Liquid Phase Hydrogenation of MIBK over M/CsPW (M = Ag, Ru, Pt, and Pd)
Abstract
:1. Introduction
2. Results
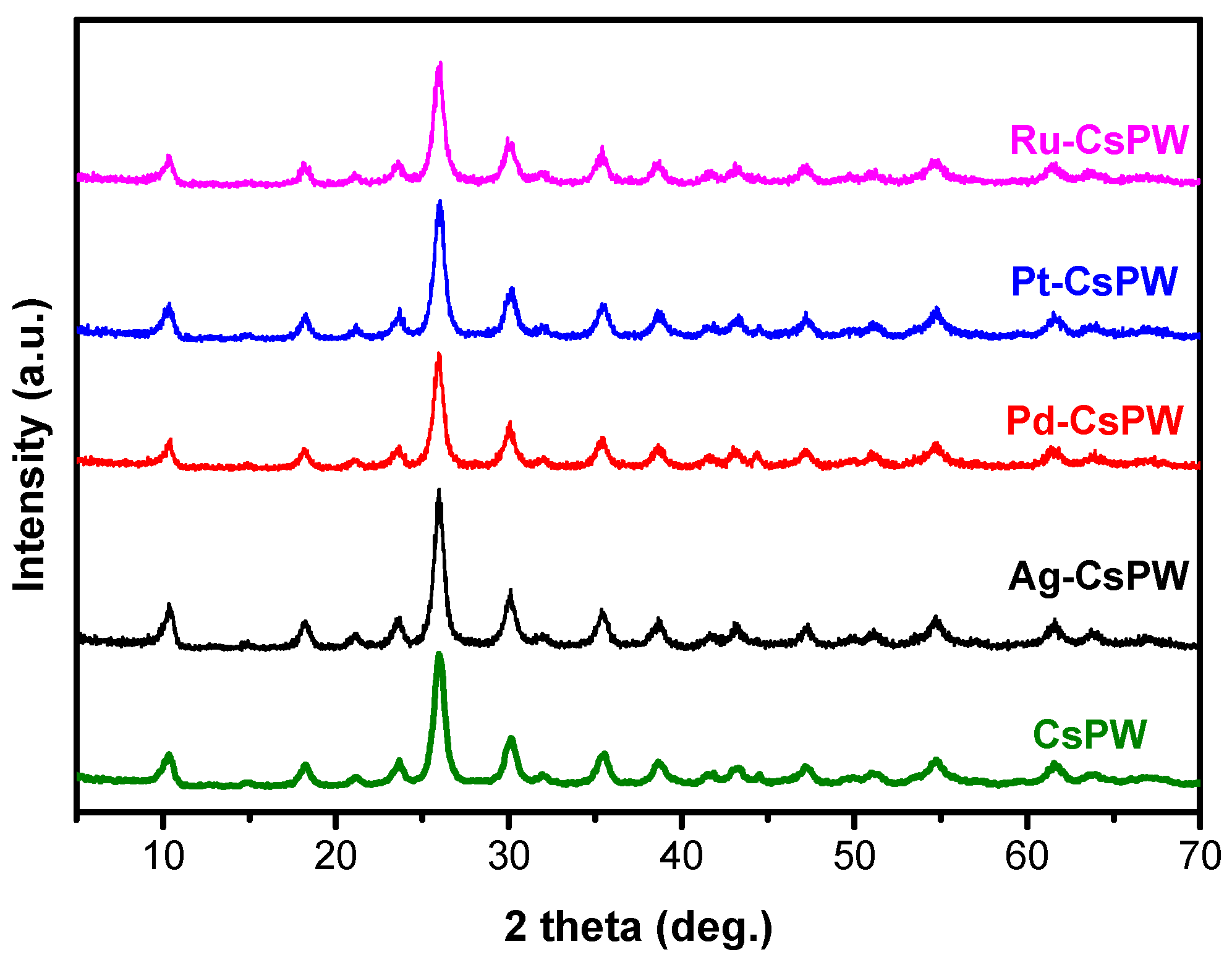
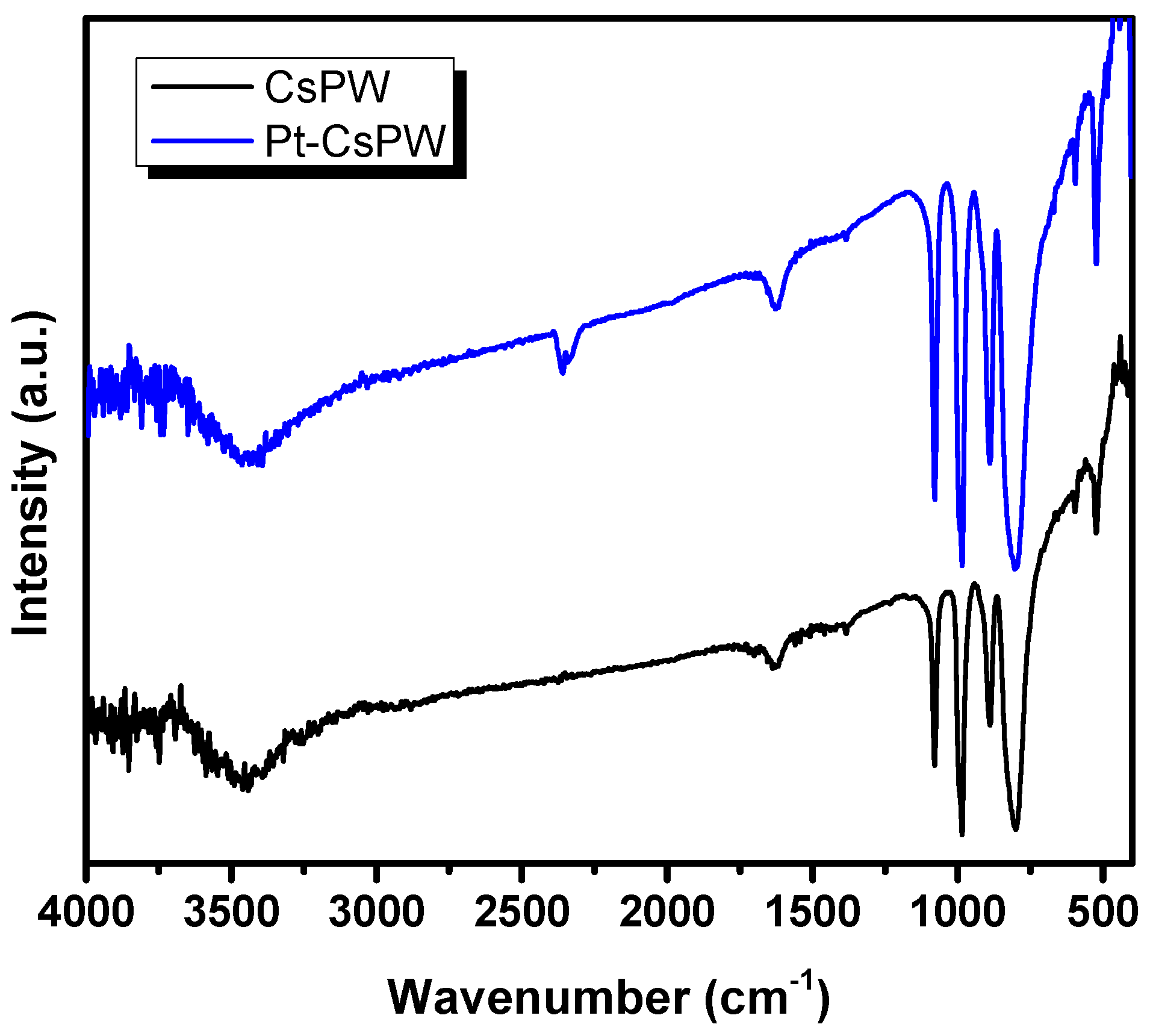
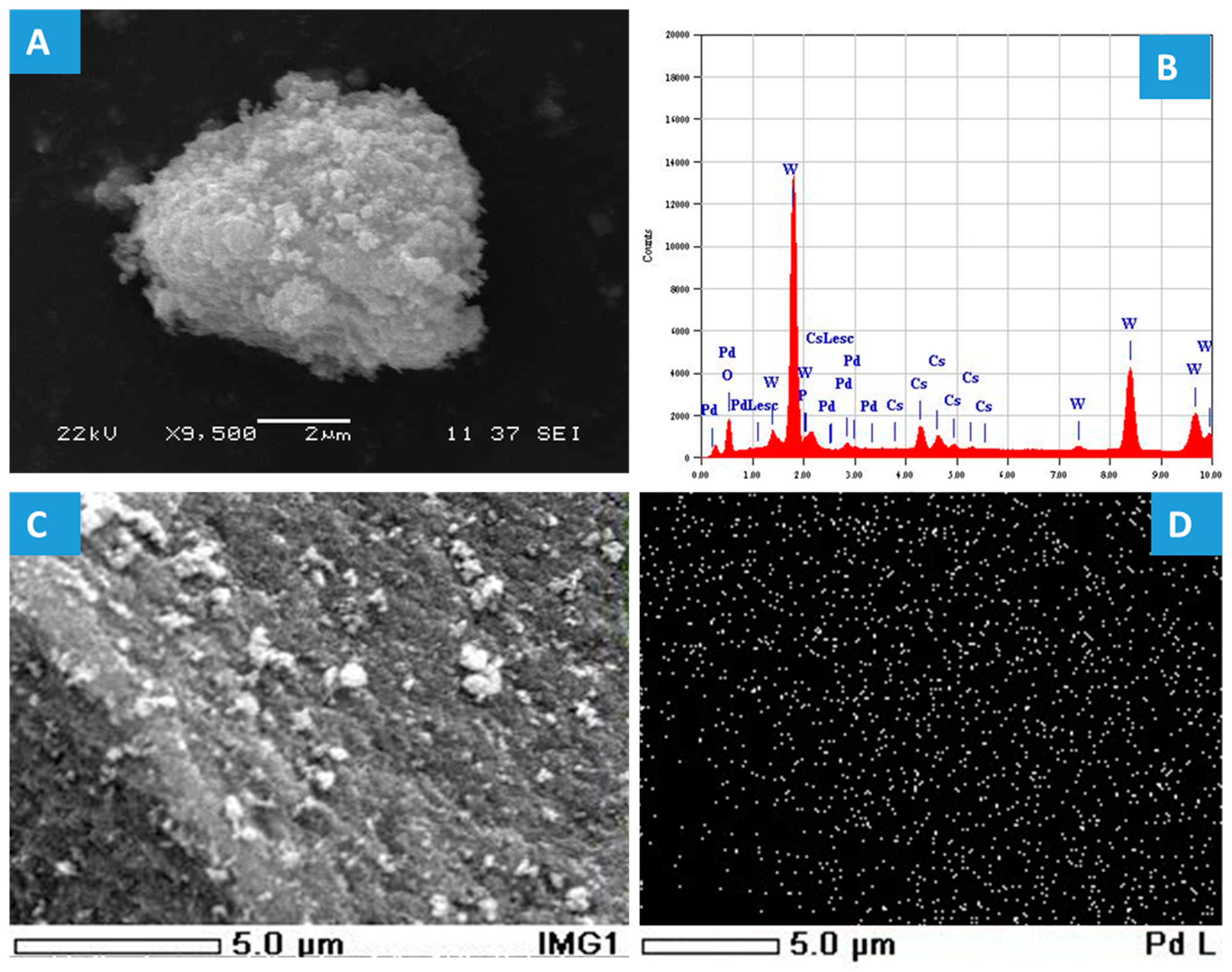
2.1. Characterization Results
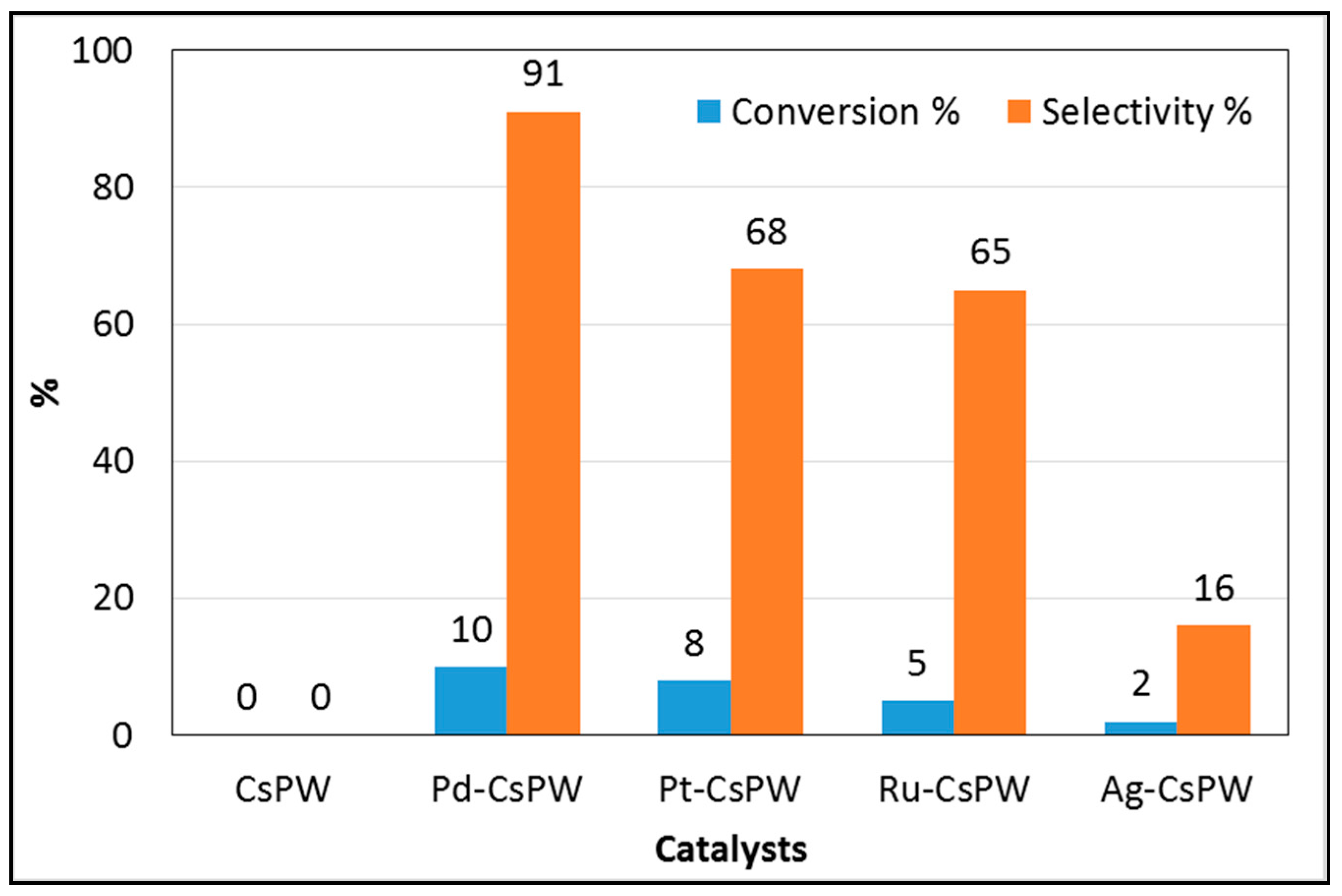
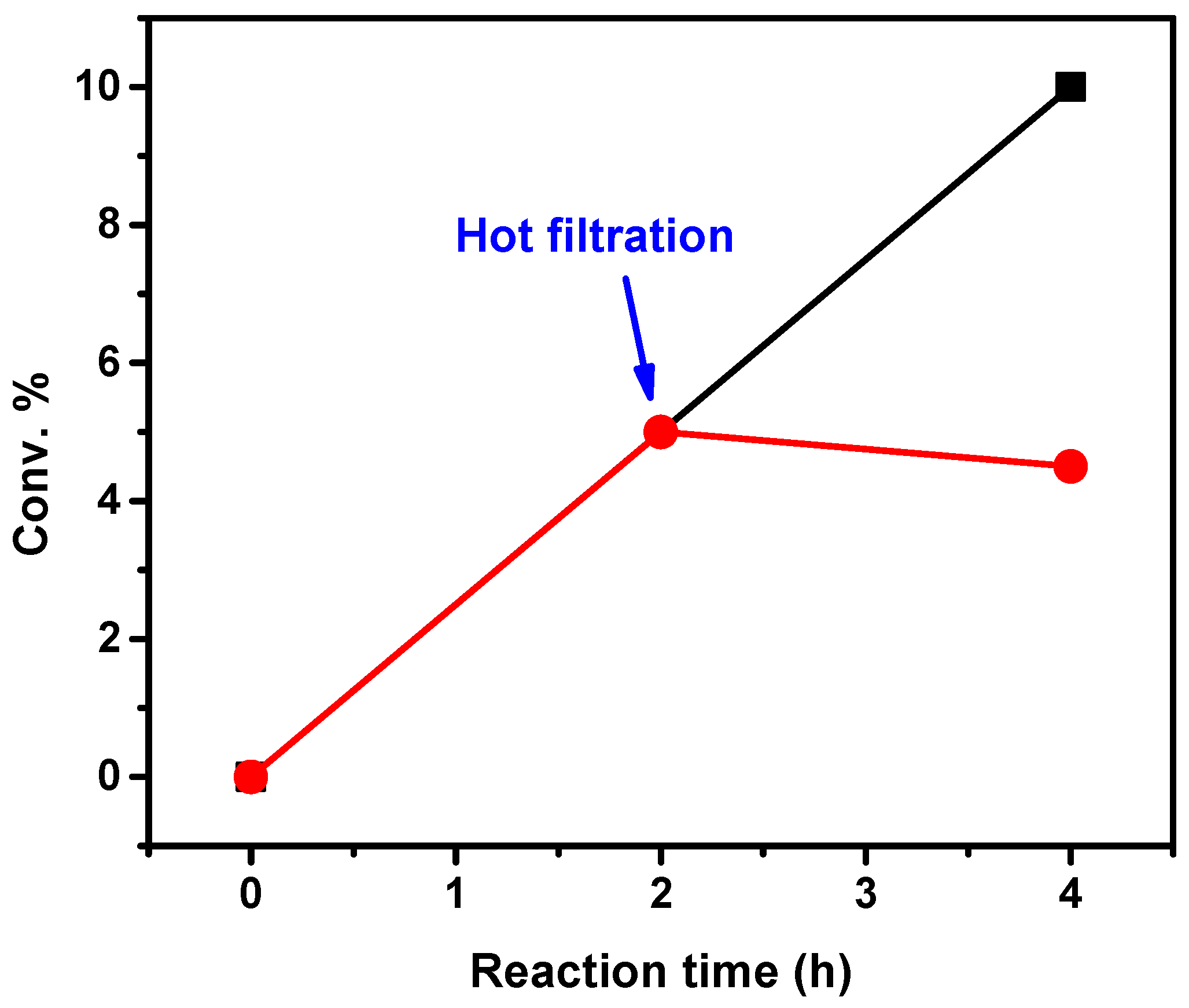
2.2. Catalytic Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of CsPW (Cs2.5H0.5 PW12O40)
4.3. Synthesis of Metal (Pt, Pd, Ru or Ag) Supported on Cs2.5H0.5PW12O40
4.4. Characterization
4.5. Catalytic Performance Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, Z. Catalytic conversion of biomass into chemicals and fuels over magnetic catalysts. ACS Catal. 2015, 6, 326–338. [Google Scholar] [CrossRef]
- Van der Wal, H.; Sperber, B.L.; Houweling-Tan, B.; Bakker, R.R.; Brandenburg, W.; López-Contreras, A.M. Production of acetone, butanol, and ethanol from biomass of the green seaweed Ulva lactuca. Biores. Technol. 2013, 128, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.R.; Kumar, V.P.; Rao, G.S.; Chary, K.V. Vapor phase selective hydrogenation of acetone to methyl isobutyl ketone (MIBK) over Ni/CeO2 catalysts. Catal. Sci. Technol. 2012, 2, 1665–1673. [Google Scholar] [CrossRef]
- Hetterley, R.D.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Multifunctional catalysis by Pd-polyoxometalate: One-step conversion of acetone to methyl isobutyl ketone. Chem. Commun. 2006, 7, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Al-Wadaani, F.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Pd supported on ZnII–CrIII mixed oxide as a catalyst for one-step synthesis of methyl isobutyl ketone. J. Catal. 2008, 257, 199–205. [Google Scholar] [CrossRef]
- Pholjaroen, B.; Li, N.; Yang, J.; Li, G.; Wang, W.; Wang, A.; Zhang, T. Production of renewable jet fuel range branched alkanes with xylose and methyl isobutyl ketone. Ind. Eng. Chem. Res. 2014, 53, 13618–13625. [Google Scholar] [CrossRef]
- Liao, T.; He, G.X.; Yang, S.H.; Luo, G.H.; Xu, X.; Guo, X.Y.; Jin, H.B. Study on the catalytic performance of supported skeletal nickel catalyst for hydrogenation of MIBK. Mod. Chem. Ind. 2017, 6, 75–80. [Google Scholar]
- Alotaibi, M.A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Efficient hydrodeoxygenation of biomass-derived ketones over bifunctional Pt-polyoxometalate catalyst. Chem. Commun. 2012, 48, 7194–7196. [Google Scholar] [CrossRef]
- Alotaibi, M.A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Hydrogenation of methyl isobutyl ketone over bifunctional Pt–zeolite catalyst. J. Catal. 2012, 293, 141–144. [Google Scholar] [CrossRef]
- Itagaki, S.; Matsuhashi, N.; Taniguchi, K.; Yamaguchi, K.; Mizuno, N. Efficient Hydrodeoxygenation of Ketones, Phenols, and Ethers Promoted by Platinum–Heteropolyacid Bifunctional Catalysts. Chem. Lett. 2014, 43, 1086–1088. [Google Scholar] [CrossRef]
- Alharbi, K.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Hydrogenation of ketones over bifunctional Pt-heteropoly acid catalyst in the gas phase. Appl. Catal. A 2015, 504, 457–462. [Google Scholar] [CrossRef]
- Eissa, M.; Alhanash, A.M.; Benaissa, M.; Hamdy, M.S. Pt-Al-TUD-1: A bifunctional catalyst for liquid phase hydrogenation of MIBK. Catal. Commun. 2016, 86, 27–31. [Google Scholar] [CrossRef]
- Okuhara, T. Microporous heteropoly compounds and their shape selective catalysis. Appl. Catal. A 2003, 256, 213–224. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Catalysis by Heteropoly Acids and Multicomponent Polyoxometalates in Liquid-Phase Reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef]
- Corma, A. Inorganic Solid Acids and Their Use in Acid-Catalyzed Hydrocarbon Reactions. Chem. Rev. 1995, 95, 559–614. [Google Scholar] [CrossRef]
- Alhanash, A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Hydrogenolysis of glycerol to propanediol over Ru: Polyoxometalate bifunctional catalyst. Catal. Lett. 2008, 120, 307–311. [Google Scholar] [CrossRef]
- Alhanash, A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Gas-phase dehydration of glycerol to acrolein catalysed by caesium heteropoly salt. Appl. Catal. A 2010, 378, 11–18. [Google Scholar] [CrossRef]
- Okuhara, T.; Watanabe, H.; Nishimura, T.; Inumaru, K.; Misono, M. Microstructure of cesium hydrogen salts of 12-tungstophosphoric acid relevant to novel acid catalysis. Chem. Mater. 2000, 12, 2230–2238. [Google Scholar] [CrossRef]
- Dias, J.A.; Caliman, E.; Dias, S.C.L. Effects of cesium ion exchange on acidity of 12-tungstophosphoric acid. Microporous Mesoporous Mater. 2004, 76, 221–232. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Heteropoly acids and related compounds as catalysts for fine chemical synthesis. Catal. Rev. 1995, 37, 311–352. [Google Scholar] [CrossRef]
- Okuhara, T.; Mizuno, N.; Misono, M. Catalytic chemistry of heteropoly compounds. Adv. Catal. 1996, 41, 113–252. [Google Scholar]
- Hashimoto, M.; Koyano, G.; Mizuno, N. In situ IR spectrum of 12-tungstophosphoric acid hexahydrate with planar H5O2+. J. Phys. Chem. B 2004, 108, 12368–12374. [Google Scholar] [CrossRef]
- Yfanti, V.L.; Vasiliadou, E.S.; Sklari, S.; Lemonidou, A.A. Hydrodeoxygenation of glycerol with in situ H2 formation over Pt catalysts supported on Fe modified Al2O3: Effect of Fe loading. J. Chem. Technol. Biotechnol. 2017, 92, 2236–2245. [Google Scholar] [CrossRef]
- Zhang, C.; Lai, Q.; Holles, J.H. Ir@ Pt bimetallic overlayer catalysts for aqueous phase glycerol hydrodeoxygenation. Appl. Catal. A 2016, 526, 113–125. [Google Scholar] [CrossRef]
- Telalović, S.; Karmee, S.K.; Ramanathan, A.; Hanefeld, U. Al-TUD-1: Introducing tetrahedral aluminium. J. Mol. Catal. A 2013, 368, 88–94. [Google Scholar] [CrossRef]
- Izumi, Y.; Ono, M.; Kitagawa, M.; Yoshida, M.; Urabe, K. Silica-included heteropoly compounds as solid acid catalysts. Microporous Mater. 1995, 5, 255–262. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhanash, A.M.; Atran, A.A.; Eissa, M.; Benaissa, M.; Hamdy, M.S. Liquid Phase Hydrogenation of MIBK over M/CsPW (M = Ag, Ru, Pt, and Pd). Catalysts 2019, 9, 47. https://doi.org/10.3390/catal9010047
Alhanash AM, Atran AA, Eissa M, Benaissa M, Hamdy MS. Liquid Phase Hydrogenation of MIBK over M/CsPW (M = Ag, Ru, Pt, and Pd). Catalysts. 2019; 9(1):47. https://doi.org/10.3390/catal9010047
Chicago/Turabian StyleAlhanash, Abdullah M., Amal A. Atran, Murad Eissa, Mhamed Benaissa, and Mohamed S. Hamdy. 2019. "Liquid Phase Hydrogenation of MIBK over M/CsPW (M = Ag, Ru, Pt, and Pd)" Catalysts 9, no. 1: 47. https://doi.org/10.3390/catal9010047




