Ozone and Photocatalytic Processes for Pathogens Removal from Water: A Review
Abstract
:1. Introduction
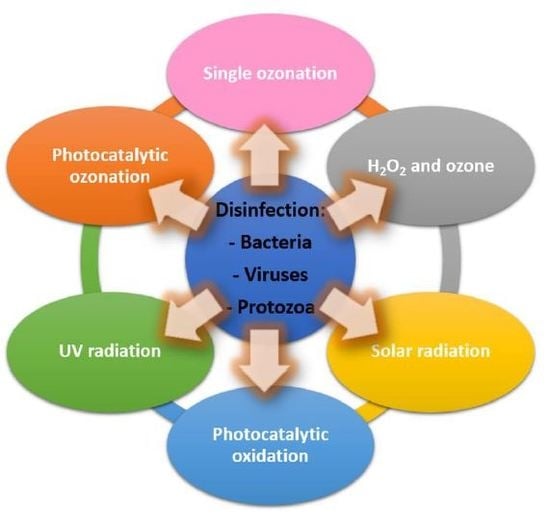
2. Ozone Based Disinfection Systems
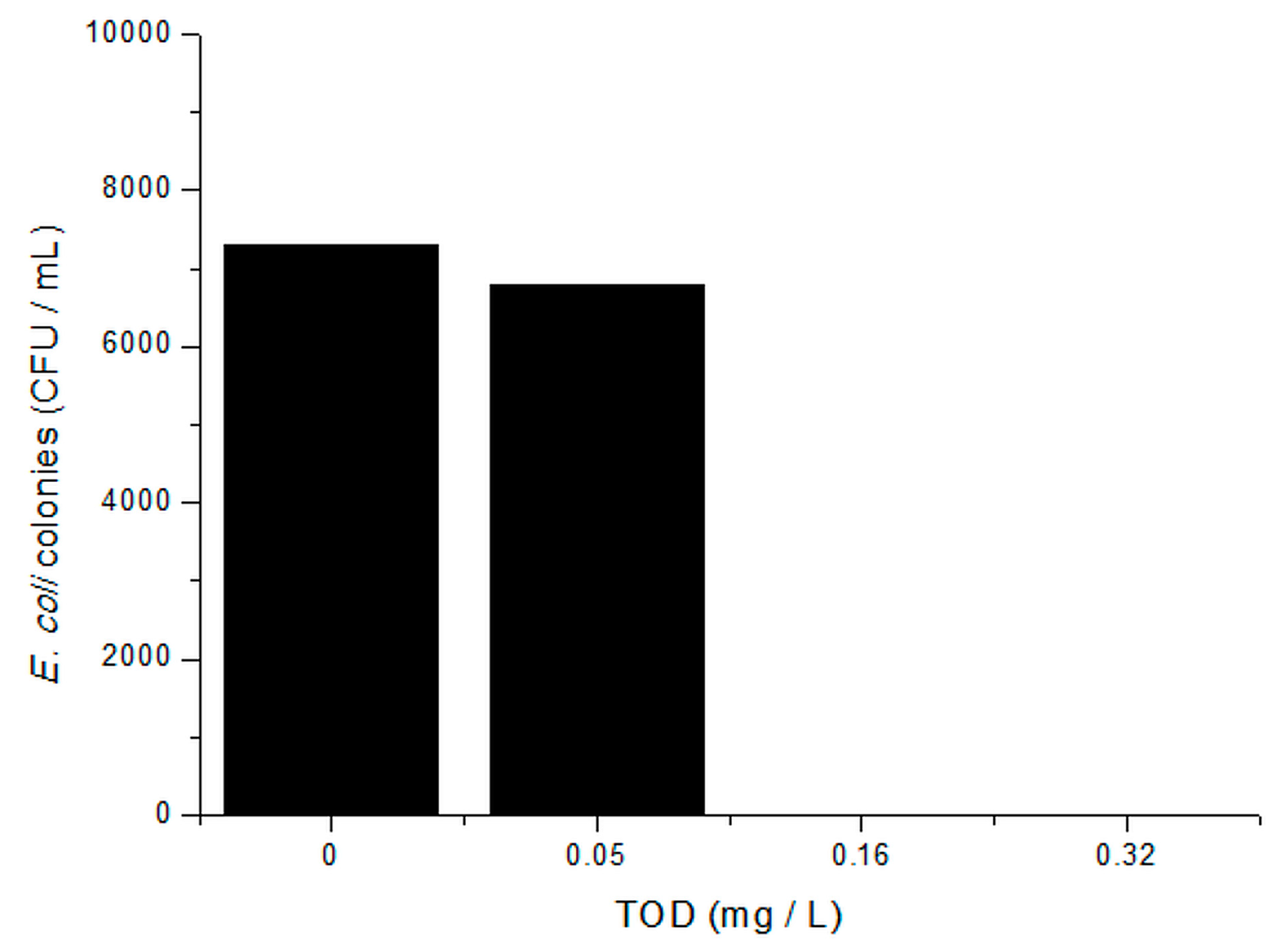
2.1. Single Ozonation
2.2. Hydrogen Peroxide Aided Ozonation
3. Light Application for Disinfection
3.1. UV Disinfection
3.2. Solar Disinfection
3.3. Photocatalytic Disinfection
3.4. Photocatalytic Ozonation
4. Application to Real Water and Integrated Disinfection Schemes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, Y.; von Gunten, U. Oxidative transformation of micropollutants during municipal wastewater treatment: Comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrateVI, and ozone) and non-selective oxidants (hydroxyl radical). Water Res. 2010, 44, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.; Beale, D.J.; Dennis, P.G.; Cook, S.; Ahmed, W. Cross-Comparison of Human Wastewater-Associated Molecular Markers in Relation to Fecal Indicator Bacteria and Enteric Viruses in Recreational Beach Waters. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sikora, P.; Rutgersson, C.; Lindh, M.; Brodin, T.; Björlenius, B.; Larsson, D.G.J.; Norder, H. Differential removal of human pathogenic viruses from sewage by conventional and ozone treatments. Int. J. Hyg. Environ. Health 2018, 221, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.; Duque, V.; Beato, S.; da Silva, J.P.; Major, E.; Meliço-Silvestre, A. Characterization of JC human polyomavirus infection in a Portuguese population. J. Med. Virol. 2010, 82, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Haramoto, E.; Kitajima, M.; Hata, A.; Torrey, J.R.; Masago, Y.; Sano, D.; Katayama, H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018, 135, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Gerba, C.P.; Betancourt, W.Q.; Kitajima, M.; Rock, C.M. Reducing uncertainty in estimating virus reduction by advanced water treatment processes. Water Res. 2018, 133, 282–288. [Google Scholar] [CrossRef]
- Brown, P.C.; Borowska, E.; Schwartz, T.; Horn, H. Impact of the particulate matter from wastewater discharge on the abundance of antibiotic resistance genes and facultative pathogenic bacteria in downstream river sediments. Sci. Total Environ. 2019, 649, 1171–1178. [Google Scholar] [CrossRef]
- Krzeminski, P.; Tomei, M.C.; Karaolia, P.; Langenhoff, A.; Almeida, C.M.R.; Felis, E.; Gritten, F.; Andersen, H.R.; Fernandes, T.; Manaia, C.M.; et al. Performance of secondary wastewater treatment methods for the removal of contaminants of emerging concern implicated in crop uptake and antibiotic resistance spread: A review. Sci. Total Environ. 2019, 648, 1052–1081. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Alexander, J.; Schwartz, T.; Fatta-Kassinos, D. Investigation of the potential of a Membrane BioReactor followed by solar Fenton oxidation to remove antibiotic-related microcontaminants. Chem. Eng. J. 2017, 310, 491–502. [Google Scholar] [CrossRef]
- Michael-Kordatou, I.; Andreou, R.; Iacovou, M.; Frontistis, Z.; Hapeshi, E.; Michael, C.; Fatta-Kassinos, D. On the capacity of ozonation to remove antimicrobial compounds, resistant bacteria and toxicity from urban wastewater effluents. J. Hazard. Mater. 2017, 323, 414–425. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Drosou, C.; Bertakis, Y.; Christofilos, D.; Armatas, G.S.; Sygellou, L.; Schwartz, T.; Xekoukoulotakis, N.P.; et al. Removal of antibiotics, antibiotic-resistant bacteria and their associated genes by graphene-based TiO2 composite photocatalysts under solar radiation in urban wastewaters. Appl. Catal. B Environ. 2018, 224, 810–824. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, Y.; Geng, J.; Ren, H.; Xu, K.; Ding, L. Reduction of antibiotic resistance genes in municipal wastewater effluent by advanced oxidation processes. Sci. Total Environ. 2016, 550, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Salcedo, D.E.; Medriano, C.A.; Kim, S. Comparison of different disinfection processes in the effective removal of antibiotic-resistant bacteria and genes. J. Environ. Sci. 2014, 26, 1238–1242. [Google Scholar] [CrossRef]
- Gomes, J.; Matos, A.; Quinta-Ferreira, R.M.; Martins, R.C. Environmentally applications of invasive bivalves for water and wastewater decontamination. Sci. Total Environ. 2018, 630, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Janex, M.-L.; Savoye, P.; Cockx, A.; Lazarova, V. Wastewater disinfection by ozone: Main parameters for process design. Water Res. 2002, 36, 1043–1055. [Google Scholar] [CrossRef]
- Korich, D.G.; Mead, J.R.; Madore, M.S.; Sinclair, N.A.; Sterling, C.R. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 1990, 56, 1423–1428. [Google Scholar]
- Xi, J.; Zhang, F.; Lu, Y.; Hu, H.-Y. A novel model simulating reclaimed water disinfection by ozonation. Sep. Purif. Technol. 2017, 179, 45–52. [Google Scholar] [CrossRef]
- Stanford, B.D.; Pisarenko, A.N.; Holbrook, R.D.; Snyder, S.A. Preozonation Effects on the Reduction of Reverse Osmosis Membrane Fouling in Water Reuse. Ozone Sci. Eng. 2011, 33, 379–388. [Google Scholar] [CrossRef]
- Ried, A.; Mielcke, J.; Wieland, A. The Potential Use of Ozone in Municipal Wastewater. Ozone Sci. Eng. 2009, 31, 415–421. [Google Scholar] [CrossRef]
- EPA. Wastewater Technology Fact Sheet Ozone Disinfection; EPA: Washington, DC, USA, 1999.
- Wolf, C.; von Gunten, U.; Kohn, T. Kinetics of Inactivation of Waterborne Enteric Viruses by Ozone. Environ. Sci. Technol. 2018, 52, 2170–2177. [Google Scholar] [CrossRef]
- Tondera, K.; Klaer, K.; Gebhardt, J.; Wingender, J.; Koch, C.; Horstkott, M.; Strathmann, M.; Jurzik, L.; Hamza, I.A.; Pinnekamp, J. Reducing pathogens in combined sewer overflows using ozonation or UV irradiation. Int. J. Hyg. Environ. Health 2015, 218, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, S.A.; Rippey, S.R.; Watkins, W.D. Inactivation of bacterial and viral indicators in secondary sewage effluents, using chlorine and ozone. Water Res. 1995, 29, 2483–2490. [Google Scholar] [CrossRef]
- Paraskeva, P.; Graham, N.J.D. Ozonation of municipal wastewater effluents. Water Environ. Res. 2002, 74, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.F. Ultraviolet Disinfection. In Microbiology of Waterborne Diseases; Elsevier: London, UK, 2014; pp. 617–630. ISBN 9780124158467. [Google Scholar]
- Bolton, J.R.; Cotton, C.A. The Ultraviolet Disinfection Handbook; American Water Works Association: Denver, CO, USA, 2008; ISBN 1613000774. [Google Scholar]
- Liu, W.; Cheung, L.-M.; Yang, X.; Shang, C. THM, HAA and CNCl formation from UV irradiation and chlor(am)ination of selected organic waters. Water Res. 2006, 40, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Dotson, A.D.; Rodriguez, C.E.; Linden, K.G. UV disinfection implementation status in US water treatment plants. J. Am. Water Works Assoc. 2012, 104, E318–E324. [Google Scholar] [CrossRef]
- Song, K.; Mohseni, M.; Taghipour, F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review. Water Res. 2016, 94, 341–349. [Google Scholar] [CrossRef]
- Rose, L.J.; O’Connell, H. UV light inactivation of bacterial biothreat agents. Appl. Environ. Microbiol. 2009, 75, 2987–2990. [Google Scholar] [CrossRef]
- Dotson, A.D.; Keen, V.(Olya)S.; Metz, D.; Linden, K.G. UV/H2O2 treatment of drinking water increases post-chlorination DBP formation. Water Res. 2010, 44, 3703–3713. [Google Scholar] [CrossRef]
- Shah, A.D.; Dotson, A.D.; Linden, K.G.; Mitch, W.A. Impact of UV Disinfection Combined with Chlorination/ Chloramination on the Formation of Halonitromethanes and Haloacetonitriles in Drinking Water. Environ. Sci. Technol. 2011, 45, 3657–3664. [Google Scholar] [CrossRef]
- Polo, D.; García-Fernández, I.; Fernández-ibáñez, P.; Romalde, J.L. Solar water disinfection (SODIS): Impact on hepatitis A virus and on a human Norovirus surrogate under natural solar conditions Solar water disinfection (SODIS): Impact on hepatitis A virus and on a human Norovirus surrogate under natural solar condit. Int. Microbiol. 2015, 18, 41–49. [Google Scholar] [CrossRef]
- Figueredo-Fernández, M.; Gutiérrez-Alfaro, S.; Acevedo-Merino, A.; Manzano, M.A. Estimating lethal dose of solar radiation for enterococcus inactivation through radiation reaching the water layer. Application to Solar Water Disinfection (SODIS). Sol. Energy 2017, 158, 303–310. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Kavitha, B.; Kumar Reddy, P.A.; Kim, K.H. TiO2-based photocatalytic disinfection of microbes in aqueous media: A review. Environ. Res. 2017, 154, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Rincón, A.-G.; Pulgarin, C. Bactericidal action of illuminated TiO2 on pure Escherichia coli and natural bacterial consortia: Post-irradiation events in the dark and assessment of the effective disinfection time. Appl. Catal. B Environ. 2004, 49, 99–112. [Google Scholar] [CrossRef]
- Fernández-Ibáñez, P.; Byrne, J.A.; Polo-López, M.I.; Dunlop, P.S.M.; Karaolia, P.; Fatta-Kassinos, D. Chapter 3. Solar Photocatalytic Disinfection of Water. In Photocatalysis: Applications; Royal Society of Chemistry: London, UK, 2016; pp. 72–91. [Google Scholar]
- Aguas, Y.; Hincapie, M.; Fernández-Ibáñez, P.; Polo-López, M.I. Solar photocatalytic disinfection of agricultural pathogenic fungi (Curvularia sp.) in real urban wastewater. Sci. Total Environ. 2017, 607–608, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.J.; Kong, S.; Orr, M.P.; Miller, G.C.; Henry, B.E. Photocatalytic inactivation of coliform bacteria and viruses in secondary wastewater effluent. Water Res. 1995, 29, 95–100. [Google Scholar] [CrossRef]
- Byrne, J.A.; Fernandez-Ibañez, P.A.; Dunlop, P.S.M.; Alrousan, D.M.A.; Hamilton, J.W.J. Photocatalytic Enhancement for Solar Disinfection of Water: A Review. Int. J. Photoenergy 2011, 2011, 1–12. [Google Scholar] [CrossRef]
- Chen, J.; Loeb, S.; Kim, J.-H. LED revolution: Fundamentals and prospects for UV disinfection applications. Environ. Sci. Water Res. Technol. 2017, 3, 188–202. [Google Scholar] [CrossRef]
- Xiong, P.; Hu, J. Inactivation/reactivation of antibiotic-resistant bacteria by a novel UVA/LED/TiO2 system. Water Res. 2013, 47, 4547–4555. [Google Scholar] [CrossRef]
- Sholtes, K.A.; Lowe, K.; Walters, G.W.; Sobsey, M.D.; Linden, K.G.; Casanova, L.M. Comparison of ultraviolet light-emitting diodes and low-pressure mercury-arc lamps for disinfection of water. Environ. Technol. 2016, 37, 2183–2188. [Google Scholar] [CrossRef]
- Hoigné, J. Chemistry of Aqueous Ozone and Transformation of Pollutants by Ozonation and Advanced Oxidation Processes. In Quality and Treatment of Drinking Water II. The Handbook of Environmental Chemistry (Part C: Water Pollution); Springer: Berlin/Heidelberg, Germany, 1998; pp. 83–141. [Google Scholar]
- Hoigné, J.; Bader, H.; Haag, W.; Staehelin, J. Rate constants of reactions of ozone with organic and inorganic compounds in water—III. Inorganic compounds and radicals. Water Res. 1985, 19, 993–1004. [Google Scholar] [CrossRef]
- Sousa, J.M.; Macedo, G.; Pedrosa, M.; Becerra-Castro, C.; Castro-Silva, S.; Pereira, M.F.R.; Silva, A.M.T.; Nunes, O.C.; Manaia, C.M. Ozonation and UV254 nm radiation for the removal of microorganisms and antibiotic resistance genes from urban wastewater. J. Hazard. Mater. 2017, 323, 434–441. [Google Scholar] [CrossRef]
- Hunt, N.K.; Mariñas, B.J. Inactivation of Escherichia coli with ozone: Chemical and inactivation kinetics. Water Res. 1999, 33, 2633–2641. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ren, H.; Geng, J.; Zhang, Y.; Zhang, Y.; Ding, L.; Xu, K. Inactivation of antibiotic resistance genes in municipal wastewater by chlorination, ultraviolet, and ozonation disinfection. Environ. Sci. Pollut. Res. 2015, 22, 7037–7044. [Google Scholar] [CrossRef] [PubMed]
- Rennecker, J.L.; Mariñas, B.J.; Owens, J.H.; Rice, E.W. Inactivation of Cryptosporidium parvum oocysts with ozone. Water Res. 1999, 33, 2481–2488. [Google Scholar] [CrossRef]
- Finch, G.R.; Black, E.K.; Labatiuk, C.W.; Gyürék, L.; Belosevic, M. Comparison of Giardia lamblia and Giardia muris cyst inactivation by ozone. Appl. Environ. Microbiol. 1993, 59, 3674–3680. [Google Scholar] [PubMed]
- Calgua, B.; Carratalà, A.; Guerrero-Latorre, L.; de Abreu Corrêa, A.; Kohn, T.; Sommer, R.; Girones, R. UVC Inactivation of dsDNA and ssRNA Viruses in Water: UV Fluences and a qPCR-Based Approach to Evaluate Decay on Viral Infectivity. Food Environ. Virol. 2014, 6, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Res. 2004, 38, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Driedger, A.; Staub, E.; Pinkernell, U.; Mariñas, B.; Köster, W.; Gunten, U. von Inactivation of bacillus subtilis spores and formation of bromate during ozonation. Water Res. 2001, 35, 2950–2960. [Google Scholar] [CrossRef]
- von Gunten, U.; Pinkernell, U. Ozonation of bromide-containing drinking waters:a delicate balance between disinfection and bromate formation. Water Sci. Technol. 2000, 41, 53–59. [Google Scholar] [CrossRef]
- EPA. Disinfection Profiling and Benchmarking Guidnace Manual, Appendix A; Rep. No. EPA 816-R-03-004; EPA: Washington, DC, USA, 2003.
- EPA. Guidance Manual for the Complance with Filtration and Disinfection Requirements; EPA: Washington, DC, USA, 1989.
- Zhang, J.; Tejada-Martinez, A.E.; Lei, H.; Zhang, Q. Indicators for technological, environmental and economic sustainability of ozone contactors. Water Res. 2016, 101, 606–616. [Google Scholar] [CrossRef]
- Zhang, J.; Tejada-Martínez, A.E.; Zhang, Q.; Lei, H. Evaluating hydraulic and disinfection efficiencies of a full-scale ozone contactor using a RANS-based modeling framework. Water Res. 2014, 52, 155–167. [Google Scholar] [CrossRef]
- EPA. EPA 625/1-86/021 Design Manual: Municipal Wastewater Disinfection; EPA: Cincinnati, OH, USA, 1986.
- Finch, G.R.; Smith, D.W. Ozone dose-response ofEscherichia coli in activated sludge effluent. Water Res. 1989, 23, 1017–1025. [Google Scholar] [CrossRef]
- Scott, D.B.; Lesher, E.C. Effect of Ozone on Survival and Permeability of Escherichia coli. J. Bacteriol. 1963, 85, 567–576. [Google Scholar] [PubMed]
- Hamelin, C.; Chung, Y.S. Optimal conditions for mutagenesis by ozone in Escherichia coli K12. Mutat. Res. Mol. Mech. Mutagen. 1974, 24, 271–279. [Google Scholar] [CrossRef]
- Zhang, F.; Xi, J.; Huang, J.-J.; Hu, H.-Y. Effect of inlet ozone concentration on the performance of a micro-bubble ozonation system for inactivation of Bacillus subtilis spores. Sep. Purif. Technol. 2013, 114, 126–133. [Google Scholar] [CrossRef]
- Rakness, K.L.; Najm, I.; Elovitz, M.; Rexing, D.; Via, S. Cryptosporidium Log-inactivation with Ozone Using Effluent CT 10, Geometric Mean CT 10, Extended Integrated CT 10 and Extended CSTR Calculations. Ozone Sci. Eng. 2005, 27, 335–350. [Google Scholar] [CrossRef]
- Rosario-Ortiz, F.L.; Mezyk, S.P.; Doud, D.F.R.; Snyder, S.A. Quantitative Correlation of Absolute Hydroxyl Radical Rate Constants with Non-Isolated Effluent Organic Matter Bulk Properties in Water. Environ. Sci. Technol. 2008, 42, 5924–5930. [Google Scholar] [CrossRef]
- Gamage, S.; Gerrity, D.; Pisarenko, A.N.; Wert, E.C.; Snyder, S.A. Evaluation of Process Control Alternatives for the Inactivation of Escherichia coli, MS2 Bacteriophage, and Bacillus subtilis Spores during Wastewater Ozonation. Ozone Sci. Eng. 2013, 35, 501–513. [Google Scholar] [CrossRef]
- Acra, A.; Jurdi, M.; Mu’allem, H.; Karahagopian, Y.; Raffoul, Z. Water Disinfection by Solar Radiation: Assessment and Application; International Development Research Centre: Ottawa, ON, Canada, 1990; ISBN 0889365555. [Google Scholar]
- Teksoy, A.; Eleren, S.Ç. Drinking Water Disinfection by Solar Radiation. Environ. Ecol. Res. 2017, 5, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Gmurek, M.; Olak-Kucharczyk, M.; Ledakowicz, S. Photochemical decomposition of endocrine disrupting compounds—A review. Chem. Eng. J. 2017, 310, 437–456. [Google Scholar] [CrossRef]
- Kozak, M.; Mazierski, P.; Żebrowska, J.; Kobylański, M.; Klimczuk, T.; Lisowski, W.; Trykowski, G.; Nowaczyk, G.; Zaleska-Medynska, A.; Kozak, M.; et al. Electrochemically Obtained TiO2/CuxOy Nanotube Arrays Presenting a Photocatalytic Response in Processes of Pollutants Degradation and Bacteria Inactivation in Aqueous Phase. Catalysts 2018, 8, 237. [Google Scholar] [CrossRef]
- Shin, G.-A.; Lee, J.-K.; Linden, K.G. Enhanced effectiveness of medium-pressure ultraviolet lamps on human adenovirus 2 and its possible mechanism. Water Sci. Technol. 2009, 60, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, O.K.; Stefanakos, E.; Trotz, M.A.; Goswami, D.Y. A review of the mechanisms and modeling of photocatalytic disinfection. Appl. Catal. B Environ. 2010, 98, 27–38. [Google Scholar] [CrossRef]
- Cruz-Ortiz, B.R.; Hamilton, J.W.J.; Pablos, C.; Díaz-Jiménez, L.; Cortés-Hernández, D.A.; Sharma, P.K.; Castro-Alférez, M.; Fernández-Ibañez, P.; Dunlop, P.S.M.; Byrne, J.A. Mechanism of photocatalytic disinfection using titania-graphene composites under UV and visible irradiation. Chem. Eng. J. 2017, 316, 179–186. [Google Scholar] [CrossRef]
- Castro-Alférez, M.; Polo-López, M.I.; Marugán, J.; Fernández-Ibáñez, P. Mechanistic model of the Escherichia coli inactivation by solar disinfection based on the photo-generation of internal ROS and the photo-inactivation of enzymes: CAT and SOD. Chem. Eng. J. 2017, 318, 214–223. [Google Scholar] [CrossRef]
- Gates, F. A study of the bactericidal action of ultra violet light: I. The reaction to monochromatic radiations. J. Gen. Physiol. 1929, 13, 231–248. [Google Scholar] [CrossRef]
- Gates, F. A study of the bactericidal action of ultra violet light ii. The effect of various environmental factors and conditions. J. Gen. Physiol. 1929, 249–260. [Google Scholar] [CrossRef]
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef]
- Abbaszadegan, M.; Hasan, M.N.; Gerba, C.P.; Roessler, P.F.; Wilson, B.R.; Kuennen, R.; Van Dellen, E. The disinfection efficacy of a point-of-use water treatment system against bacterial, viral and protozoan waterborne pathogens. Water Res. 1997, 31, 574–582. [Google Scholar] [CrossRef]
- Bourrouet, A.; García, J.; Mujeriego, R.; Peñuelas, G. Faecal bacteria and bacteriophage inactivation in a full-scale UV disinfection system used for wastewater reclamation. Water Sci. Technol. 2001, 43, 187–194. [Google Scholar] [CrossRef]
- Green, C.F.; Scarpino, P.V.; Jensen, P.; Jensen, N.J.; Gibbs, S.G. Disinfection of selected Aspergillus spp. using ultraviolet germicidal irradiation. Can. J. Microbiol. 2004, 50, 221–224. [Google Scholar] [CrossRef]
- Passantino, L.; Malley, J.; Knudson, M.; Ward, R.; Kim, J. Effect of low turbidity and algae on UV disinfection performance. J. Am. Water Work. Assoc. 2004, 96, 128–137. [Google Scholar] [CrossRef]
- Hamilton, K.A.; Waso, M.; Reyneke, B.; Saeidi, N.; Levine, A.; Lalancette, C.; Besner, M.-C.; Khan, W.; Ahmed, W. Cryptosporidium and Giardia in Wastewater and Surface Water Environments. J. Environ. Qual. 2018, 47, 1006. [Google Scholar] [CrossRef]
- Koutchma, T.; Forney, L.J.; Moraru, C.I. Ultraviolet Light in food Technology: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781420059502. [Google Scholar]
- Makarova, K.S.; Aravind, L.; Wolf, Y.I.; Tatusov, R.L.; Minton, K.W.; Koonin, E.V.; Daly, M.J. Genome of the Extremely Radiation-Resistant Bacterium Deinococcus radiodurans Viewed from the Perspective of Comparative Genomics. Microbiol. Mol. Biol. Rev. 2001, 65, 44–79. [Google Scholar] [CrossRef]
- Lindenauer, K.G.; Darby, J.L. Ultraviolet disinfection of wastewater: Effect of dose on subsequent photoreactivation. Water Res. 1994, 28, 805–817. [Google Scholar] [CrossRef]
- Rodriguez, R.A.; Bounty, S.; Beck, S.; Chan, C.; McGuire, C.; Linden, K.G. Photoreactivation of bacteriophages after UV disinfection: Role of genome structure and impacts of UV source. Water Res. 2014, 55, 143–149. [Google Scholar] [CrossRef]
- Guo, M.; Hu, H.; Bolton, J.R.; El-Din, M.G. Comparison of low- and medium-pressure ultraviolet lamps: Photoreactivation of Escherichia coli and total coliforms in secondary effluents of municipal wastewater treatment plants. Water Res. 2009, 43, 815–821. [Google Scholar] [CrossRef] [PubMed]
- ÖNORM. Austrian National Standard: ÖNORM M 5873-1 E, Plants for Disinfection of Water Using Ultraviolet Radiation: Requirements and Testing, Part 1: Low Pressure Mercury Lamp Plants; ÖNORM: Vienna, Austria, 2001. [Google Scholar]
- ÖNORM. Austrian National Standard: prÖNORM M 5873-2 E, Plants for dIsinfection of Water Using Ultraviolet Radiation: Requirements and Testing, Part 2: Medium Pressure Mercury Lamp Plants; ÖNORM: Vienna, Austria, 2003. [Google Scholar]
- DVGW W 294 Teile 1–3. UV-Geräte zur Desinfektion in der Wasserversorgung; DVGW: Bonn, Germany, 2006. [Google Scholar]
- Stanfield, G.; Lechevallier, M.; Snozzi, M. Treatment efficiency. In Assessing Microbial Safety of Drinking Water: Improving Approaches and Methods; Dufour, A., Snozzi, M., Koster, W., Bartram, J., Ronchi, E., Fewtrell, L., Eds.; IWA Publishing: London, UK, 2003; pp. 159–178. [Google Scholar]
- Ultraviolet Disinfection Guidance Manual For The Final Long Term 2 Enhanced Surface Water Treatment Rule Uv Disinfection Guidance Manual; EPA-LT2ESWTR; EPA: Washington, DC, USA, 2006.
- NSF/ANSI. NSF/ANSI Standard 55:Ultraviolet Microbiological Water Treatment Systems; NSF: Ann Arbor, MI, USA, 2002. [Google Scholar]
- Beck, S.E.; Wright, H.B.; Hargy, T.M.; Larason, T.C.; Linden, K.G.; Linden, K.G. Action spectra for validation of pathogen disinfection in medium-pressure ultraviolet (UV) systems. Water Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Okpara, C.G.; Oparaku, N.F.; Ibeto, C.N. An overview of water disinfection in developing countries and potentials of renewable energy. J. Environ. Sci. Technol. 2011, 4, 18–30. [Google Scholar] [CrossRef]
- Das, T.K. Ultraviolet disinfection application to a wastewater treatment plant. Clean Prod. Process. 2001, 3, 69–80. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011; Volume 38. [Google Scholar]
- Hollaender, A. Effect of long ultraviolet and short visible radiation (3500 to 4900A) on Escherichia coli. J. Bacteriol. 1943, 46, 531–541. [Google Scholar] [PubMed]
- Oates, P.M.; Shanahan, P.; Polz, M.F. Solar disinfection (SODIS): Simulation of solar radiation for global assessment and application for point-of-use water treatment in Haiti. Water Res. 2003, 37, 47–54. [Google Scholar] [CrossRef]
- Meierhofer, R.; Wegelin, M. Solar Water Disinfection: A Guide for the Application of SODIS; SANDEC: Duebendorf, Switzerland, 2002. [Google Scholar]
- Castro-Alférez, M.; Polo-López, M.I.; Fernández-Ibáñez, P. Intracellular mechanisms of solar water disinfection. Sci. Rep. 2016, 6, 38145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, J.; Malato, S.; Fernández-Ibañez, P.; Alarcón, D.; Gernjak, W.; Maldonado, M.I. Review of feasible solar energy applications to water processes. Renew. Sustain. Energy Rev. 2009, 13, 1437–1445. [Google Scholar] [CrossRef]
- McGuigan, K.G.; Conroy, R.M.; Mosler, H.J.; du Preez, M.; Ubomba-Jaswa, E.; Fernandez-Ibañez, P. Solar water disinfection (SODIS): A review from bench-top to roof-top. J. Hazard. Mater. 2012, 235–236, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.B.; Iriarte, M.; Nelson, K.L. Solar water disinfection (SODIS) of Escherichia coli, Enterococcus spp., and MS2 coliphage: Effects of additives and alternative container materials. Water Res. 2012, 46, 1745–1754. [Google Scholar] [CrossRef]
- Keogh, M.B.; Castro-Alférez, M.; Polo-López, M.I.; Fernández Calderero, I.; Al-Eryani, Y.A.; Joseph-Titus, C.; Sawant, B.; Dhodapkar, R.; Mathur, C.; McGuigan, K.G.; et al. Capability of 19-L polycarbonate plastic water cooler containers for efficient solar water disinfection (SODIS): Field case studies in India, Bahrain and Spain. Sol. Energy 2015, 116, 1–11. [Google Scholar] [CrossRef]
- Boyle, M.; Sichel, C.; Fernández-Ibáñez, P.; Arias-Quiroz, G.B.; Iriarte-Puña, M.; Mercado, A.; Ubomba-Jaswa, E.; McGuigan, K.G. Bactericidal effect of solar water disinfection under real sunlight conditions. Appl. Environ. Microbiol. 2008, 74, 2997–3001. [Google Scholar] [CrossRef]
- Loeb, S.; Hofmann, R.; Kim, J.H. Beyond the Pipeline: Assessing the Efficiency Limits of Advanced Technologies for Solar Water Disinfection. Environ. Sci. Technol. Lett. 2016, 3, 73–80. [Google Scholar] [CrossRef]
- Freuze, I.; Brosillon, S.; Laplanche, A.; Tozza, D.; Cavard, J. Effect of chlorination on the formation of odorous disinfection by-products. Water Res. 2005, 39, 2636–2642. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Choi, Y. The effects of UV disinfection on drinking water quality in distribution systems. Water Res. 2010, 44, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D.; Thruston, A.D.; Caughran, T.V.; Chen, P.H.; Collette, T.W.; Floyd, T.L.; Schenck, K.M.; Lykins, B.W.; Sun, G.R.; Majetich, G. Identification of new ozone disinfection byproducts in drinking water. Environ. Sci. Technol. 1999, 33, 3368–3377. [Google Scholar] [CrossRef]
- Cho, M.; Chung, H.; Yoon, J. Disinfection of water containing natural organic matter by using ozone-initiated radical reactions. Appl. Environ. Microbiol. 2003, 69, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-J.; Fang, G.-C.; Wang, C.-C. The determination and fate of disinfection by-products from ozonation of polluted raw water. Sci. Total Environ. 2005, 345, 261–272. [Google Scholar] [CrossRef]
- Gmurek, M.; Olak-Kucharczyk, M.; Ledakowicz, S. Influence of dissolved organic matter in natural and simulated water on the photochemical decomposition of butylparaben. J. Environ. Heal. Sci. Eng. 2015, 13. [Google Scholar] [CrossRef]
- Frimmel, F.H. Impact of light on the properties of aquatic natural organic matter. Environ. Int. 1998, 24, 559–571. [Google Scholar] [CrossRef]
- Buchanan, W.; Roddick, F.; Porter, N. Formation of hazardous by-products resulting from the irradiation of natural organic matter: Comparison between UV and VUV irradiation. Chemosphere 2006, 63, 1130–1141. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Lonnen, J.; Kilvington, S.; Kehoe, S.C.; Al-Touati, F.; McGuigan, K.G. Solar and photocatalytic disinfection of protozoan, fungal and bacterial microbes in drinking water. Water Res. 2005, 39, 877–883. [Google Scholar] [CrossRef]
- Méndez-Hermida, F.; Ares-Mazás, E.; McGuigan, K.G.; Boyle, M.; Sichel, C.; Fernández-Ibáñez, P. Disinfection of drinking water contaminated with Cryptosporidium parvum oocysts under natural sunlight and using the photocatalyst TiO2. J. Photochem. Photobiol. B Biol. 2007, 88, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Foszpańczyk, M.; Bednarczyk, K.; Drozdek, E.; Martins, R.C.; Ledakowicz, S.; Gmurek, M. Comparison of Photocatalytic and Photosensitized Oxidation of Paraben Aqueous Solutions Under Sunlight. Water Air Soil Pollut. 2018, 229, 362. [Google Scholar] [CrossRef] [PubMed]
- Maness, P.C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal activity of photocatalytic TiO2 reaction: Toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [PubMed]
- Rincón, A.G.; Pulgarin, C. Photocatalytical inactivation of E. coli: Effect of (continuous–intermittent) light intensity and of (suspended–fixed) TiO2 concentration. Appl. Catal. B Environ. 2003, 44, 263–284. [Google Scholar] [CrossRef]
- Sunada, K.; Kikuchi, Y.; Hashimoto, K.; Fujishima, A. Bactericidal and Detoxification Effects of TiO2 Thin Film Photocatalysts. Environ. Sci. Technol. 1998, 32, 726–728. [Google Scholar] [CrossRef]
- Bogdan, J.; Zarzyńska, J.; Pławińska-Czarnak, J. Comparison of Infectious Agents Susceptibility to Photocatalytic Effects of Nanosized Titanium and Zinc Oxides: A Practical Approach. Nanoscale Res. Lett. 2015, 10, 1023. [Google Scholar] [CrossRef]
- El Saliby, I.; McDonagh, A.; Erdei, L.; Kyong Shon, H. Water Reclamation by Heterogeneous Photocatalysis over Titanium Dioxide. In Green Technologies for Sustainable Water Management; American Society of Civil Engineers: Reston, VA, USA, 2016; pp. 679–704. [Google Scholar]
- Gomes, J.F.; Lopes, A.; Gonçalves, D.; Luxo, C.; Gmurek, M.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C.; Matos, A. Biofiltration using C. fluminea for E.coli removal from water: Comparison with ozonation and photocatalytic oxidation. Chemosphere 2018, 208. [Google Scholar] [CrossRef]
- Cho, M.; Yoon, J. Measurement of OH radical CT for inactivating Cryptosporidium parvum using photo/ferrioxalate and photo/TiO2 systems. J. Appl. Microbiol. 2008, 104, 759–766. [Google Scholar] [CrossRef]
- Abeledo-Lameiro, M.J.; Ares-Mazás, E.; Gómez-Couso, H. Evaluation of solar photocatalysis using TiO2 slurry in the inactivation of Cryptosporidium parvum oocysts in water. J. Photochem. Photobiol. B Biol. 2016, 163, 92–99. [Google Scholar] [CrossRef]
- Mac Mahon, J.; Pillai, S.C.; Kelly, J.M.; Gill, L.W. Solar photocatalytic disinfection of E. coli and bacteriophages MS2, ΦX174 and PR772 using TiO2, ZnO and ruthenium based complexes in a continuous flow system. J. Photochem. Photobiol. B Biol. 2017, 170, 79–90. [Google Scholar] [CrossRef]
- Ndounla, J.; Kenfack, S.; Wéthé, J.; Pulgarin, C. Relevant impact of irradiance (vs. dose) and evolution of pH and mineral nitrogen compounds during natural water disinfection by photo-Fenton in a solar CPC reactor. Appl. Catal. B Environ. 2014, 148–149, 144–153. [Google Scholar] [CrossRef]
- Rincón, A.-G.; Pulgarin, C. Field solar E. coli inactivation in the absence and presence of TiO2: Is UV solar dose an appropriate parameter for standardization of water solar disinfection? Sol. Energy 2004, 77, 635–648. [Google Scholar] [CrossRef]
- Castro, C.A.; Jurado, A.; Sissa, D.; Giraldo, S.A. Performance of Ag-TiO 2 photocatalysts towards the photocatalytic disinfection of water under interior-lighting and solar-simulated light irradiations. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef]
- Rincón, A.-G.; Pulgarin, C. Use of coaxial photocatalytic reactor (CAPHORE) in the TiO2 photo-assisted treatment of mixed E. coli and Bacillus sp. and bacterial community present in wastewater. Catal. Today 2005, 101, 331–344. [Google Scholar] [CrossRef]
- Shang, C.; Cheung, L.M.; Ho, C.-M.; Zeng, M. Repression of photoreactivation and dark repair of coliform bacteria by TiO2-modified UV-C disinfection. Appl. Catal. B Environ. 2009, 89, 536–542. [Google Scholar] [CrossRef]
- Dunlop, P.S.M.; Ciavola, M.; Rizzo, L.; Mcdowell, D.A.; Byrne, J.A. Effect of photocatalysis on the transfer of antibiotic resistance genes in urban wastewater. Catal. Today 2015, 240, 55–60. [Google Scholar] [CrossRef]
- Rizzo, L.; Sannino, D.; Vaiano, V.; Sacco, O.; Scarpa, A.; Pietrogiacomi, D. Effect of solar simulated N-doped TiO 2 photocatalysis on the inactivation and antibiotic resistance of an E. coli strain in biologically treated urban wastewater. Appl. Catal. B Environ. 2014, 144, 369–378. [Google Scholar] [CrossRef]
- Venieri, D.; Gounaki, I.; Bikouvaraki, M.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Mantzavinos, D. Solar photocatalysis as disinfection technique: Inactivation of Klebsiella pneumoniae in sewage and investigation of changes in antibiotic resistance profile. J. Environ. Manag. 2017, 195, 140–147. [Google Scholar] [CrossRef]
- Guo, C.; Wang, K.; Hou, S.; Wan, L.; Lv, J.; Zhang, Y.; Qu, X.; Chen, S.; Xu, J. H2O2 and/or TiO2 photocatalysis under UV irradiation for the removal of antibiotic resistant bacteria and their antibiotic resistance genes. J. Hazard. Mater. 2017, 323, 710–718. [Google Scholar] [CrossRef]
- Moreira, N.F.; Narciso-da-Rocha, C.; Polo-López, M.I.; Pastrana-Martínez, L.M.; Faria, J.L.; Manaia, C.M.; Fernández-Ibáñez, P.; Nunes, O.C.; Silva, A.M.T. Solar treatment (H2O2, TiO2-P25 and GO-TiO2 photocatalysis, photo-Fenton) of organic micropollutants, human pathogen indicators, antibiotic resistant bacteria and related genes in urban wastewater. Water Res. 2018, 135, 195–206. [Google Scholar] [CrossRef]
- Mecha, A.C.; Onyango, M.S.; Ochieng, A.; Fourie, C.J.S.; Momba, M.N.B. Synergistic effect of UV–vis and solar photocatalytic ozonation on the degradation of phenol in municipal wastewater: A comparative study. J. Catal. 2016, 341, 116–125. [Google Scholar] [CrossRef]
- Moreira, N.F.F.; Sousa, J.M.; Macedo, G.; Ribeiro, A.R.; Barreiros, L.; Pedrosa, M.; Faria, J.L.; Pereira, M.F.R.; Castro-Silva, S.; Segundo, M.A.; et al. Photocatalytic ozonation of urban wastewater and surface water using immobilized TiO2 with LEDs: Micropollutants, antibiotic resistance genes and estrogenic activity. Water Res. 2016, 94, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Lopes, A.; Bednarczyk, K.; Gmurek, M.; Stelmachowski, M.; Zaleska-Medynska, A.; Quinta-Ferreira, M.; Costa, R.; Quinta-Ferreira, R.; Martins, R.; et al. Effect of Noble Metals (Ag, Pd, Pt) Loading over the Efficiency of TiO2 during Photocatalytic Ozonation on the Toxicity of Parabens. ChemEngineering 2018, 2, 4. [Google Scholar] [CrossRef]
- Gomes, J.F.; Leal, I.; Bednarczyk, K.; Gmurek, M.; Stelmachowski, M.; Zaleska-Medynska, A.; Quinta-Ferreira, M.E.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Detoxification of parabens using UV-A enhanced by noble metals—TiO2 supported catalysts. J. Environ. Chem. Eng. 2017, 5. [Google Scholar] [CrossRef]
- Mecha, A.C.; Onyango, M.S.; Ochieng, A.; Momba, M.N.B. Evaluation of synergy and bacterial regrowth in photocatalytic ozonation disinfection of municipal wastewater. Sci. Total Environ. 2017, 601–602, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Imminger, S.; Czekalski, N.; von Gunten, U.; Hammes, F. Inactivation efficiency of Escherichia coli and autochthonous bacteria during ozonation of municipal wastewater effluents quantified with flow cytometry and adenosine tri-phosphate analyses. Water Res. 2016, 101, 617–627. [Google Scholar] [CrossRef]
- Zimmermann, S.G.; Wittenwiler, M.; Hollender, J.; Krauss, M.; Ort, C.; Siegrist, H.; von Gunten, U. Kinetic assessment and modeling of an ozonation step for full-scale municipal wastewater treatment: Micropollutant oxidation, by-product formation and disinfection. Water Res. 2011, 45, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Von Gunten, U. Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 2003, 37, 1469–1487. [Google Scholar] [CrossRef]
- Blatchley, E.R.; Weng, S.; Afifi, M.Z.; Chiu, H.-H.; Reichlin, D.B.; Jousset, S.; Erhardt, R.S. Ozone and UV254 radiation for municipal wastewater disinfection. Water Environ. Res. 2012, 84, 2017–2027. [Google Scholar] [CrossRef]
- Colberg, P.J.; Lingg, A.J. Effect of Ozonation on Microbial Fish Pathogens, Ammonia, Nitrate, Nitrite, and BOD in Simulated Reuse Hatchery Water. J. Fish. Res. Board Can. 1978, 35, 1290–1296. [Google Scholar] [CrossRef]
- Liltved, H.; Hektoen, H.; Efraimsen, H. Inactivation of bacterial and viral fish pathogens by ozonation or UV irradiation in water of different salinity. Aquac. Eng. 1995, 14, 107–122. [Google Scholar] [CrossRef]
- Wedemeyer, G.A.; Nelson, N.C.; Smith, C.A. Survival of the Salmonid Viruses Infectious Hematopoietic Necrosis (IHNV) and Infectious Pancreatic Necrosis (IPNV) in Ozonated, Chlorinated, and Untreated Waters. J. Fish. Res. Board Can. 1978, 35, 875–879. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Sharrer, M.J.; Tsukuda, S.M.; Gearheart, M. Process requirements for achieving full-flow disinfection of recirculating water using ozonation and UV irradiation. Aquac. Eng. 2009, 40, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Sharrer, M.J.; Summerfelt, S.T. Ozonation followed by ultraviolet irradiation provides effective bacteria inactivation in a freshwater recirculating system. Aquac. Eng. 2007, 37, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Tao, Y.; Wang, L.; Zhang, X.; Lei, Y.; Wang, Z.; Noguchi, H. Performance of an integrated process combining ozonation with ceramic membrane ultra-filtration for advanced treatment of drinking water. Desalination 2014, 335, 47–54. [Google Scholar] [CrossRef]
- Zhang, J.; Knight, A.; Duke, M.; Northcott, K.; Packer, M.; Scales, P.J.; Gray, S.R. A new integrated potable reuse process for a small remote community in Antarctica. Process Saf. Environ. Prot. 2016, 104, 196–208. [Google Scholar] [CrossRef]
- Gomes, J.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Application of ozonation for pharmaceuticals and personal care products removal from water. Sci. Total Environ. 2017, 586, 265–283. [Google Scholar] [CrossRef]
- du Preez, M.; Conroy, R.M.; Ligondo, S.; Hennessy, J.; Elmore-Meegan, M.; Soita, A.; McGuigan, K.G. Randomized Intervention Study of Solar Disinfection of Drinking Water in the Prevention of Dysentery in Kenyan Children Aged under 5 Years. Environ. Sci. Technol. 2011, 45, 9315–9323. [Google Scholar] [CrossRef]
- McGuigan, K.G.; Samaiyar, P.; du Preez, M.; Conroy, R.M. High Compliance Randomized Controlled Field Trial of Solar Disinfection of Drinking Water and Its Impact on Childhood Diarrhea in Rural Cambodia. Environ. Sci. Technol. 2011, 45, 7862–7867. [Google Scholar] [CrossRef]
- Polo-López, M.I.; Fernández-Ibáñez, P.; Ubomba-Jaswa, E.; Navntoft, C.; García-Fernández, I.; Dunlop, P.S.M.; Schmid, M.; Byrne, J.A.; McGuigan, K.G. Elimination of water pathogens with solar radiation using an automated sequential batch CPC reactor. J. Hazard. Mater. 2011, 196, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Schwab, K.J.; Harding, A.S. Using Limes and Synthetic Psoralens to Enhance Solar Disinfection of Water (SODIS): A Laboratory Evaluation with Norovirus, Escherichia coli, and MS2. Am. J. Trop. Med. Hyg. 2012, 86, 566–572. [Google Scholar] [CrossRef]

| Type of Water | Water Characteristics | Pathogens Removal | Ozone Dose | Reference |
|---|---|---|---|---|
| Secondary municipal effluent | COD = 42–49 mg/L TSS = 4.5–6 mg/L E. coli = 1.8 × 103 CFU/mL | E. coli total removal | 0.3 mg/L (transferred dose) | [10] |
| Synthetic and Actual secondary urban effluent | Synthetic effluent: COD = 300 mg/L Actual effluent: COD = 25–50 mg/L TSS = 5.2 mg/L | Synthetic effluent: ~3.3 log fungi ~6.5 log bacteria Actual effluent: ~2 log fungi ~3–4 log bacteria | 225 mg/L (injected dose) | [46] |
| 2 secondary and 1 tertiary municipal effluent | COD = 30–71 mg/L TSS = 2.3–18 mg/L E. coli = 2.7–4.3 log CFU/100 mL Clostridum = 3.0–5.5 log CFU/100 mL | Viruses always removed Clostridium the most difficult pathogen | 2–15 mg/L (transferred dose, depending water quality) | [15] |
| Pathogen | CT (mgO3 min/L) | Reference |
|---|---|---|
| E. coli (bacteria) | 6.0 × 10−3 | [53] |
| C. parvum (protozoa) | 3.08 (25 °C) | [49] |
| G. lamblia (protozoa) | 0.65 | [50] |
| G. muris (protozoa) | 0.24 | [50] |
| Echovirus (virus) | 1.9 × 10−3 | [21] |
| Adenovirus (virus) | 4.1 × 10−3 | [21] |
| Coxsackievirus (virus) | 8.0 × 10−3 | [21] |
| Process | Operational Conditions | Most Relevant Results | Bacteria | ARG | References |
|---|---|---|---|---|---|
| Photocatalysis/UVA | TiO2 (P25) [TiO2] = 0.5 mg/cm2 immobilised onto borosilicate plate, 2 × UVA lamp (9 W 80 W/m2) DW and ASE from UWWTP | Bacteria declined from 3 log to 0.5 log (180 min). Gene pair conjugant numbers after treatment in DW showed a four-fold increase. In effluent, a lower reduction in ARG gene pair conjugates was observed. | E. coli | 9:1 mixture of J-53R (recipient) to HT-99 (donor) (i.e., the ARG recipient within the conjugated pair was present in 10-fold excess) | [135] |
| photocatalysis/simulated Sunlight | N-doped TiO2 [TiO2] = 25–500 mg/L Simulated sunlight (250 W lamp equipped with a UV filter) Effluents from UWWTP | Total inactivation of E. coli (60 min) N-TiO2 lead to higher efficiency than TiO2 | E. coli | cip, cef, tet and van according to Kirby–Bauer test. | [136] |
| photocatalysis/Simulated sunlight | TiO2 TiO2-rGO-PH TiO2-rGO-HD [Cat] = 100 mg/L Solar simulator Newport type 91193 (Xe Lamp (Vis, 1000 W, 63 W/m2), pH 5.2–6.2 effluents after MBR from WWTP | Total inactivation of E. coli (180 min) | E. coli | sul1, ampC, ermB, mecA, ecfX, 23S rRNA | [11] |
| Photocatalysis/UVC | TiO2 thin film UVC (254 nm, 300 W, 800 W) fluence = 0–120 mJ/cm2 PBS solution (pH = 7.4) NW from drinking water source (pH = 7.2) | No difference was observed between PBS and NW on bacteria inactivation. 5.8 log mecA reduction and 4.7 log ampC reduction were achieved (120 mJ/cm2) in the presence of TiO2 for both matrixes. | Staphylococcus aureus, Pseudomonas aeruginosa | mecA, ampC | [138] |
| photocatalysis/simulated and natural sunlight | Mn-, Co- and binary Mn/CoeTiO2 Natural and simulated solar irradiation (150 W), effluents after CAS from WWTP spiked with K. pneumonia | Simulated solar irradiation, yielding 6 log reduction after 30 min. Slower under natural solar irradiation (2 log). Only sul1 and ampC remained after Mn/Co-TiO2 photocatalysis. | Klebsiella pneumoniae | tetA, tetM, sul1, blaTEM, ampC | [137] |
| Photocatalysis/UVC/H2O2 | TiO2 thin film UVC (254 nm, 300 W, 800 W) fluence = 0–120 mJ/cm2 [H2O2] = 10–100 mmol/L PBS solution (pH = 7.4) NW from drinking water source (pH = 7.2) | Increasing H2O2 concentration increased efficiency. | Staphylococcus aureus, Pseudomonas aeruginosa | mecA, ampC | [138] |
| Photocatalysis/natural sunlight TiO2/H2O2 Solar/H2O2 | TiO2 (P25), GO-TiO2, [Cat.] = 200 mg/L [H2O2] = 20 mg/L Effluent from UWWTP | P25/H2O2 and Solar/H2O2 most eficiente processes for ARGs reduction. | Faecal coliforms and enterococci | 16S rRNA, intI1, qnrS, blaCTX-M, sul1, blaTEM and vanA | [139] |
| CT # (mg min/L) for 2 log Inactivation | ||||
|---|---|---|---|---|
| Escherichia coli | Bacillus subtilis spores | Cryptosporidium parvum | ||
| HO• radical driven | 0.000015 | 0.000082 | 0.000093 | |
| ozone | 0.006 | 4.1 | 3.5 | |
| Chlorine driven | ClO2 | 0.08 | 50 | 70 |
| Cl2 | 0.13 | 450 | 4200 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, J.; Matos, A.; Gmurek, M.; Quinta-Ferreira, R.M.; Martins, R.C. Ozone and Photocatalytic Processes for Pathogens Removal from Water: A Review. Catalysts 2019, 9, 46. https://doi.org/10.3390/catal9010046
Gomes J, Matos A, Gmurek M, Quinta-Ferreira RM, Martins RC. Ozone and Photocatalytic Processes for Pathogens Removal from Water: A Review. Catalysts. 2019; 9(1):46. https://doi.org/10.3390/catal9010046
Chicago/Turabian StyleGomes, João, Ana Matos, Marta Gmurek, Rosa M. Quinta-Ferreira, and Rui C. Martins. 2019. "Ozone and Photocatalytic Processes for Pathogens Removal from Water: A Review" Catalysts 9, no. 1: 46. https://doi.org/10.3390/catal9010046