Dry Reforming of Propane over γ-Al2O3 and Nickel Foam Supported Novel SrNiO3 Perovskite Catalyst
Abstract
:1. Introduction
2. Result and Discussion
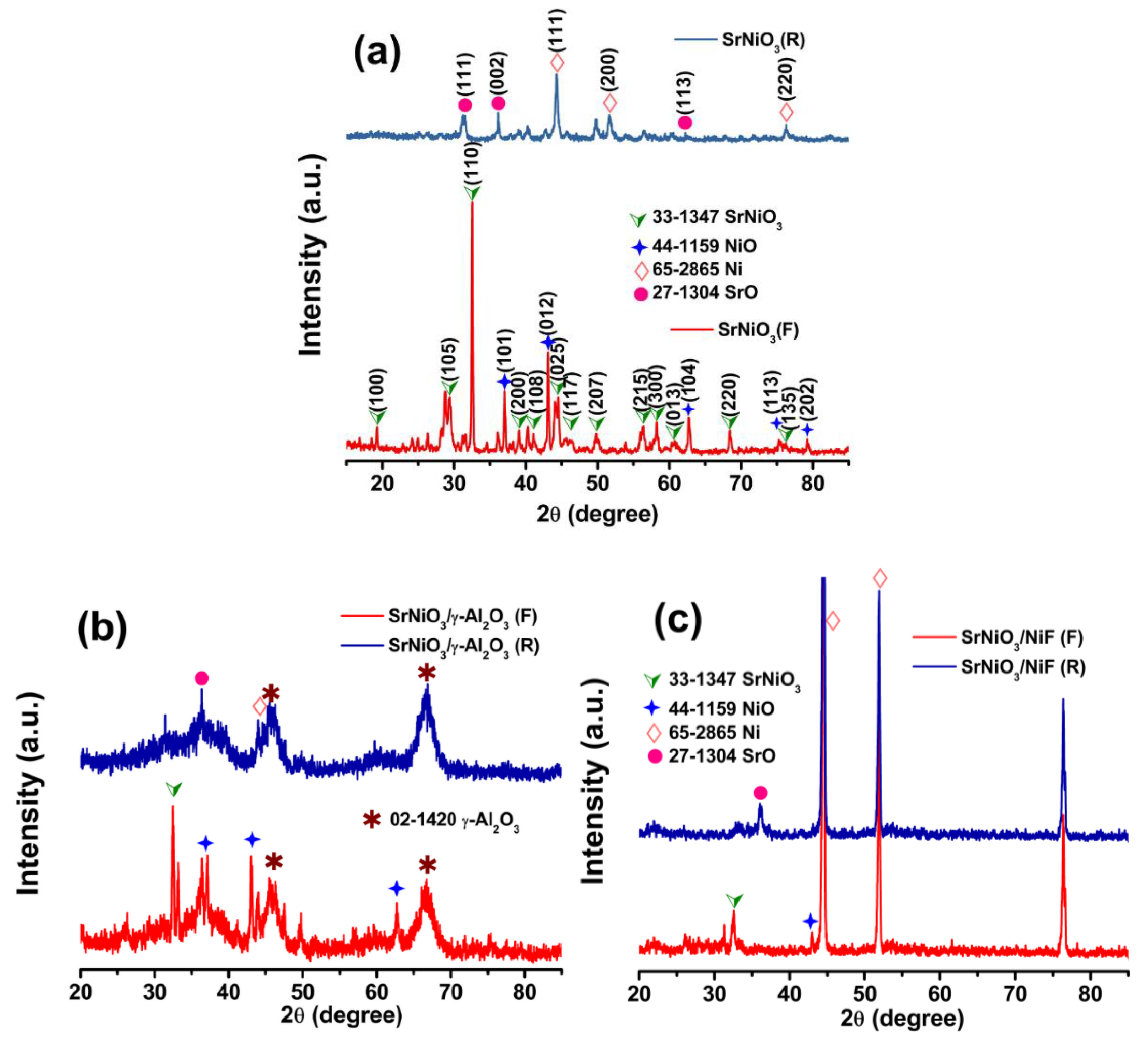
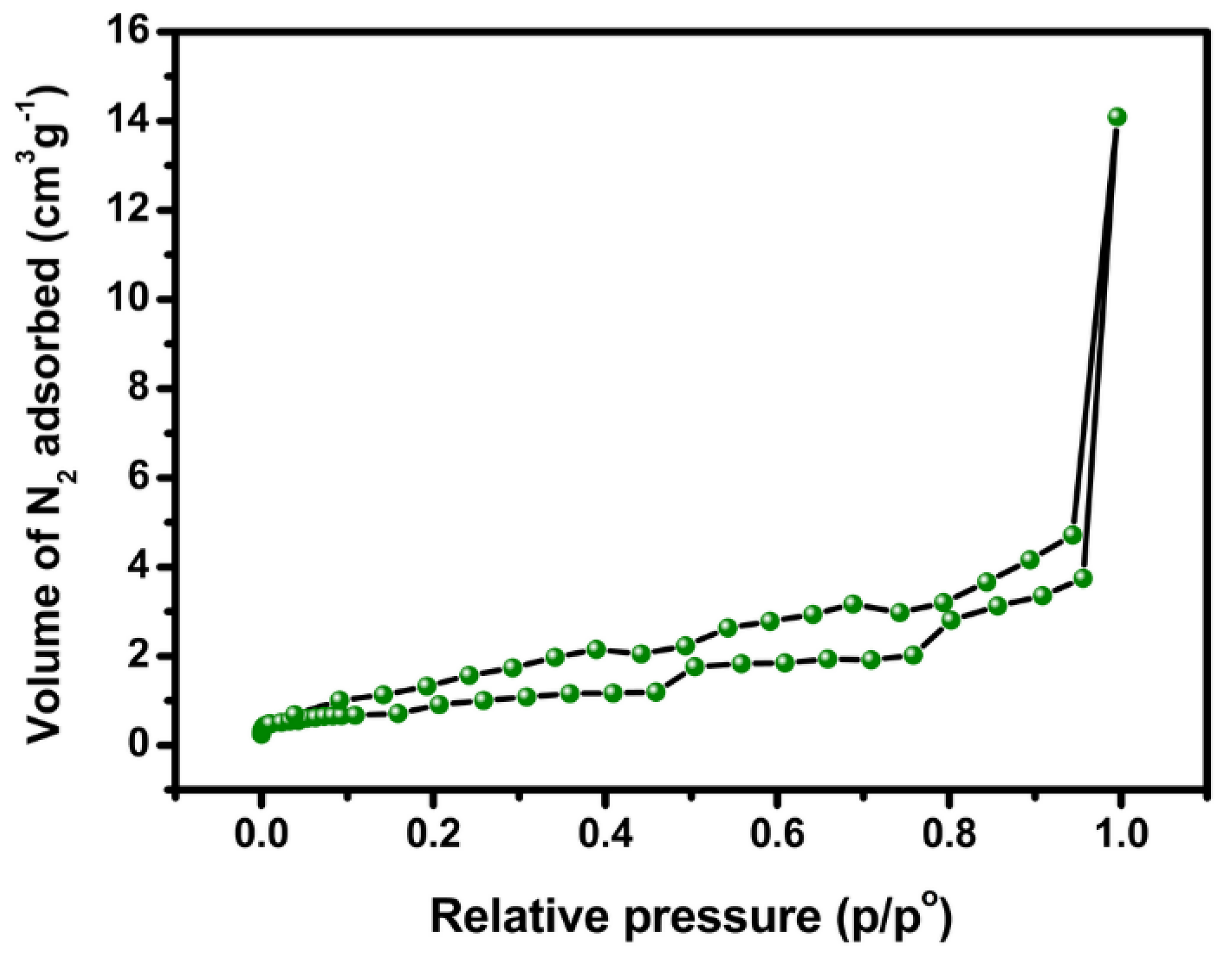
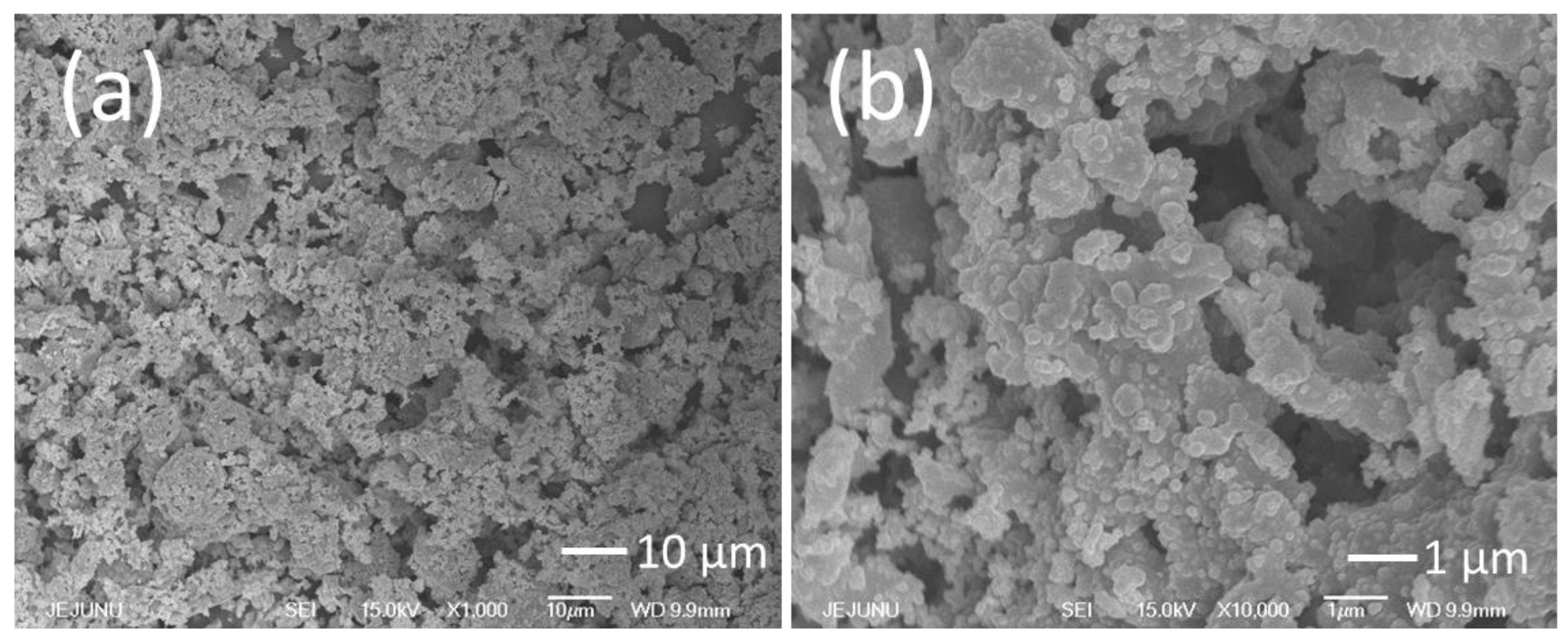
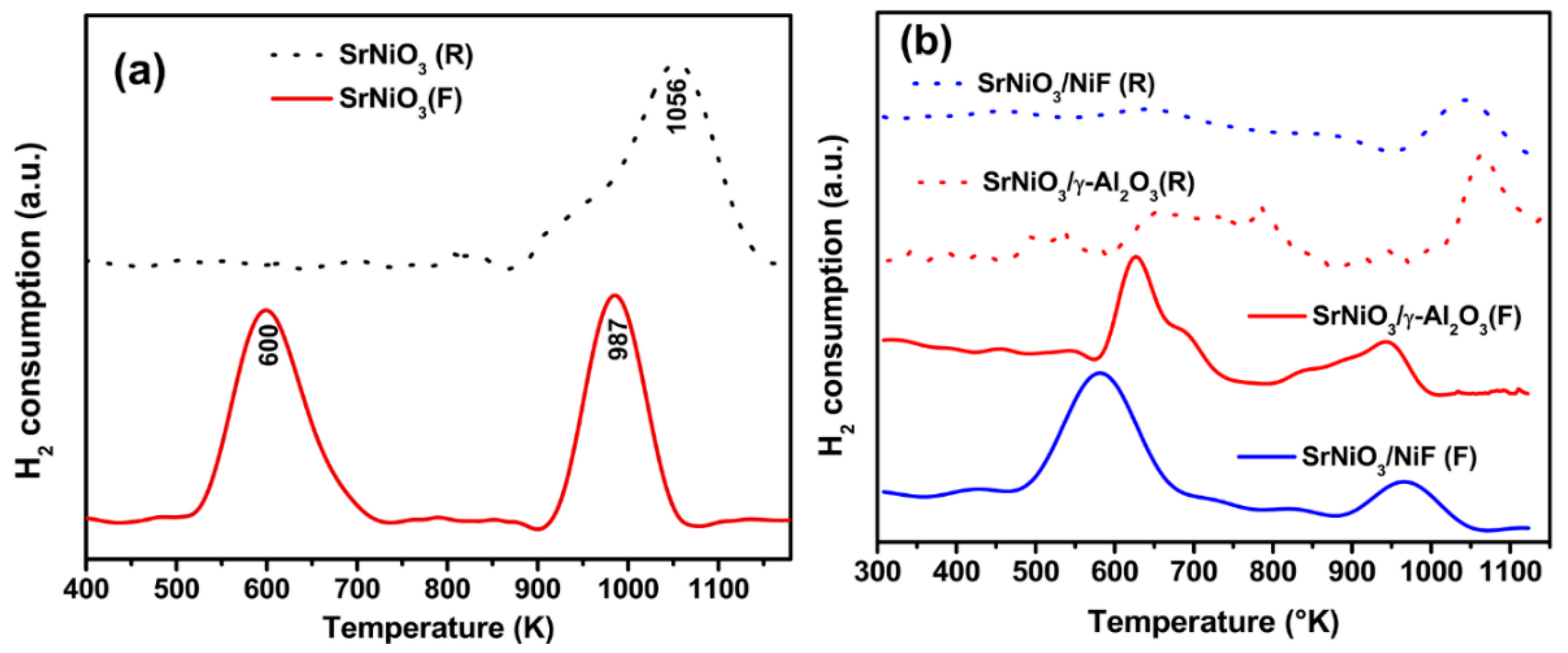
2.1. Materials Characterization
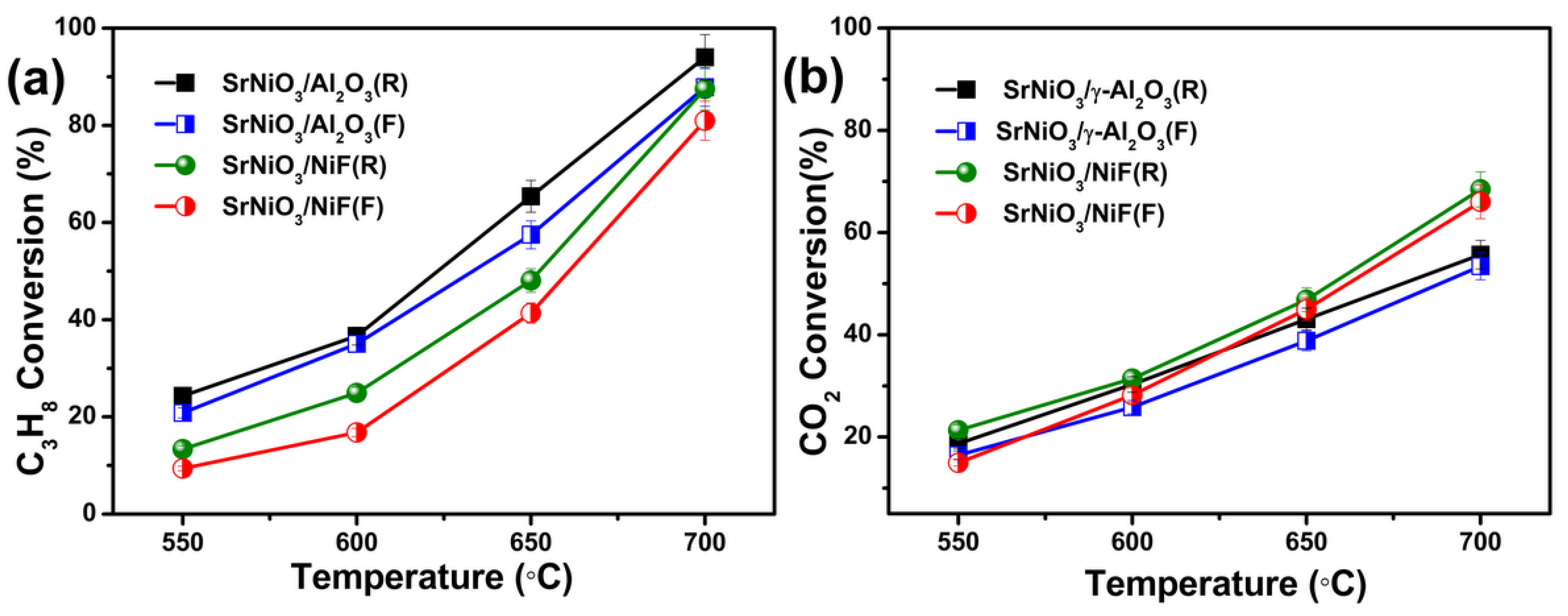
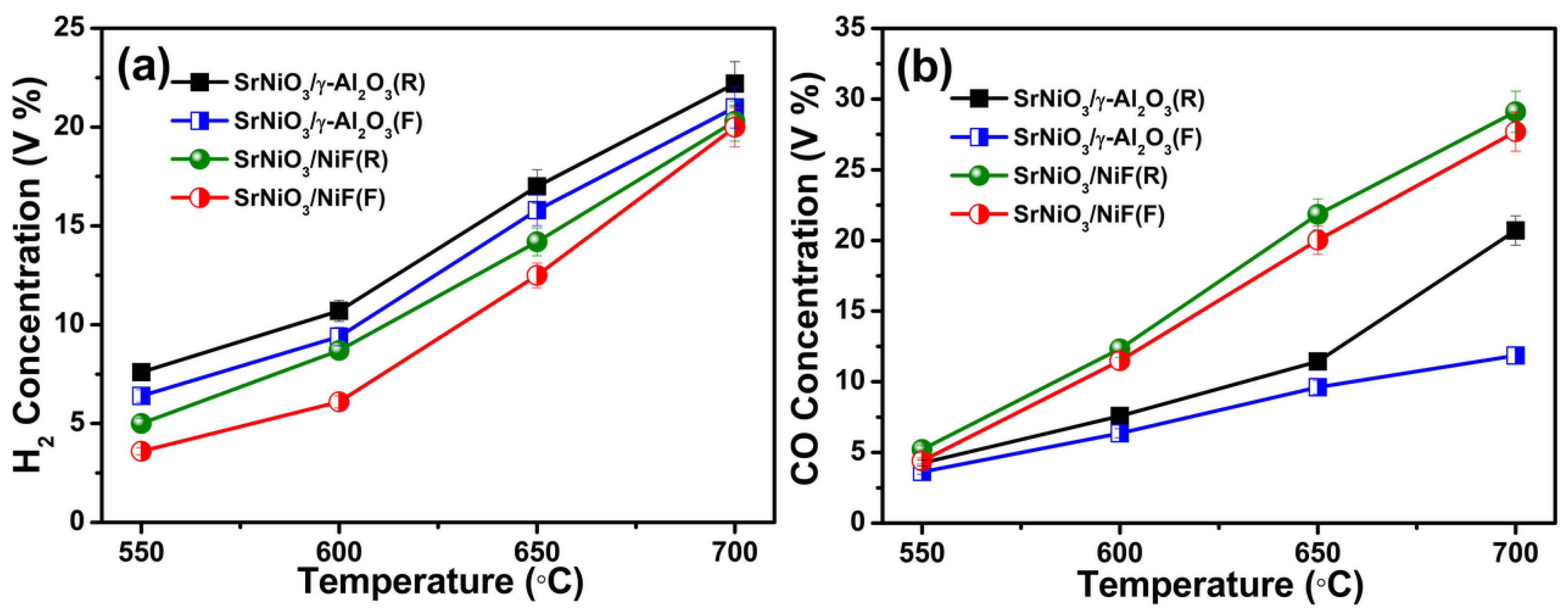
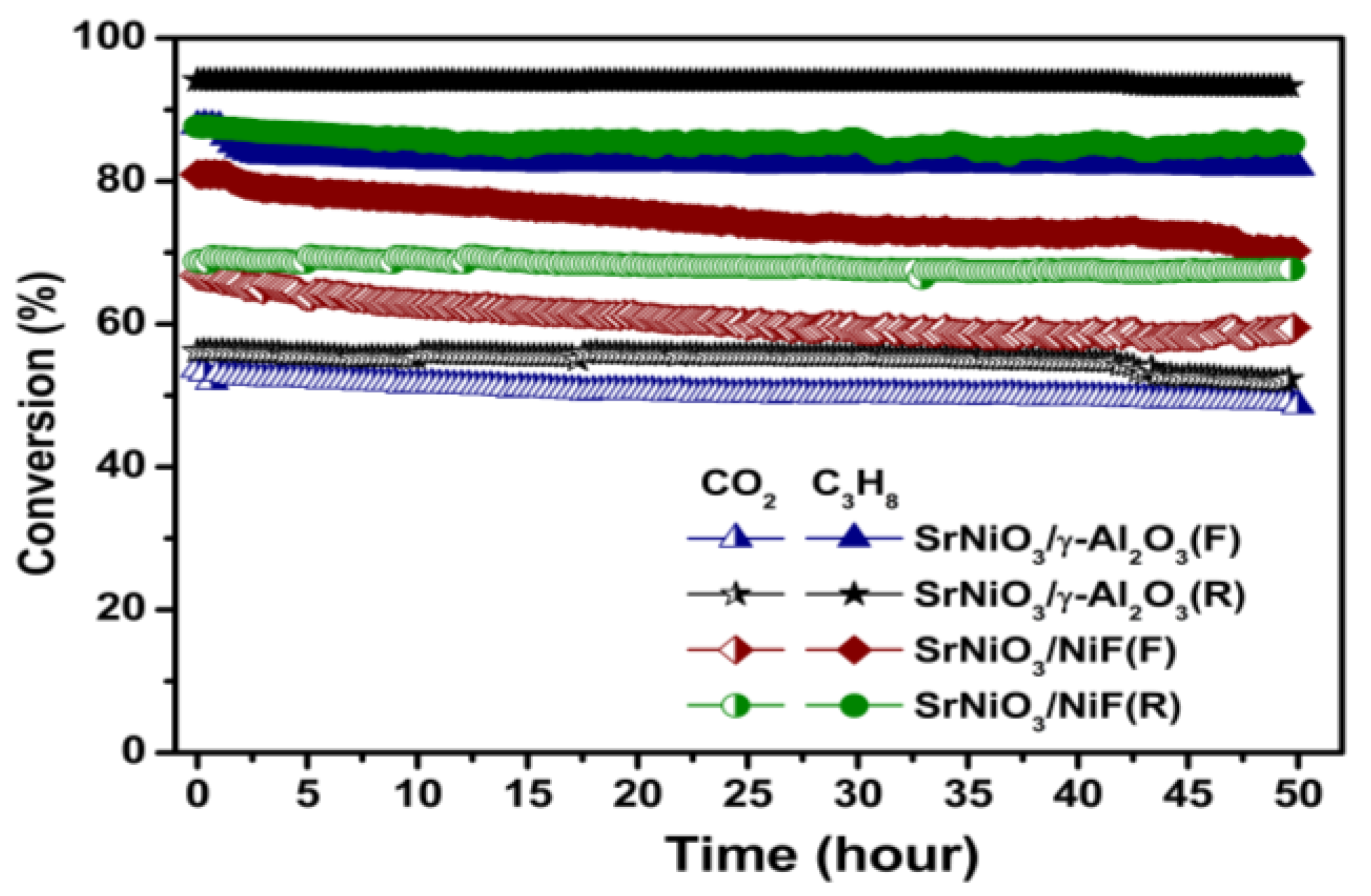
2.2. Catalytic Activity
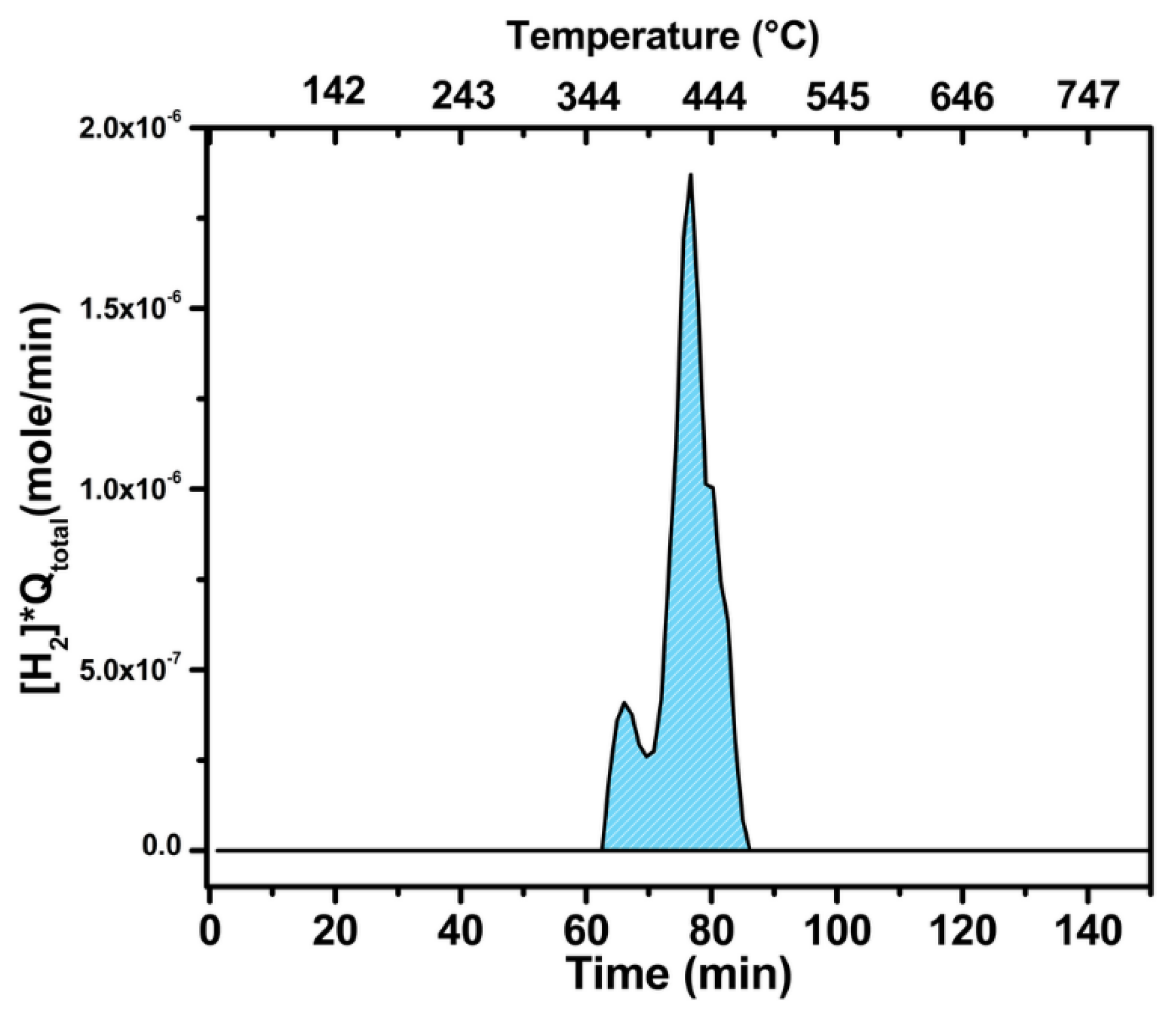
2.3. Catalytic Characterization of Spent Catalyst
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of SrNiO3 Compound
3.3. Preparation of Catalyst
3.4. Material Characterization
3.5. Catalytic Activity and Selectivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Yeung, C.M.Y.; Ni, J.; Meunier, F.; Acerbi, N.; Fowles, M.; Tsang, S.C. Methane steam reforming for hydrogen production using low water-ratios without carbon formation over ceria coated Ni catalysts. Appl. Catal. A Gen. 2008, 345, 119–127. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Maneerung, T.; Kathiraser, Y.; Kawi, S. CO2 dry-reforming of methane over La0.8Sr0.2Ni0.8M0.2O3 perovskite (M = Bi, Co, Cr, Cu, Fe): Roles of lattice oxygen on C–H activation and carbon suppression. Int. J. Hydrogen Energy 2012, 37, 11195–11207. [Google Scholar] [CrossRef]
- Sudhakaran, M.S.P.; Sultana, L.; Hossain, M.M.; Pawlat, J.; Diatczyk, J.; Brüser, V.; Reuter, S.; Mok, Y.S. Iron–ceria spinel (FeCe2O4) catalyst for dry reforming of propane to inhibit carbon formation. J. Ind. Eng. Chem. 2018, 61, 142–151. [Google Scholar] [CrossRef]
- Todic, B.; Nowicki, L.; Nikacevic, N.; Bukur, D.B. Fischer–Tropsch synthesis product selectivity over an industrial iron-based catalyst: Effect of process conditions. Catal. Today 2016, 261, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Siahvashi, A.; Adesina, A.A. Synthesis gas production via propane dry (CO2) reforming: Influence of potassium promotion on bimetallic Mo-Ni/Al2O3. Catal. Today 2013, 214, 30–41. [Google Scholar] [CrossRef]
- Staniforth, J.; Evans, S.E.; Good, O.J.; Darton, R.J.; Ormerod, R.M. A novel perovskite based catalyst with high selectivity and activity for partial oxidation of methane for fuel cell applications. Dalton Trans. 2014, 43, 15022–15027. [Google Scholar] [CrossRef] [PubMed]
- Nojoumi, H.; Dincer, I.; Naterer, G.F. Greenhouse gas emissions assessment of hydrogen and kerosene-fueled aircraft propulsion. Int. J. Hydrogen Energy 2009, 34, 1363–1369. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Shafer, W.D.; Davis, B.H. Fischer–Tropsch synthesis: Activity of metallic phases of cobalt supported on silica. Catal. Today 2013, 215, 13–17. [Google Scholar] [CrossRef]
- Pashchenko, D. Thermodynamic equilibrium analysis of combined dry and steam reforming of propane for thermochemical waste-heat recuperation. Int. J. Hydrogen Energy 2017, 42, 14926–14935. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Karuppiah, J.; Mok, Y.S. Plasma-reduced Ni/γ–Al2O3 and CeO2–Ni/γ–Al2O3 catalysts for improving dry reforming of propane. Int. J. Hydrogen Energy 2014, 39, 16329–16338. [Google Scholar] [CrossRef]
- Malaibari, Z.O.; Amin, A.; Croiset, E.; Epling, W. Performance characteristics of Mo–Ni/Al2O3 catalysts in LPG oxidative steam reforming for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 10061–10073. [Google Scholar] [CrossRef]
- Barroso-Quiroga, M.M.; Castro-Luna, A.E. Catalytic activity and effect of modifiers on Ni-based catalysts for the dry reforming of methane. Int. J. Hydrogen Energy 2010, 35, 6052–6056. [Google Scholar] [CrossRef]
- Foo, S.Y.; Cheng, C.K.; Nguyen, T.-H.; Adesina, A.A. Kinetic study of methane CO2 reforming on Co–Ni/Al2O3 and Ce–Co–Ni/Al2O3 catalysts. Catal. Today 2011, 164, 221–226. [Google Scholar] [CrossRef]
- Corthals, S.; Van Nederkassel, J.; Geboers, J.; De Winne, H.; Van Noyen, J.; Moens, B.; Sels, B.; Jacobs, P. Influence of composition of MgAl2O4 supported NiCeO2ZrO2 catalysts on coke formation and catalyst stability for dry reforming of methane. Catal. Today 2008, 138, 28–32. [Google Scholar] [CrossRef]
- Yamazoe, N.; Teraoka, Y. Oxidation catalysis of perovskites—Relationships to bulk structure and composition (valency, defect, etc.). Catal. Today 1990, 8, 175–199. [Google Scholar] [CrossRef]
- Rynkowski, J.; Samulkiewicz, P.; Ladavos, A.K.; Pomonis, P.J. Catalytic performance of reduced La2−xSrxNiO4 perovskite-like oxides for CO2 reforming of CH4. Appl. Catal. A Gen. 2004, 263, 1–9. [Google Scholar] [CrossRef]
- Mawdsley, J.R.; Krause, T.R. Rare earth-first-row transition metal perovskites as catalysts for the autothermal reforming of hydrocarbon fuels to generate hydrogen. Appl. Catal. A Gen. 2008, 334, 311–320. [Google Scholar] [CrossRef]
- Dinka, P.; Mukasyan, A.S. Perovskite catalysts for the auto-reforming of sulfur containing fuels. J. Power Sources 2007, 167, 472–481. [Google Scholar] [CrossRef]
- Gallego, G.S.; Mondragón, F.; Barrault, J.; Tatibouët, J.-M.; Batiot-Dupeyrat, C. CO2 reforming of CH4 over La–Ni based perovskite precursors. Appl. Catal. A Gen. 2006, 311, 164–171. [Google Scholar] [CrossRef]
- Chawl, S.K.; George, M.; Patel, F.; Patel, S. Production of Synthesis Gas by Carbon Dioxide Reforming of Methane over Nickel based and Perovskite Catalysts. Procedia Eng. 2013, 51, 461–466. [Google Scholar] [CrossRef]
- Nair, M.M.; Kaliaguine, S.; Kleitz, F. Nanocast LaNiO3 Perovskites as Precursors for the Preparation of Coke-Resistant Dry Reforming Catalysts. ACS Catal. 2014, 4, 3837–3846. [Google Scholar] [CrossRef]
- Kondakindi, R.R.; Kundu, A.; Karan, K.; Peppley, B.A.; Qi, A.; Thurgood, C.; Schurer, P. Characterization and activity of perovskite catalysts for autothermal reforming of dodecane. Appl. Catal. A Gen. 2010, 390, 271–280. [Google Scholar] [CrossRef]
- Osazuwa, O.U.; Setiabudi, H.D.; Rasid, R.A.; Cheng, C.K. Syngas production via methane dry reforming: A novel application of SmCoO3 perovskite catalyst. J. Nat. Gas Sci. Eng. 2017, 37, 435–448. [Google Scholar] [CrossRef]
- Goldwasser, M.R.; Rivas, M.E.; Pietri, E.; Pérez-Zurita, M.J.; Cubeiro, M.L.; Gingembre, L.; Leclercq, L.; Leclercq, G. Perovskites as catalysts precursors: CO2 reforming of CH4 on Ln1−xCaxRu0.8Ni0.2O3 (Ln = La, Sm, Nd). Appl. Catal. A Gen. 2003, 255, 45–57. [Google Scholar] [CrossRef]
- Pichas, C.; Pomonis, P.; Petrakis, D.; Ladavos, A. Kinetic study of the catalytic dry reforming of CH4 with CO2 over La2−xSrxNiO4 perovskite-type oxides. Appl. Catal. A Gen. 2010, 386, 116–123. [Google Scholar] [CrossRef]
- Valderrama, G.; Kiennemann, A.; Goldwasser, M.R. La-Sr-Ni-Co-O based perovskite-type solid solutions as catalyst precursors in the CO2 reforming of methane. J. Power Sources 2010, 195, 1765–1771. [Google Scholar] [CrossRef]
- Smorygo, O.; Mikutski, V.; Marukovich, A.; Vialiuha, Y.; Ilyushchanka, A.; Mezentseva, N.; Alikina, G.; Vostrikov, Z.; Fedorova, Y.; Pelipenko, V.; et al. Structured catalyst supports and catalysts for the methane indirect internal steam reforming in the intermediate temperature SOFC. Int. J. Hydrogen Energy 2009, 34, 9505–9514. [Google Scholar] [CrossRef]
- Podyacheva, O.Y.; Ketov, A.A.; Ismagilov, Z.R.; Ushakov, V.A.; Bos, A.; Veringa, H.J. Metal foam supported perovskite catalysts. React. Kinet. Catal. Lett. 1997, 60, 243–250. [Google Scholar] [CrossRef]
- Takeda, Y.; Hashino, T.; Miyamoto, H.; Kanamaru, F.; Kume, S.; Koizumi, M. Synthesis of SrNiO3 and related compound, Sr2Ni2O5. J. Inorg. Nucl. Chem. 1972, 34, 1599–1601. [Google Scholar] [CrossRef]
- De Abreu, W.C.; De Moura, C.V.R.; Costa, J.C.S.; De Moura, E.M. Strontium and nickel heterogeneous catalysts for biodiesel production from macaw oil. J. Braz. Chem. Soc. 2017, 28, 319–327. [Google Scholar] [CrossRef]
- Luo, N.; Liu, K.X.; Li, X.; Shen, H.; Wu, S.; Fu, Z. Systematic study of detonation synthesis of Ni-based nanoparticles. Chem. Eng. J. 2012, 210, 114–119. [Google Scholar] [CrossRef]
- Rivas, M.E.; Fierro, J.L.G.; Goldwasser, M.R.; Pietri, E.; Pérez-Zurita, M.J.; Griboval-Constant, A.; Leclercq, G. Structural features and performance of LaNi1−xRhxO3 system for the dry reforming of methane. Appl. Catal. A Gen. 2008, 344, 10–19. [Google Scholar] [CrossRef]
- Pereñíguez, R.; González-DelaCruz, V.M.; Holgado, J.P.; Caballero, A. Synthesis and characterization of a LaNiO3 perovskite as precursor for methane reforming reactions catalysts. Appl. Catal. B Environ. 2010, 93, 346–353. [Google Scholar] [CrossRef]
- Sudhakaran, M.S.P.; Trinh, H.Q.; Karuppiah, J.; Hossain, M.M.; Mok, Y.S. Plasma Catalytic Removal of p-Xylene from Air Stream Using γ-Al2O3 Supported Manganese Catalyst. Top. Catal. 2017, 60, 944–954. [Google Scholar] [CrossRef]
- Tang, C.; Pu, Z.; Liu, Q.; Asiri, A.M.; Sun, X.; Luo, Y.; He, Y. In Situ Growth of NiSe Nanowire Film on Nickel Foam as an Electrode for High-Performance Supercapacitors. ChemElectroChem 2015, 2, 1903–1907. [Google Scholar] [CrossRef]
- Kumar, M.; Srikanth, S.; Ravikumar, B.; Alex, T.C.; Das, S.K. Synthesis of pure and Sr-doped LaGaO3, LaFeO3 and LaCoO3 and Sr,Mg-doped LaGaO3 for ITSOFC application using different wet chemical routes. Mater. Chem. Phys. 2009, 113, 803–815. [Google Scholar] [CrossRef]
- Valderrama, G.; Goldwasser, M.R.; de Navarro, C.U.; Tatibouët, J.M.; Barrault, J.; Batiot-Dupeyrat, C.; Martínez, F. Dry reforming of methane over Ni perovskite type oxides. Catal. Today 2005, 107–108, 785–791. [Google Scholar] [CrossRef]
- Chen, H.-Y.T.; Tosoni, S.; Pacchioni, G. Hydrogen Adsorption, Dissociation, and Spillover on Ru10 Clusters Supported on Anatase TiO2 and Tetragonal ZrO2 (101) Surfaces. ACS Catal. 2015, 5, 5486–5495. [Google Scholar] [CrossRef]
- Boudjahem, A.-G.; Monteverdi, S.; Mercy, M.; Bettahar, M.M. Nanonickel Particles Supported on Silica. Morphology Effects on Their Surface and Hydrogenating Properties. Catal. Lett. 2004, 97, 177–183. [Google Scholar] [CrossRef]
- Zhan, H.; Li, F.; Xin, C.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. Performance of the La–Mn–Zn–Cu–O Based Perovskite Precursors for Methanol Synthesis from CO2 Hydrogenation. Catal. Lett. 2015, 145, 1177–1185. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, H.; Lin, G.; Yuan, Y. Highly Active CNT-Promoted Cu–ZnO–Al2 O3 Catalyst for Methanol Synthesis from H2/CO/CO2. Catal. Lett. 2003, 85, 237–246. [Google Scholar] [CrossRef]
- Lu, X.; Gu, F.; Liu, Q.; Gao, J.; Liu, Y.; Li, H.; Jia, L.; Xu, G.; Zhong, Z.; Su, F. VOx promoted Ni catalysts supported on the modified bentonite for CO and CO2 methanation. Fuel Process. Technol. 2015, 135, 34–46. [Google Scholar] [CrossRef]
- Al-Doghachi, F.A.J.; Rashid, U.; Taufiq-Yap, Y.H. Investigation of Ce(iii) promoter effects on the tri-metallic Pt, Pd, Ni/MgO catalyst in dry-reforming of methane. RSC Adv. 2016, 6, 10372–10384. [Google Scholar] [CrossRef]
- Batiot-Dupeyrat, C.; Gallego, G.A.S.; Mondragon, F.; Barrault, J.; Tatibouët, J.-M. CO2 reforming of methane over LaNiO3 as precursor material. Catal. Today 2005, 107–108, 474–480. [Google Scholar] [CrossRef]
- Luo, J.Z.; Yu, Z.L.; Ng, C.F.; Au, C.T. CO2/CH4 reforming over Ni-La2O3/5 A: An investigation on carbon deposition and reaction steps. J. Catal. 2000, 194, 198–210. [Google Scholar] [CrossRef]
- Gennequin, C.; Safariamin, M.; Siffert, S.; Aboukaïs, A.; Abi-Aad, E. CO2 reforming of CH4 over Co–Mg–Al mixed oxides prepared via hydrotalcite like precursors. Catal. Today 2011, 176, 139–143. [Google Scholar] [CrossRef]
- Djinović, P.; Batista, J.; Pintar, A. Efficient catalytic abatement of greenhouse gases: Methane reforming with CO2 using a novel and thermally stable Rh–CeO2 catalyst. Int. J. Hydrogen Energy 2012, 37, 2699–2707. [Google Scholar] [CrossRef]
- Slagtern, A.; Schuurman, Y.; Leclercq, C.; Verykios, X.; Mirodatos, C. Specific Features Concerning the Mechanism of Methane Reforming by Carbon Dioxide over Ni/La2O3 Catalyst. J. Catal. 1997, 172, 118–126. [Google Scholar] [CrossRef]
- Olafsen, A.; Slagtern, Å.; Dahl, I.M.; Olsbye, U.; Schuurman, Y.; Mirodatos, C. Mechanistic features for propane reforming by carbon dioxide over a Ni/Mg(Al)O hydrotalcite-derived catalyst. J. Catal. 2005, 229, 163–175. [Google Scholar] [CrossRef]
- Pakhare, D.; Schwartz, V.; Abdelsayed, V.; Haynes, D.; Shekhawat, D.; Poston, J.; Spivey, J. Kinetic and mechanistic study of dry (CO2) reforming of methane over Rh-substituted La2Zr2O7 pyrochlores. J. Catal. 2014, 316, 78–92. [Google Scholar] [CrossRef]
- Rosen, B.A.; Gileadi, E.; Eliaz, N. Electrodeposited Re-promoted Ni foams as a catalyst for the dry reforming of methane. Catal. Commun. 2016, 76, 23–28. [Google Scholar] [CrossRef]
- Yang, E.; Noh, Y.; Ramesh, S.; Lim, S.S.; Moon, D.J. The effect of promoters in La0.9M0.1Ni0.5Fe0.5O3 (M=Sr, Ca) perovskite catalysts on dry reforming of methane. Fuel Process. Technol. 2015, 134, 404–413. [Google Scholar] [CrossRef]
- Kwon, B.W.; Oh, J.H.; Kim, G.S.; Yoon, S.P.; Han, J.; Nam, S.W.; Ham, H.C. The novel perovskite-type Ni-doped Sr0.92Y0.08TiO3 as a reforming biogas (CH4+CO2) for H2 production. Appl. Energy 2018, 227, 213–219. [Google Scholar] [CrossRef]
- Gurav, H.R.; Bobade, R.; Das, V.L.; Chilukuri, S. Carbon dioxide reforming of methane over ruthenium substituted strontium titanate perovskite catalysts. Indian J. Chem. Sect. A Inorg. Phys. Theor. Anal. Chem. 2012, 51, 1339–1347. [Google Scholar]
- Sutthiumporn, K.; Kawi, S. Promotional effect of alkaline earth over Ni-La2O3 catalyst for CO2 reforming of CH4: Role of surface oxygen species on H2 production and carbon suppression. Int. J. Hydrogen Energy 2011, 36, 14435–14446. [Google Scholar] [CrossRef]
- de Sousa, F.F.; de Sousa, H.S.A.; Oliveira, A.C.; Junior, M.C.C.; Ayala, A.P.; Barros, E.B.; Viana, B.C.; Filho, J.M.; Oliveira, A.C. Nanostructured Ni-containing spinel oxides for the dry reforming of methane: Effect of the presence of cobalt and nickel on the deactivation behaviour of catalysts. Int. J. Hydrogen Energy 2012, 37, 3201–3212. [Google Scholar] [CrossRef]
- de Sousa, H.S.A.; da Silva, A.N.; Castro, A.J.R.; Campos, A.; Filho, J.M.; Oliveira, A.C. Mesoporous catalysts for dry reforming of methane: Correlation between structure and deactivation behaviour of Ni-containing catalysts. Int. J. Hydrogen Energy 2012, 37, 12281–12291. [Google Scholar] [CrossRef]
- Pinheiro, A.L.; Pinheiro, A.N.; Valentini, A.; Filho, J.M.; de Sousa, F.F.; de Sousa, J.R.; Maria da Graça, C.R.; Bargiela, P.; Oliveira, A.C. Analysis of coke deposition and study of the structural features of MAl2O4 catalysts for the dry reforming of methane. Catal. Commun. 2009, 11, 11–14. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef]
- Xu, L.; Song, H.; Chou, L. Carbon dioxide reforming of methane over ordered mesoporous NiO–MgO–Al2O3 composite oxides. Appl. Catal. B Environ. 2011, 108–109, 177–190. [Google Scholar] [CrossRef]
- Swaan, H.M.; Kroll, V.C.H.; Martin, G.A.; Mirodatos, C. Deactivation of supported nickel catalysts during the reforming of methane by carbon dioxide. Catal. Today 1994, 21, 571–578. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M.; Sahebdelfar, S.; Yan, Z.-F. Effects of CO2 content on the activity and stability of nickel catalyst supported on mesoporous nanocrystalline zirconia. J. Nat. Gas Chem. 2008, 17, 278–282. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Zaikovskii, V.I.; Buyanov, R.A.; Molchanov, V.V.; Plyasova, L.M. Morphology of carbon from methane on nickel-containing catalysts. Catal. Today 1995, 24, 265–267. [Google Scholar] [CrossRef]
- Beglaryan, H.A.; Melikyan, S.A.; Terzyan, A.M.; Isahakyan, A.R.; Zulumyan, N.H. Preparation of Hydrated Strontium Silicates from Silica Hydrogel Isolated from Serpentines and Their Thermal Transformations. Russ. J. Inorg. Chem. 2018, 63, 1395–1402. [Google Scholar] [CrossRef]
- Hu, B.; Mahapatra, M.K.; Keane, M.; Zhang, H.; Singh, P. Effect of CO2 on the stability of strontium doped lanthanum manganite cathode. J. Power Sources 2014, 268, 404–413. [Google Scholar] [CrossRef]
- Mok, Y.S.; Jwa, E.; Hyun, Y.J. Regeneration of C4H10 dry reforming catalyst by nonthermal plasma. J. Energy Chem. 2013, 22, 394–402. [Google Scholar] [CrossRef]


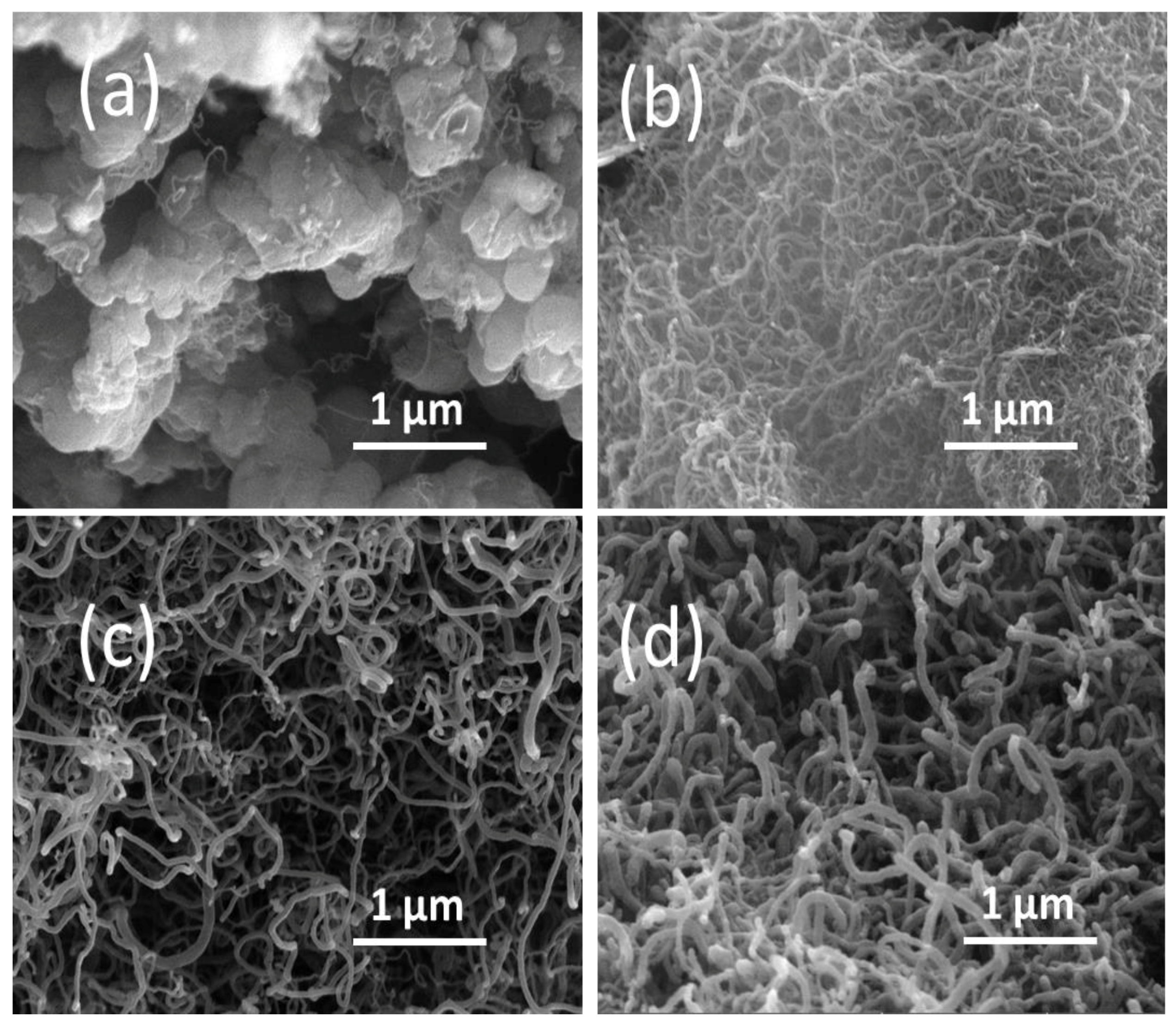
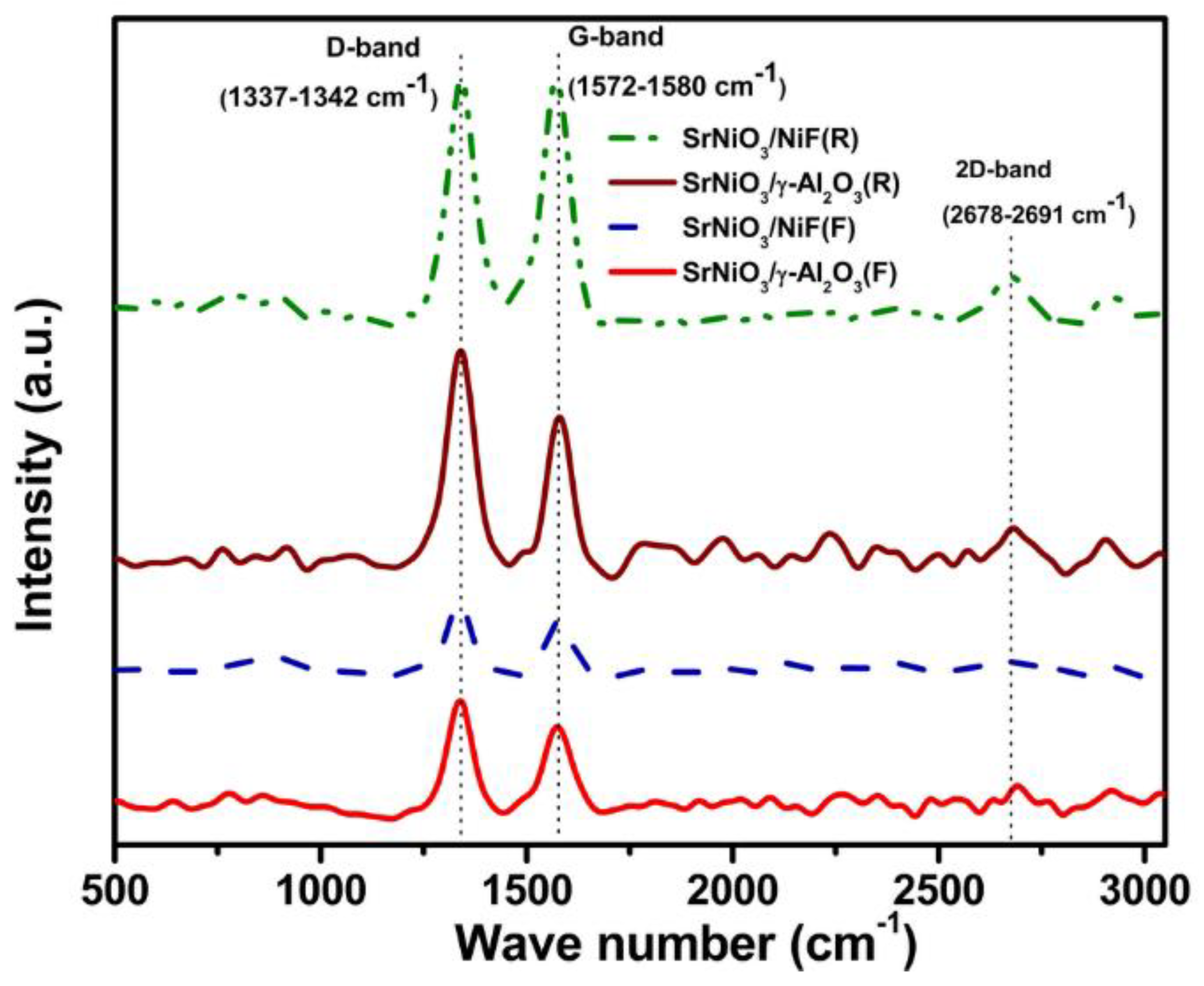


| Catalyst | SBET (m2/g) |
|---|---|
| SrNiO3/γ-Al2O3(F) | 205.1 |
| SrNiO3/γ-Al2O3(R) | 223.5 |
| SrNiO3/NiF(F) | 6.2 |
| SrNiO3/NiF(R) | 23.7 |
| Catalyst | H/CO2 | ||||
|---|---|---|---|---|---|
| SrNiO3/γ-Al2O3 (F) | 82.0 | 48.0 | 20.2 | 61.0 | 1.9 |
| SrNiO3/γ-Al2O3 (R) | 92.0 | 52.1 | 46.2 | 63.2 | 1.0 |
| SrNiO3/NiF(F) | 70.2 | 59.5 | 94.1 | 64.3 | 0.7 |
| SrNiO3/NiF(R) | 85.3 | 67.0 | 97.7 | 67.7 | 0.7 |
| Composition of Catalysts | Catalyst Preparation | Reforming Conditions | Conversion at 700 °C (%) | H2/CO Ratio | References |
|---|---|---|---|---|---|
| LaNiO3 | Sol-gel | 700 °C, CO2:CH4 = 1 | 65 (CH4) | 0.82 | [20] |
| Ni-Re/SS 316 | Electrodeposition | 700–800 °C, CO2:CH4 = 1 | 60 (CH4) | 0.8 | [52] |
| La0.8Sr0.2NiO3 | Sol-gel | 600–800°C, CO2:CH4 = 1 | 80.1 (CH4) | NA | [2] |
| La1−xSrxNi0.4Co0.6O3 | Sol-gel | 700 °C, CO2:CH4 = 1 | 75 (CH4) | 1.0 | [27] |
| La0.9Sr0.1Ni0.5Fe0.5O3 | Sol-gel | 700–900 °C, CO2:CH4 = 1 | 47.2 (CH4) | 0.7 | [53] |
| Sr0.92Y0.08TiO3-3%wt Ni | Sol-gel | 625–745 °C, CO2:CH4 = 1 | 60 (CH4) | NA | [54] |
| SrTi0.85Ru0.15O3-δ | Sol-gel | 600–800 °C, CO2:CH4 = 1 | 75 (CH4) | 0.8 | [55] |
| SrNiO3/NiF(R) | Sol-gel | 550–700 °C, CO2:C3H8 = 3 | 85.3 (C3H8) | 0.7 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M.S.P, S.; Hossain, M.M.; Gnanasekaran, G.; Mok, Y.S. Dry Reforming of Propane over γ-Al2O3 and Nickel Foam Supported Novel SrNiO3 Perovskite Catalyst. Catalysts 2019, 9, 68. https://doi.org/10.3390/catal9010068
M.S.P S, Hossain MM, Gnanasekaran G, Mok YS. Dry Reforming of Propane over γ-Al2O3 and Nickel Foam Supported Novel SrNiO3 Perovskite Catalyst. Catalysts. 2019; 9(1):68. https://doi.org/10.3390/catal9010068
Chicago/Turabian StyleM.S.P, Sudhakaran, Md. Mokter Hossain, Gnanaselvan Gnanasekaran, and Young Sun Mok. 2019. "Dry Reforming of Propane over γ-Al2O3 and Nickel Foam Supported Novel SrNiO3 Perovskite Catalyst" Catalysts 9, no. 1: 68. https://doi.org/10.3390/catal9010068





