Improving Interfacial Charge-Transfer Transitions in Nb-Doped TiO2 Electrodes with 7,7,8,8-Tetracyanoquinodimethane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization of Nb-Doped TiO2
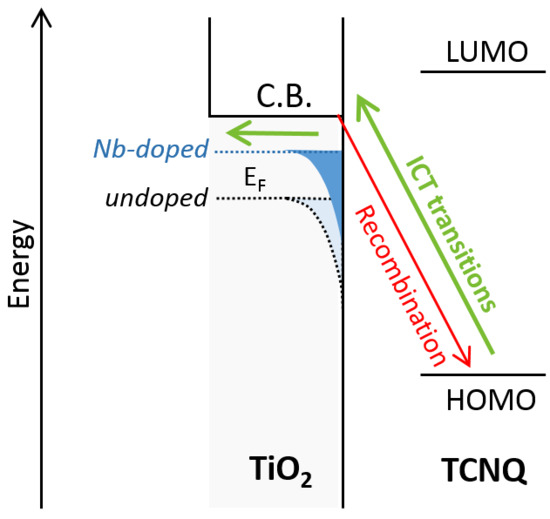
2.2. Optical Properties of Nb-Doped TiO2
2.3. Photovoltaic Performance of Interfacial Charge-Transfer (ICT) Photoconversion Devices
3. Experimental Section
3.1. Preparation of Nb-Doped TiO2
3.2. Cell Fabrication
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fujisawa, J.-I. Large Impact of Reorganization Energy on Photovoltaic Conversion Due to Interfacial Charge-Transfer Transitions. Phys. Chem. Chem. Phys. 2015, 17, 12228–12237. [Google Scholar] [CrossRef] [PubMed]
- Xagas, A.P.; Bernard, M.C.; Hugot-Le Goff, A.; Spyrellis, N.; Loizos, Z.; Falaras, P. Surface Modification and Photosensitisation of TiO2 Nanocrystalline Films with Ascorbic Acid. J. Photochem. Photobiol. A 2000, 132, 115–120. [Google Scholar] [CrossRef]
- Tae, E.L.; Lee, S.H.; Lee, J.K.; Yoo, S.S.; Kang, E.J.; Yoon, K.B. A Strategy to Increase the Efficiency of the Dye-Sensitized TiO2 Solar Cells Operated by Photoexcitation of Dye-to-TiO2 Charge-Transfer Bands. J. Phys. Chem. B 2005, 109, 22513–22522. [Google Scholar] [CrossRef] [PubMed]
- Manzhos, S.; Jono, R.; Yamashita, K.; Fujisawa, J.-I.; Nagata, M.; Segawa, H. Study of Interfacial Charge Transfer Bands and Electron Recombination in the Surface Complexes of TCNE, TCNQ, and TCNAQ with TiO2. J. Phys. Chem. C 2011, 115, 21487–21493. [Google Scholar] [CrossRef]
- Manzhos, S.; Fujisawa, J.-I.; Segawa, H.; Yamashita, K. Isotopic Substitution as a Strategy to Control Non-Adiabatic Dynamics in Photoelectrochemical Cells: Surface Complexes between TiO2 and Dicyanomethylene Compounds. J. Appl. Phys. 2012, 51, 10NE03. [Google Scholar] [CrossRef]
- Fujisawa, J.-I.; Nagata, M.; Hanaya, M. Charge-Transfer Complex Versus [Sigma]-Complex Formed between TiO2 and Bis (Dicyanomethylene) Electron Acceptors. Phys. Chem. Chem. Phys. 2015, 17, 27343–27356. [Google Scholar] [CrossRef] [PubMed]
- Michinobu, T.; Satoh, N.; Cai, J.; Li, Y.; Han, L. Novel Design of Organic Donor-Acceptor Dyes without Carboxylic Acid Anchoring Groups for Dye-Sensitized Solar Cells. J. Mater. Chem. C 2014, 2, 3367–3372. [Google Scholar] [CrossRef]
- Fujisawa, J.-I.; Hanaya, M. Electronic Structures of TiO2–TCNE, –TCNQ, and –2,6-TCNAQ Surface Complexes Studied by Ionization Potential Measurements and DFT Calculations: Mechanism of the Shift of Interfacial Charge-Transfer Bands. Chem. Phys. Lett. 2016, 653, 11–16. [Google Scholar] [CrossRef]
- Wang, Y.; Hang, K.; Anderson, N.A.; Lian, T. Comparison of Electron Transfer Dynamics in Molecule-to-Nanoparticle and Intramolecular Charge Transfer Complexes. J. Phys. Chem. B 2003, 107, 9434–9440. [Google Scholar] [CrossRef]
- Hod, I.; Shalom, M.; Tachan, Z.; Rühle, S.; Zaban, A. SrTiO3 Recombination-Inhibiting Barrier Layer for Type II Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 10015–10018. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Numata, Y.; Han, L. Highly Efficient Dye-Sensitized Solar Cells: Progress and Future Challenges. Energy Environ. Sci. 2013, 6, 1443–1464. [Google Scholar] [CrossRef]
- Nikolay, T.; Larina, L.; Shevaleevskiy, O.; Ahn, B.A. Electronic Structure Study of Lightly Nb-Doped TiO2 Electrode for Dye-Sensitized Solar Cells. Energy Environ. Sci. 2011, 4, 1480–1486. [Google Scholar] [CrossRef]
- Ghartavol, H.M.; Mohammadi, M.R.; Afshar, A.; Chau-Nan Hong, F.; Jeng, Y.-R. Efficient Dye-Sensitized Solar Cells Based on CNT-Derived TiO2 Nanotubes and Nb-Doped TiO2 Nanoparticles. RSC Adv. 2016, 6, 101737–101744. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.-G.; Wang, X.; Zhang, M.; Guo, M. Titanium Mesh Supported TiO2 Nanowire Arrays/Nb-Doped TiO2 Nanoparticles for Fully Flexible Dye-Sensitized Solar Cells with Improved Photovoltaic Properties. J. Mater. Chem. C 2016, 4, 11118–11128. [Google Scholar] [CrossRef]
- Su, H.; Huang, Y.-T.; Chang, Y.-H.; Zhai, P.; Hau, N.Y.; Cheung, P.C.H.; Yeh, W.-T.; Wei, N.T.-C.; Feng, S.-P. The Synthesis of Nb-Doped TiO2 Nanoparticles for Improved-Performance Dye Sensitized Solar Cells. Electrochim. Acta 2015, 182, 230–237. [Google Scholar] [CrossRef]
- Lü, X.; Mou, M.X.; Wu, J.; Zhang, D.; Zhang, L.; Huang, F.; Xu, F.; Huang, S. Improved-Performance Dye-Sensitized Solar Cells Using Nb-Doped TiO2 Electrodes: Efficient Electron Injection and Transfer. Adv. Funct. Mater. 2010, 20, 509–515. [Google Scholar] [CrossRef]
- Kim, S.G.; Ju, M.J.; Choi, I.T.; Choi, W.S.; Choi, H.-J.; Baek, J.-B.; Kim, H.K. Nb-Doped TiO2 Nanoparticles for Organic Dye-Sensitized Solar Cells. RSC Adv. 2013, 3, 16380–16386. [Google Scholar] [CrossRef]
- Liu, J.; Duan, Y.; Zhou, X.; Lin, Y. Influence of VB Group Doped TiO2 on Photovoltaic Performance of Dye-Sensitized Solar Cells. Appl. Surf. Sci. 2013, 277, 231–236. [Google Scholar] [CrossRef]
- Hishita, S.; Mutoh, I.; Koumoto, K.; Yanagida, H. Inhibition Mechanism of the Anatase-Rutile Phase Transformation by Rare Earth Oxides. Ceram. Int. 1983, 9, 61–67. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-Ray Diffraction. Am. J. Phys. 1957, 25, 394–395. [Google Scholar] [CrossRef]
- Jono, R.; Fujisawa, J.-I.; Segawa, H.; Yamashita, K. Theoretical Study of the Surface Complex between TiO2 and TCNQ Showing Interfacial Charge-Transfer Transitions. J. Phys. Chem. Lett. 2011, 2, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Taro, H.; Hideyuki, K.; Koichi, Y.; Hiroyuki, N.; Yutaka, F.; Shoichiro, N.; Naoomi, Y.; Akira, C.; Hiroshi, K.; Masaharu, O.; et al. Electronic Band Structure of Transparent Conductor: Nb-Doped Anatase TiO2. Appl. Phys. Express 2008, 1, 111203. [Google Scholar]
- Bisquert, J. Theory of the Impedance of Electron Diffusion and Recombination in a Thin Layer. J. Phys. Chem. B 2002, 106, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zakeeruddin, S.M.; O’Regan, B.C.; Humphry-Baker, R.; Grätzel, M. Influence of 4-Guanidinobutyric Acid as Coadsorbent in Reducing Recombination in Dye-Sensitized Solar Cells. J. Phys. Chem. B 2005, 109, 21818–21824. [Google Scholar] [CrossRef] [PubMed]
- Karvinen, S. The Effects of Trace Elements on the Crystal Properties of TiO2. Solid State Sci. 2003, 5, 811–819. [Google Scholar] [CrossRef]
- Ito, S.; Murakami, T.N.; Comte, P.; Liska, P.; Grätzel, C.; Nazeeruddin, M.K.; Grätzel, M. Fabrication of Thin Film Dye Sensitized Solar Cells with Solar to Electric Power Conversion Efficiency over 10%. Thin Solid Films 2008, 516, 4613–4619. [Google Scholar] [CrossRef]
- Kubelka, P. New Contributions to the Optics of Intensely Light-Scattering Materials. Part I. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef]
- Yang, L.; Kruse, B. Revised Kubelka–Munk Theory. I. Theory and Application. J. Opt. Soc. Am. A 2004, 21, 1933–1941. [Google Scholar] [CrossRef]
- Yang, L.; Miklavcic, S.J. Revised Kubelka–Munk Theory. III. A General Theory of Light Propagation in Scattering and Absorptive Media. J. Opt. Soc. Am. A 2005, 22, 1866–1873. [Google Scholar] [CrossRef]




| Nb Content (mol %) | 0 | 1.5 | 3.0 | 5.0 |
| Eg (eV) | 3.06 | 3.07 | 3.11 | 3.14 |
| ECBM–EF (eV) | 0.46 | 0.37 | 0.41 | 0.64 |
| Sample | Jsc (mA/cm2) | Voc (V) | FF (%) | H (%) |
|---|---|---|---|---|
| Nb 0 mol % | 4.5 | 0.40 | 63 | 1.1 |
| Nb 1.5 mol % | 5.5 | 0.41 | 59 | 1.3 |
| Nb 3.0 mol % | 5.7 | 0.38 | 57 | 1.2 |
| Nb 5.0 mol % | 4.4 | 0.36 | 56 | 0.87 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eguchi, R.; Takekuma, Y.; Ochiai, T.; Nagata, M. Improving Interfacial Charge-Transfer Transitions in Nb-Doped TiO2 Electrodes with 7,7,8,8-Tetracyanoquinodimethane. Catalysts 2018, 8, 367. https://doi.org/10.3390/catal8090367
Eguchi R, Takekuma Y, Ochiai T, Nagata M. Improving Interfacial Charge-Transfer Transitions in Nb-Doped TiO2 Electrodes with 7,7,8,8-Tetracyanoquinodimethane. Catalysts. 2018; 8(9):367. https://doi.org/10.3390/catal8090367
Chicago/Turabian StyleEguchi, Reo, Yuya Takekuma, Tsuyoshi Ochiai, and Morio Nagata. 2018. "Improving Interfacial Charge-Transfer Transitions in Nb-Doped TiO2 Electrodes with 7,7,8,8-Tetracyanoquinodimethane" Catalysts 8, no. 9: 367. https://doi.org/10.3390/catal8090367