar] [CrossRef]


| Sample | Fe/Ti (%) | Ag/Ti (%) |
|---|---|---|
| Pristine TIO2 | 0.0 | 0.0 |
| Fe3O4/TiO2 | 2.9 | 0.0 |
| Ag/Fe3O4/ TiO2 | 3.1 | 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-H.; Wu, M.-C. Enhanced Photocatalytic Reduction of Cr(VI) by Combined Magnetic TiO2-Based NFs and Ammonium Oxalate Hole Scavengers. Catalysts 2019, 9, 72. https://doi.org/10.3390/catal9010072
Chang Y-H, Wu M-C. Enhanced Photocatalytic Reduction of Cr(VI) by Combined Magnetic TiO2-Based NFs and Ammonium Oxalate Hole Scavengers. Catalysts. 2019; 9(1):72. https://doi.org/10.3390/catal9010072
Chicago/Turabian StyleChang, Yin-Hsuan, and Ming-Chung Wu. 2019. "Enhanced Photocatalytic Reduction of Cr(VI) by Combined Magnetic TiO2-Based NFs and Ammonium Oxalate Hole Scavengers" Catalysts 9, no. 1: 72. https://doi.org/10.3390/catal9010072