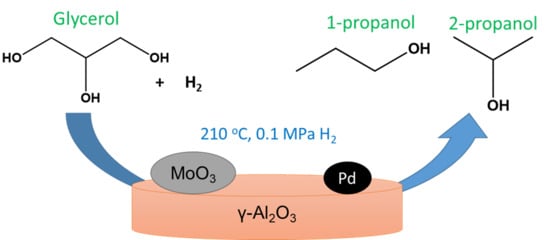
Toward the Sustainable Synthesis of Propanols from Renewable Glycerol over MoO3-Al2O3 Supported Palladium Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization Techniques
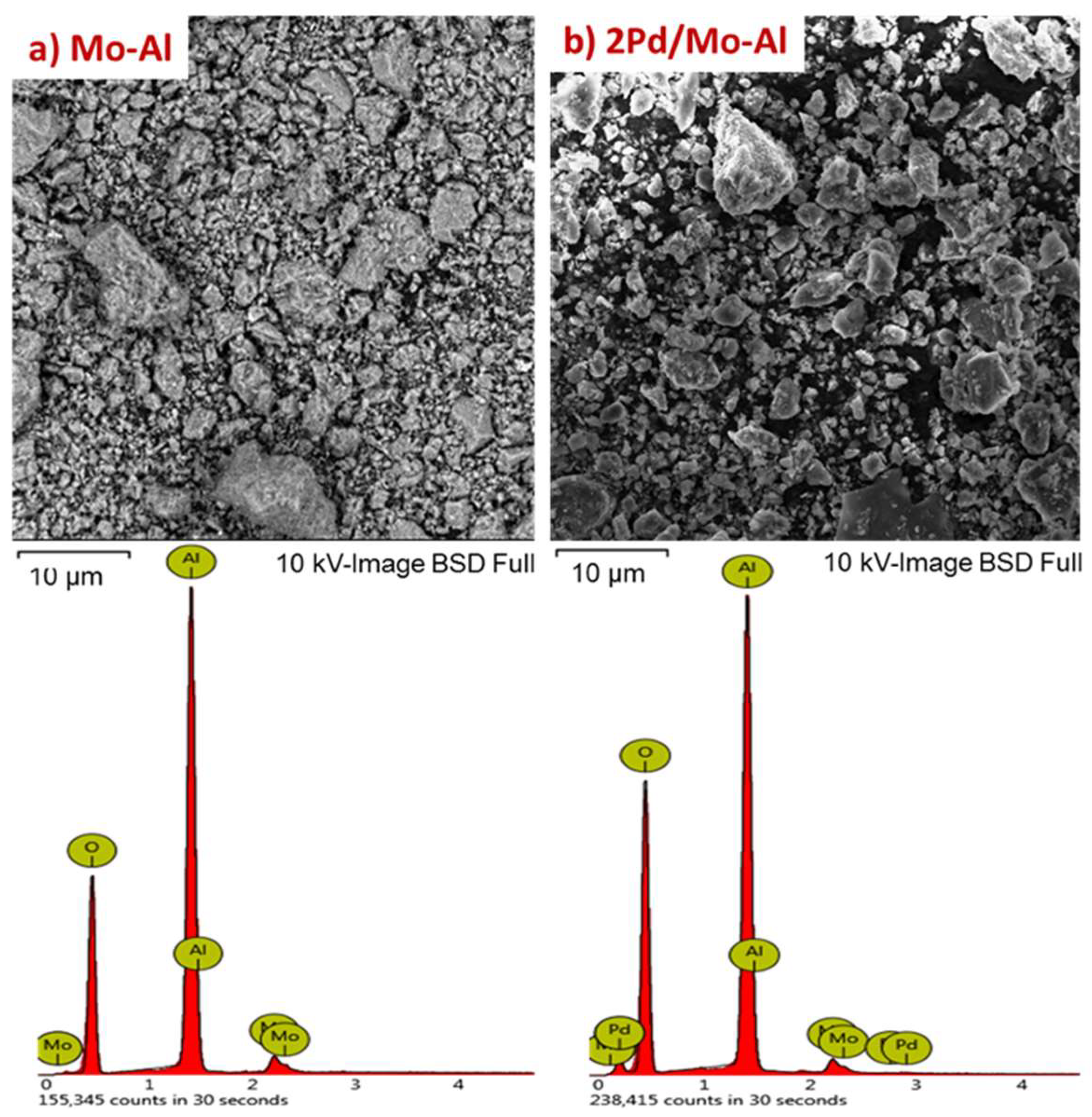
2.1.1. Structural Characterizations of the Catalysts
2.1.2. Physicochemical Properties of Catalysts
2.1.3. Acidity Measurement by NH3–TPD Analysis
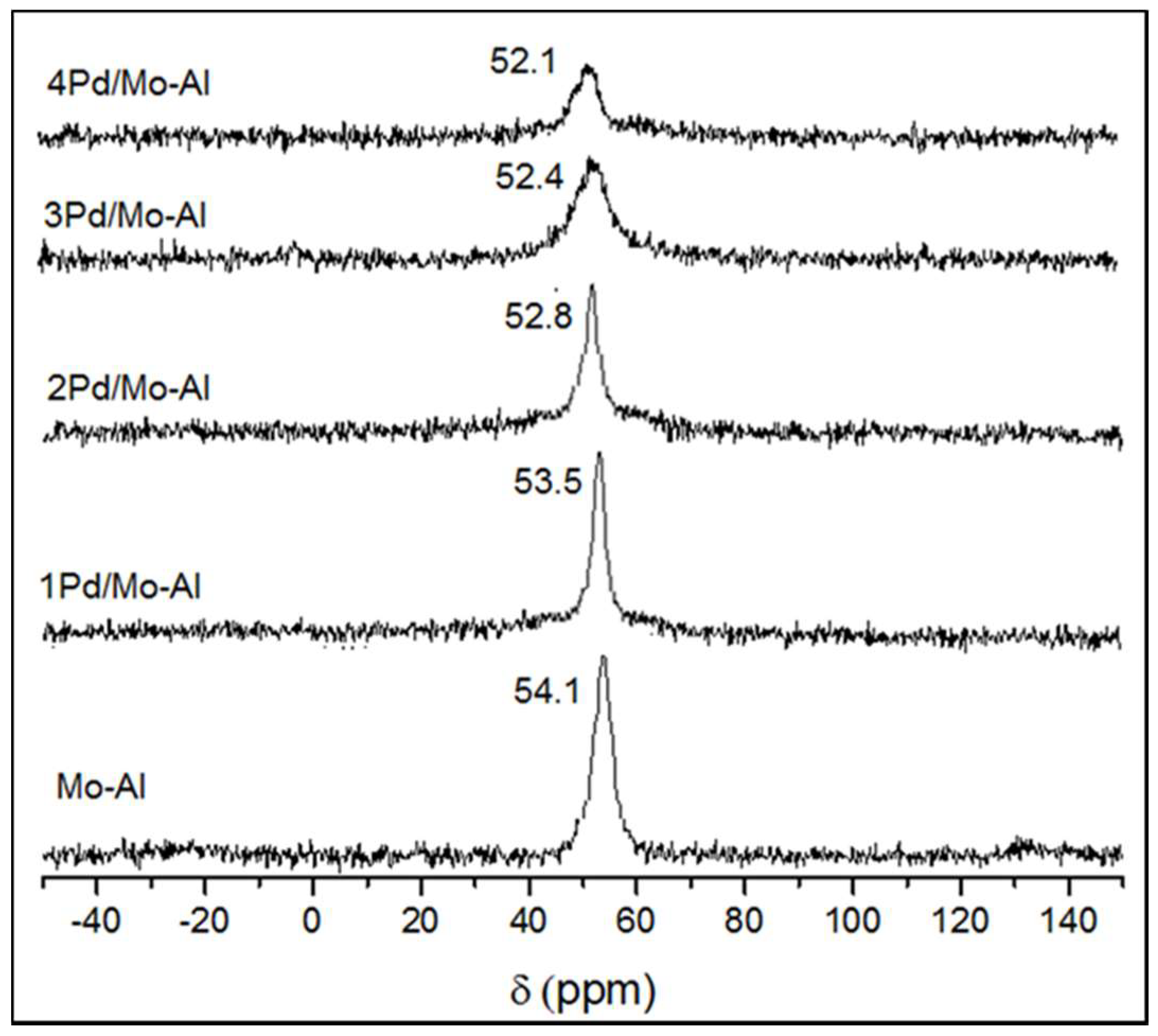
2.1.4. 27Al NMR Spectroscopy
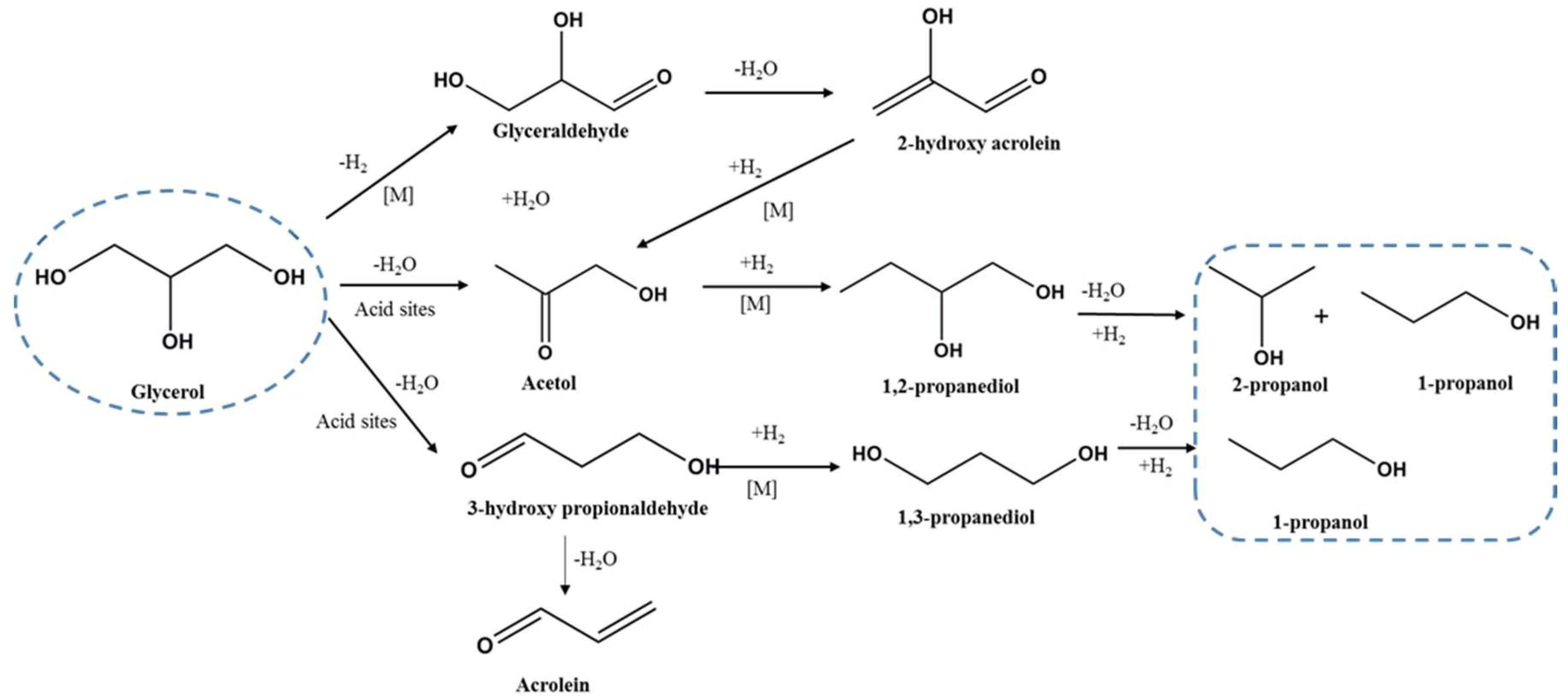
2.2. Catalytic Studies
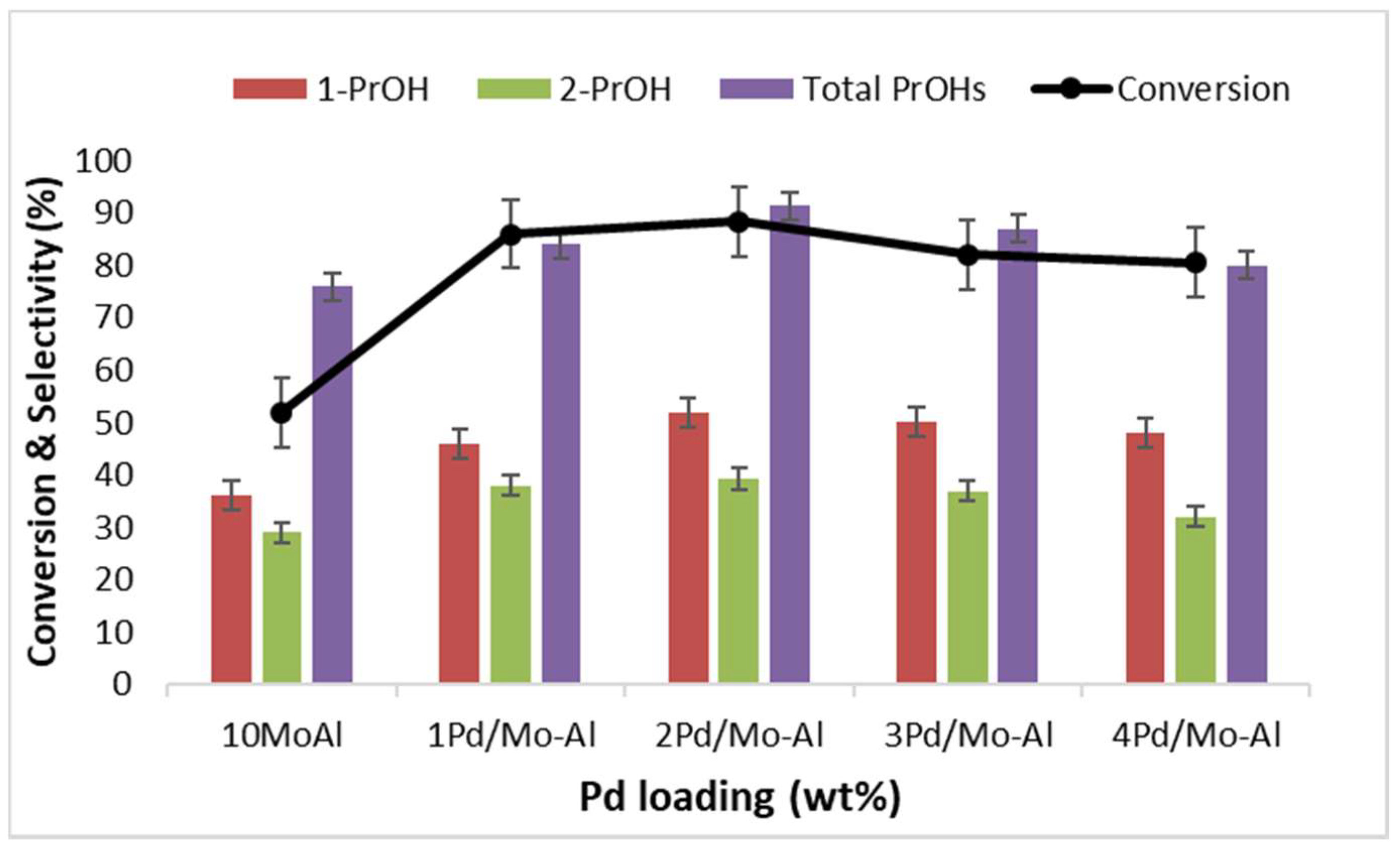
2.2.1. Effect of Pd Loading
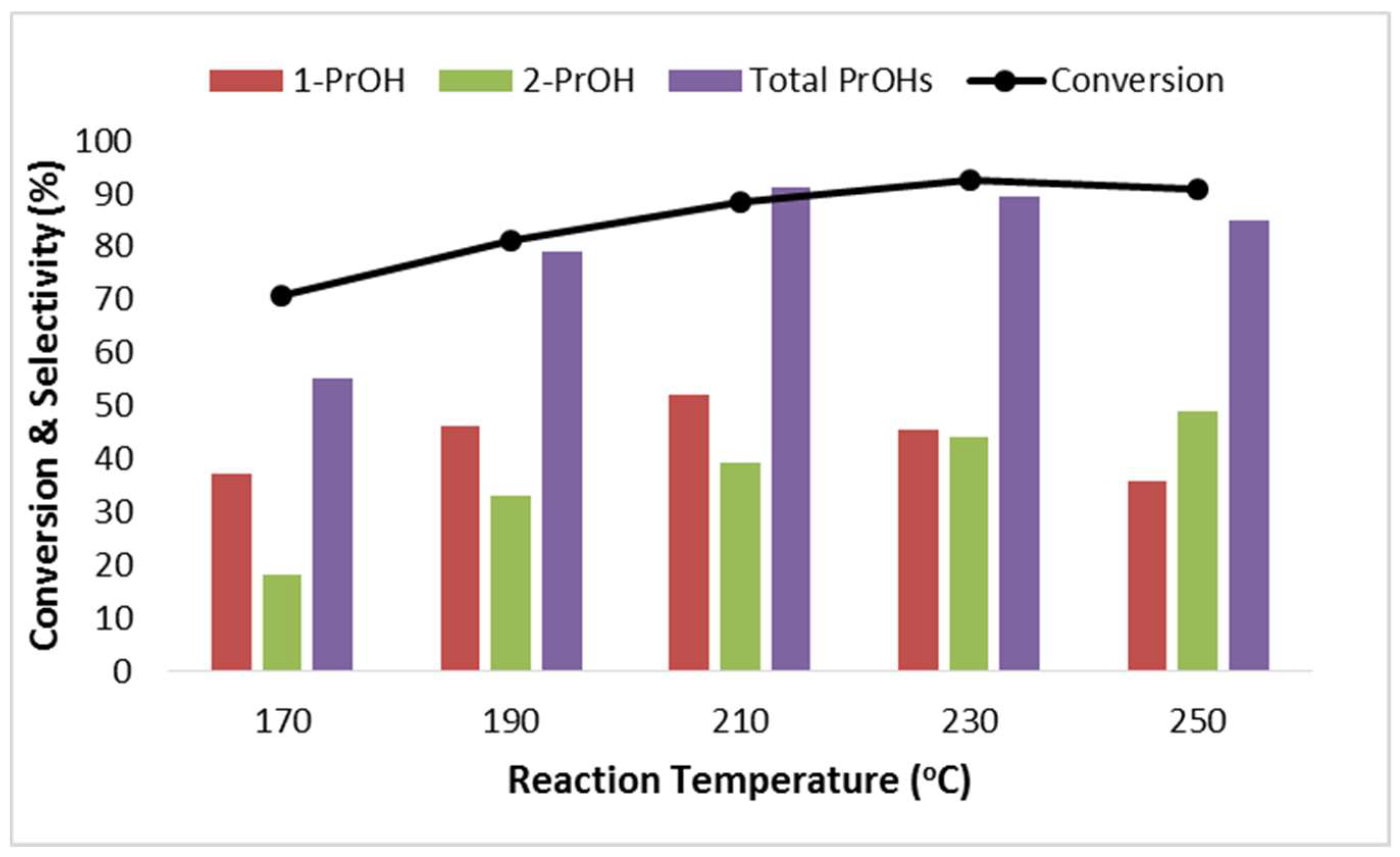
2.2.2. Effect of Reaction Temperature
2.2.3. Effect of Hydrogen Flow Rate
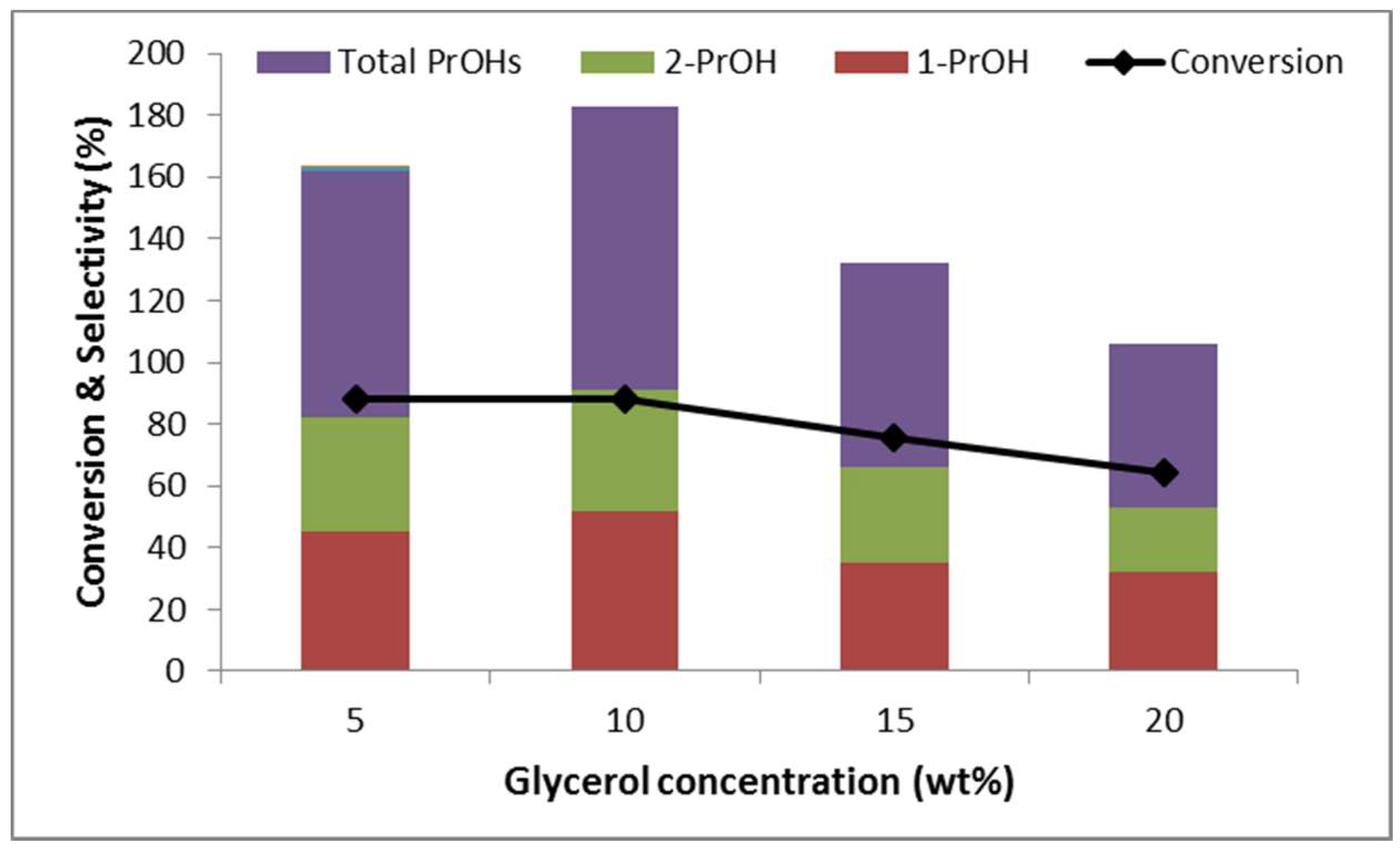
2.2.4. Effect of Glycerol Concentration
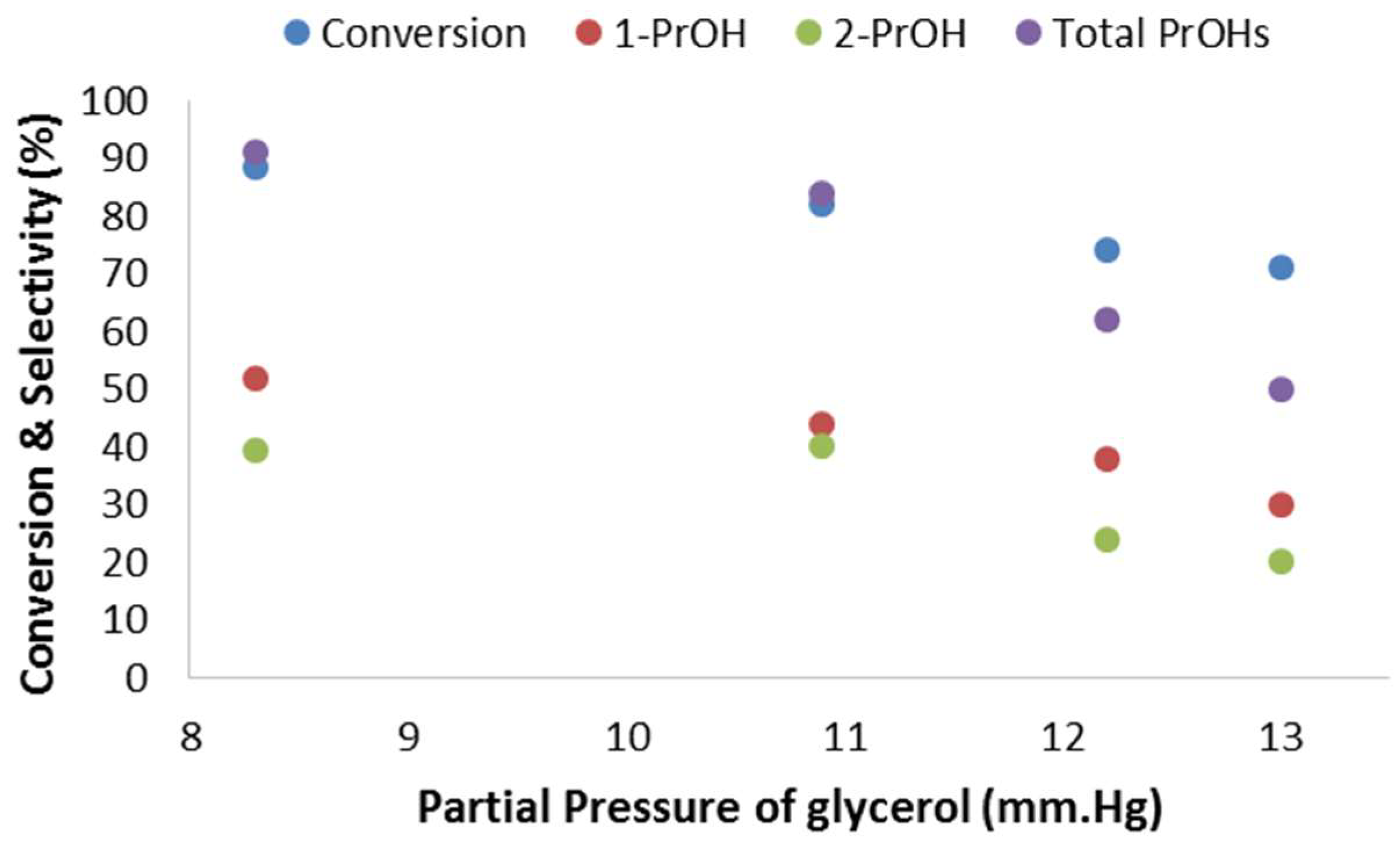
2.2.5. Effect of the Partial Pressure of Glycerol
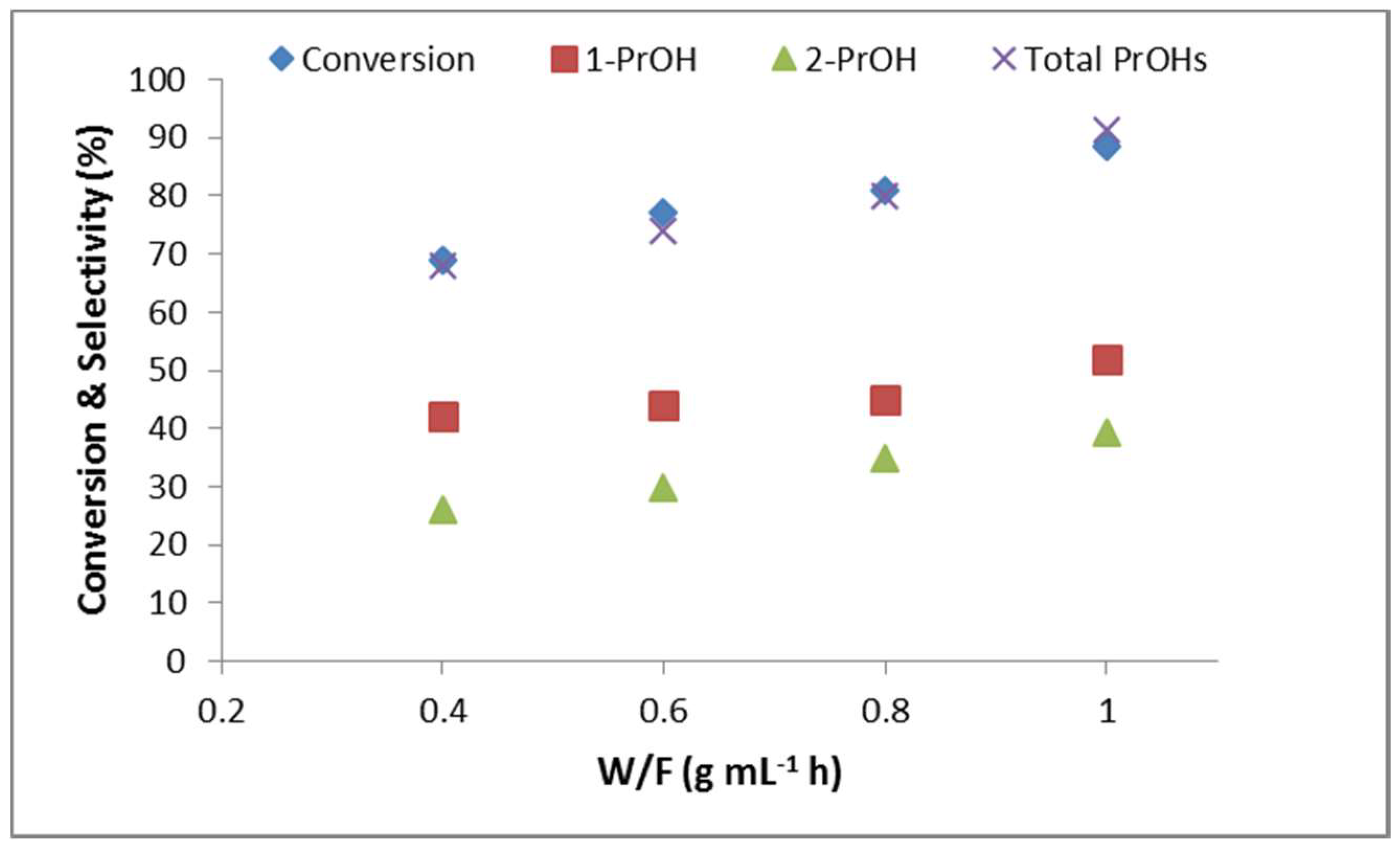
2.2.6. Effect of Contact Time (W/F)
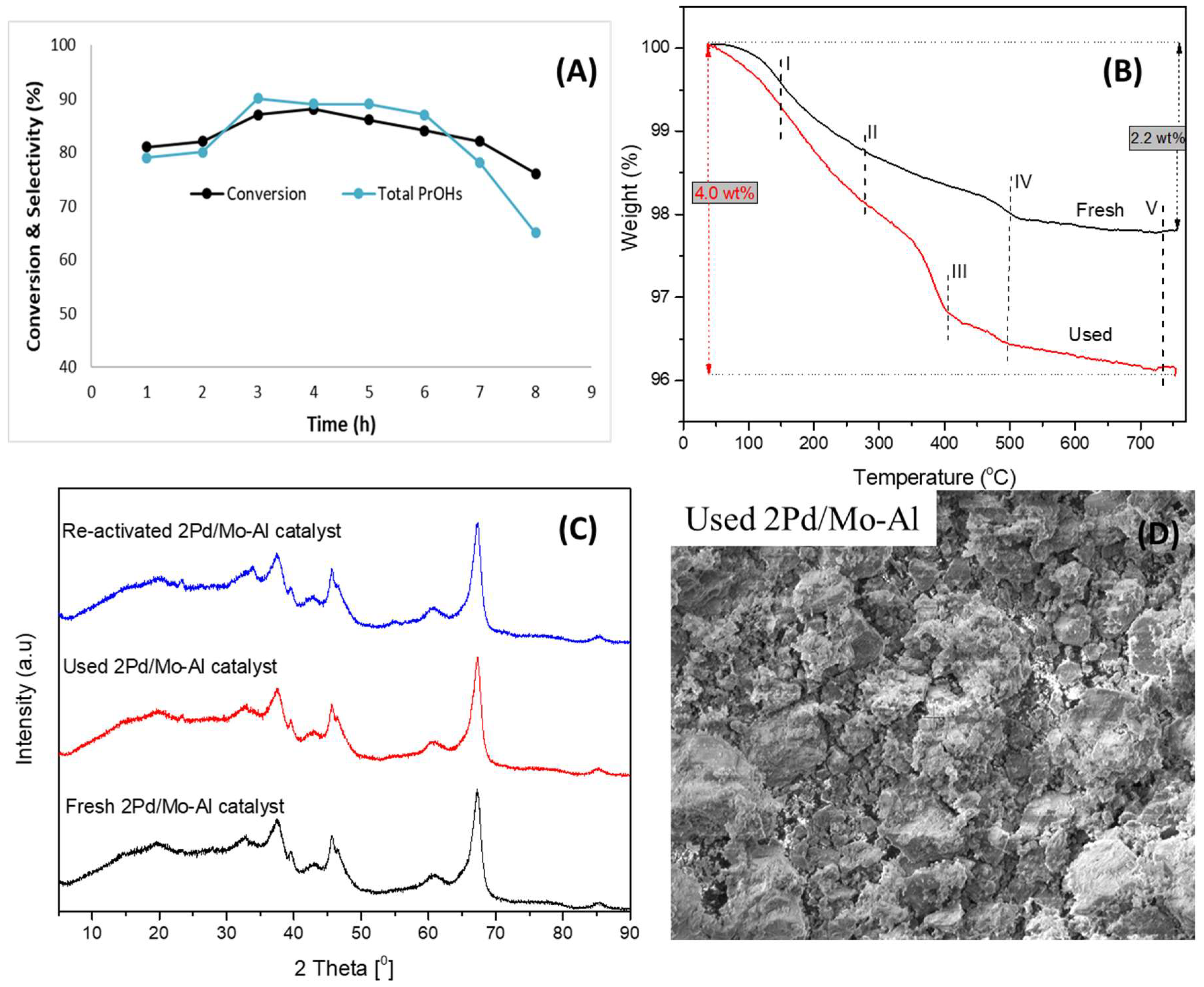
2.2.7. Time on Stream Experiment
2.3. Reusability of the Catalyst
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Testing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Werpy, T.; Petersen, G.; Aden, A.; Bozell, J.; Holladay, J.; White, J.; Manheim, A.; Eliot, D.; Lasure, L.; Jones, S. Top Value Added Chemicals from Biomass. Results of Screening for Potential Candidates from Sugars and Synthesis Gas; US Department of Energy: Southwest Washington, DC, USA, 2004; Volume 1. [Google Scholar]
- Zhou, C.H.C.; Beltramini, J.N.; Fan, Y.X.; Lu, G.Q.M. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.S.; Selvakannan, P.R.; Chary, K.V.R.; Kantam, M.L.; Bhargava, S.K. Solvent-Free Microwave-Assisted Synthesis of Solketal from Glycerol Using Transition Metal Ions Promoted Mordenite Solid Acid Catalysts. J. Mol. Catal. A Chem. 2017, 434, 184–193. [Google Scholar] [CrossRef]
- Li, R.; Song, H.; Chen, J. Propylsulfonic Acid Functionalized SBA-15 Mesoporous Silica as Efficient Catalysts for the Acetalization of Glycerol. Catalysts 2018, 8, 297. [Google Scholar] [CrossRef]
- Bossaert, W.D.; De Vos, D.E.; Rhijn, W.M.V.; Bullen, J.; Grobet, P.J.; Jacobs, P.A. Mesoporous Sulfonic Acids as Selective Heterogeneous Catalysts for the Synthesis of Monoglycerides. J. Catal. 1999, 182, 156–164. [Google Scholar] [CrossRef]
- Karinen, R.S.; Krause, A.O.I. New biocomponents from glycerol. Appl. Catal. A Gen. 2006, 306, 128–133. [Google Scholar] [CrossRef]
- Demirel, S.; Lucas, M.; Warna, J.; Salmi, T.; Murzin, D.; Claus, P. Reaction kinetics and modeling of the gold catalysed glycerol oxidation. Top. Catal. 2005, 44, 299–305. [Google Scholar] [CrossRef]
- Kim, Y.T.; Jung, K.D.; Park, E.D. Gas-phase dehydration of glycerol over ZSM-5 catalysts. Microporous Mesoporous Mater. 2010, 131, 28–36. [Google Scholar] [CrossRef]
- Priya, S.S.; Kandasamy, S.; Bhattacharya, S. Turning Biodiesel Waste Glycerol into 1,3-Propanediol: Catalytic Performance of Sulphuric acid-Activated Montmorillonite Supported Platinum Catalysts in Glycerol Hydrogenolysis. Nat. Sci. Rep. 2018, 8, 7484. [Google Scholar]
- King, D.L.; Zhang, L.; Xia, G.; Karim, A.M.; Heldebrant, D.J.; Wang, X.; Peterson, T.; Wang, Y. Aqueous phase reforming of glycerol for hydrogen production over Pt–Re supported on carbon. Appl. Catal. B Environ. 2010, 99, 206–213. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. Appl. Catal. B Environ. 2010, 193, 75–92. [Google Scholar] [CrossRef]
- Unruh, J.D.; Pearson, D. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Logsdon, J.E.; Loke, R.A. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Dam, J.T.; Djanashvili, K.; Kapteijn, F.; Hanefeld, U. Pt/Al2O3 Catalyzed 1,3-Propanediol Formation from Glycerol using Tungsten Additives. ChemCatChem 2013, 5, 497–505. [Google Scholar]
- Arundhathi, R.; Mizugaki, T.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. Highly Selective Hydrogenolysis of Glycerol to 1,3-Propanediol over a Boehmite-Supported Platinum/Tungsten Catalyst. ChemSusChem 2013, 6, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Qiu, Y.; Zhu, Y.; Hao, S.; Zheng, H.; Li, Y. Hydrogenolysis of glycerol to 1,3-propanediol over bifunctional catalysts containing Pt and heteropolyacids. Catal. Today 2013, 212, 120–126. [Google Scholar] [CrossRef]
- Priya, S.S.; Kumar, V.P.; Kantam, M.L.; Bhargava, S.K.; Chary, K.V.R. Catalytic performance of Pt/AlPO4 catalysts for selective hydrogenolysis of glycerol to 1,3- propanediol in the vapour phase. RSC Adv. 2014, 4, 51893–51903. [Google Scholar] [CrossRef]
- Ryneveld, E.V.; Mahomed, A.S.; Heerden, P.S.V.; Green, M.J.; Friedrich, H.B. A catalytic route to lower alcohols from glycerol using Ni-supported catalysts. Green Chem. 2011, 13, 1819–1827. [Google Scholar] [CrossRef]
- Tamura, M.; Amada, Y.; Liu, S.; Yuan, Z.; Nakagawa, Y.; Tomishige, K. Promoting effect of Ru on Ir-ReOx/SiO2catalyst in hydrogenolysisof glycerol. J. Mol. Catal. A Chem. 2014, 388–399, 177–187. [Google Scholar] [CrossRef]
- Furikado, I.; Miyazawa, T.; Koso, S.; Shimao, A.; Kunimori, K.; Tomishige, K. Catalytic performance of Rh/SiO2 in glycerol reaction under hydrogen. Green Chem. 2007, 9, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Amada, Y.; Koso, S.; Nakagawa, Y.; Tomishige, K. Hydrogenolysis of 1,2-Propanediol for the Production of Biopropanols from Glycerol. ChemSusChem 2010, 3, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Ryneveld, E.V.; Mahomed, A.S.; Heerden, P.S.V.; Friedrich, H.B. Direct Hydrogenolysis of Highly Concentrated Glycerol Solutions over Supported Ru, Pd and Pt Catalyst Systems. Catal. Lett. 2011, 141, 958–967. [Google Scholar] [CrossRef]
- Li, C.; He, B.; Ling, Y.; Tsang, C.W.; Lian, C. Glycerol hydrogenolysis to n-propanol over Zr-Al composite oxide-supported Pt catalysts. Chin. J. Catal. 2011, 39, 1121–1128. [Google Scholar] [CrossRef]
- Wang, M.; Yang, H.; Xie, Y.; Wu, X.; Chen, C.; Ma, W.; Dong, Q.; Hou, Z. Catalytic transformation of glycerol to 1-propanol by combining zirconium phosphate and supported Ru catalysts. RSC Adv. 2016, 6, 29769. [Google Scholar] [CrossRef]
- Schlaf, M.; Ghosh, P.; Fagan, P.J.; Hauptman, E.; Bullock, R.M. Catalytic Deoxygenation of 1,2-Propanediol to Give n-Propanol. Adv. Synth. Catal. 2009, 351, 789–800. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, Y.; Hao, S.; Zheng, H.; Mo, T.; Li, Y. One-step hydrogenolysis of glycerol to biopropanols over Pt–H4SiW12O40/ZrO2 catalysts. Green Chem. 2012, 14, 2607–2616. [Google Scholar] [CrossRef]
- Priya, S.S.; Kumar, V.P.; Kantam, M.L.; Bhargava, S.K.; Periasamy, S.; Chary, K.V.R. Metal–acid bifunctional catalysts for selective hydrogenolysis of glycerol under atmospheric pressure: A highly selective route toproduce propanols. Appl. Catal. A Gen. 2015, 498, 88–98. [Google Scholar] [CrossRef]
- Lin, X.; Lv, Y.; Xi, Y.; Qu, Y.; Phillips, D.L.; Liu, C. Hydrogenolysis of Glycerol by the Combined Use of Zeolite and Ni/Al2O3 as Catalysts: A Route for Achieving High Selectivity to 1-Propanol. Energy Fuels 2014, 28, 3345–3351. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, W.; Long, X.; Nie, H. Redispersion effects of citric acid on CoMo/-Al2O3 hydrodesulfurization catalysts. Catal. Commun. 2016, 82, 20–23. [Google Scholar] [CrossRef]
- Meng, D.; Wang, B.; Yu, W.; Wang, W.; Li, Z.; Ma, X. Effect of Citric Acid on MoO3/Al2O3 Catalysts for Sulfur-Resistant Methanation. Catalysts 2017, 7, 151. [Google Scholar] [CrossRef]
- Halasz, I.; Brenner, A. Preparation and characterization of PdO-MoO & Al2O3 catalysts. Appl. Catal. A Gen. 1992, 82, 51–63. [Google Scholar]
- Del Arco, M.; Carrazan, S.R.G.; Martin, C.; Martin, I.; Rives, V.; Maletb, P. Surface dispersion of molybdena supported on silica, alumina and titania. J. Mater. Chem. 1993, 3, 1313–1318. [Google Scholar] [CrossRef]
- Yang, X.L.; Dai, W.L.; Gao, R.; Fan, K. Characterization and catalytic behavior of highly active tungsten-doped SBA-15 catalyst in the synthesis of glutaraldehyde using an anhydrous approach. J. Catal. 2007, 249, 278–288. [Google Scholar] [CrossRef]
- Barras, J.; Klinowski, J. 27AI and 29Si Solid-state NMR Studies of Dealuminated Mordenite. J. Chem. Soc. Faraday Trans. 1994, 90, 3719–3723. [Google Scholar] [CrossRef]
- Priya, S.S.; Bhanuchander, P.; Kumar, V.P.; Deepa, D.; Selvakannan, P.R.; Kantam, M.L.; Bhargava, S.K.; Chary, K.V.R. Platinum supported on H-Mordenite: A highly efficient catalyst for selective hydrogenolysis of glycerol to 1,3-PDO. ACS Sustain. Chem. Eng. 2016, 4, 1212–1222. [Google Scholar] [CrossRef]
- Oh, J.; Dash, S.; Lee, H. Selective conversion of glycerol to 1,3-propanediol using Pt-sulfated Zirconia. Green Chem. 2011, 13, 2004–2007. [Google Scholar] [CrossRef]
- Priya, S.S.; Kumar, V.P.; Kantam, M.L.; Bhargava, S.K.; Srikanth, A.; Chary, K.V.R. High Efficiency Conversion of Glycerol to 1,3-Propanediol Using a Novel Platinum−Tungsten Catalyst Supported on SBA-15. Ind. Eng. Chem. Res. 2015, 54, 9104–9115. [Google Scholar] [CrossRef]
- Priya, S.S.; Bhanuchander, P.; Kumar, V.P.; Bhargava, S.K.; Chary, K.V.R. Activity & Selectivity of Platinum-Copper bimetallic catalysts supported on mordenite for glycerol hydrogenolysis to 1,3-propanediol. Ind. Eng. Chem. Res. 2016, 55, 4461–4472. [Google Scholar]
- Priya, S.S.; Kumar, V.P.; Kantam, M.L.; Bhargava, S.K.; Chary, K.V.R. Vapour-Phase Hydrogenolysis of Glycerol to 1,3-Propanediol Over Supported Pt Catalysts: The Effect of Supports on the Catalytic Functionalities. Catal. Lett. 2014, 144, 2129–2143. [Google Scholar] [CrossRef]
- Amada, Y.; Shinmi, Y.; Koso, S.; Kubota, T.; Nakagawa, Y.; Tomishige, K. Reaction mechanism of the glycerol hydrogenolysis to 1,3-propanediol over Ir-ReOx/SiO2 catalyst. Appl. Catal. B Environ. 2011, 105, 117–127. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, Y.; Zheng, H.; Ding, G.; Li, Y. Direct conversion of glycerol into 1,3-propanediol over Cu-H4SiW12O40/SiO2 in vapor phase. Catal. Lett. 2009, 131, 312–320. [Google Scholar] [CrossRef]
- Cavani, F.; Guidetti, S.; Trevisanut, C.; Ghedini, E.; Signoretto, M. Unexpected events in sulfated zirconia catalyst during glycerol-to-acrolein conversion. Appl. Catal. A Gen. 2011, 409–410, 267–278. [Google Scholar] [CrossRef]
- Miyazawa, T.; Kusunoki, Y.; Kunimori, K.; Tomishige, K. Glycerol conversion in the aqueous solution under hydrogen over Ru/C + an ion-exchange resin and its reaction mechanism. J. Catal. 2006, 240, 213–221. [Google Scholar] [CrossRef]
- Kumar, V.P.; Priya, S.S.; Harikrishna, Y.; Kumar, A.; Chary, K.V.R. Catalytic Functionalities of Nano Ruthenium/γ-Al2O3 Catalysts for the Vapour Phase Hydrogenolysis of Glycerol. J. Nanosci. Nanotechnol. 2016, 16, 1952–1960. [Google Scholar] [CrossRef]
- El-Shobakya, H.G.; Mokhtarb, M.; Ahmeda, A.S. Effect of MgO-doping on solid-solid interactions in MoO3/Al2O3 system. Thermochim. Acta 1999, 327, 39–46. [Google Scholar] [CrossRef]
- Toniolo, F.S.; Barbosa-Coutinho, E.; Schwaab, M.; Leocadio, I.C.L.; Aderne, R.S.; Schmal, M.; Pinto, J.C. Kinetics of the catalytic combustion of diesel soot with MoO3/Al2O3 catalyst from thermogravimetric analyses. Appl. Catal. A Gen. 2008, 342, 87–92. [Google Scholar]





| Catalyst | Weight Percentage (%) a | ICP-AES b (wt%) | ||||
|---|---|---|---|---|---|---|
| Mo | Al | O | Pd | N | ||
| Mo-Al | 7.83 | 48.17 | 44.00 | -- | -- | -- |
| 1Pd/Mo-Al | 6.99 | 43.02 | 48.82 | 0.67 | 0.50 | 0.59 |
| 2Pd/Mo-Al | 7.12 | 40.86 | 50.22 | 1.22 | 0.58 | 1.18 |
| 3Pd/Mo-Al | 7.01 | 40.33 | 49.70 | 2.41 | 0.55 | 2.20 |
| 4Pd/Mo-Al | 7.24 | 40.99 | 48.21 | 3.07 | 0.49 | 2.97 |
| Catalyst | BET Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| Mo-Al | 189 | 5.9 | 0.29 |
| 1Pd/Mo-Al | 181 | 5.8 | 0.28 |
| 2Pd/Mo-Al | 170 | 5.6 | 0.28 |
| 3Pd/Mo-Al | 162 | 5.3 | 0.26 |
| 4Pd/Mo-Al | 158 | 5.3 | 0.23 |
| Catalyst | NH3 Uptake (μmol/g) | Total NH3 Uptake (μmol/g) | ||
|---|---|---|---|---|
| Weak | Moderate | Strong | ||
| γ-Al2O3 | 298.7 | 426.2 | -- | 724.9 |
| Mo-Al | 323.5 | 350.6 | -- | 674.1 |
| 1Pd/Mo-Al | 297.3 | 361.2 | -- | 658.5 |
| 2Pd/Mo-Al | 273.3 | 372.2 | -- | 645.5 |
| 3Pd/Mo-Al | 194.2 | 369.0 | -- | 563.2 |
| 4Pd/Mo-Al | 195.0 | 364.9 | -- | 559.9 |
| Catalyst | Conversion (%) | Selectivity of Total PrOHs (%) | BET Surface Area (m2/g) | Total Acidity (NH3 μmol/g) |
|---|---|---|---|---|
| Fresh | 88.4 | 91.3 | 170 | 645.5 |
| Used | 87.0 | 90.0 | 162 | 594.0 |
| Reactivated | 88.0 | 90.9 | 168 | 617.2 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samudrala, S.P.; Bhattacharya, S. Toward the Sustainable Synthesis of Propanols from Renewable Glycerol over MoO3-Al2O3 Supported Palladium Catalysts. Catalysts 2018, 8, 385. https://doi.org/10.3390/catal8090385
Samudrala SP, Bhattacharya S. Toward the Sustainable Synthesis of Propanols from Renewable Glycerol over MoO3-Al2O3 Supported Palladium Catalysts. Catalysts. 2018; 8(9):385. https://doi.org/10.3390/catal8090385
Chicago/Turabian StyleSamudrala, Shanthi Priya, and Sankar Bhattacharya. 2018. "Toward the Sustainable Synthesis of Propanols from Renewable Glycerol over MoO3-Al2O3 Supported Palladium Catalysts" Catalysts 8, no. 9: 385. https://doi.org/10.3390/catal8090385