Improvement of the Catalytic Efficiency of Butene Oligomerization Using Alkali Metal Hydroxide-Modified Hierarchical ZSM-5 Catalysts
Abstract
:1. Introduction
2. Results
2.1. Characterization of Catalysts
2.1.1. X-ray Diffraction Analysis
2.1.2. X-ray Fluorescence Analysis
2.1.3. Porous Structure Characterization
2.1.4. TEM Investigations
2.1.5. NH3-TPD Analysis
2.1.6. Pyridine FT-IR Analysis
2.2. Catalytic Performance
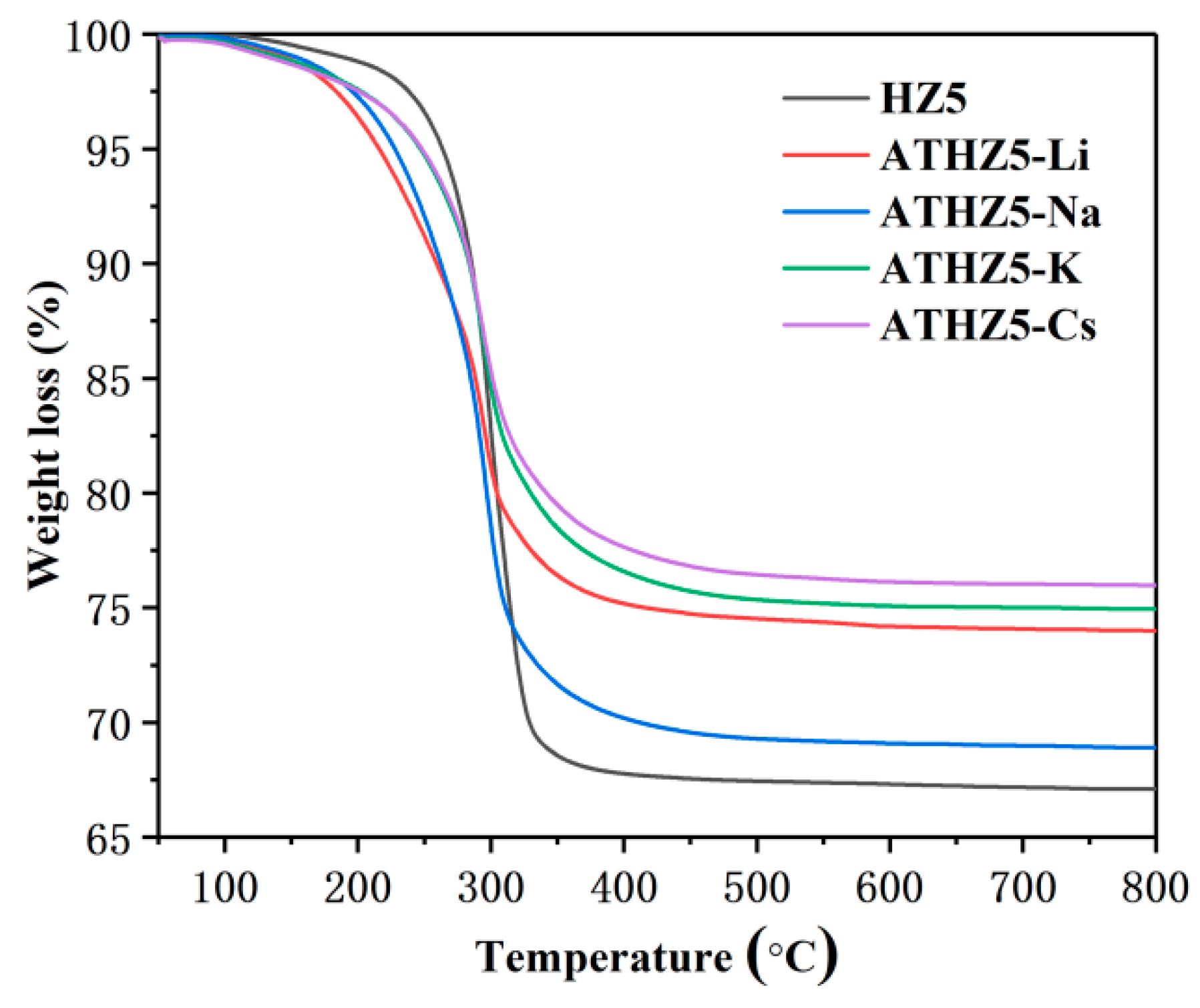
2.3. Thermogravimetric Analysis
2.4. Schematic Illustration of the Improved Diffusion
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl. Catal. A Gen. 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Park, Y.-K.; Lee, C.W.; Kang, N.Y.; Choi, W.C.; Choi, S.; Oh, S.H.; Park, D.S. Catalytic cracking of lower-valued hydrocarbons for producing light olefins. Catal. Surv. Asia 2010, 14, 75–84. [Google Scholar] [CrossRef]
- Tanabe, K.; Hölderich, W.F. Industrial application of solid acid–base catalysts. Appl. Catal. A Gen. 1999, 181, 399–434. [Google Scholar] [CrossRef]
- Skupinska, J. Oligomerization of a-olefins to higher oligomers. Chem. Rev. 1991, 91, 613–648. [Google Scholar] [CrossRef]
- Ding, X.; Li, C.; Yang, C. Study on the oligomerization of ethylene in fluidized catalytic cracking (FCC) dry gas over metal-loaded HZSM-5 catalysts. Energy Fuels 2010, 24, 3760–3763. [Google Scholar] [CrossRef]
- Catani, R.; Mandreoli, M.; Rossini, S.; Vaccari, A. Mesoporous catalysts for the synthesis of clean diesel fuels by oligomerisation of olefins. Catal. Today 2002, 75, 125–131. [Google Scholar] [CrossRef]
- Schmidt, R.; Welch, M.B.; Randolph, B.B. Oligomerization of C5 olefins in light catalytic naphtha. Energy Fuels 2008, 22, 1148–1155. [Google Scholar] [CrossRef]
- Wulfers, M.J.; Lobo, R.F. Assessment of mass transfer limitations in oligomerization of butene at high pressure on H-beta. Appl. Catal. A Gen. 2015, 505, 394–401. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Serrano-Ruiz, J.C.; Dumesic, J.A. Production of liquid hydrocarbon transportation fuels by oligomerization of biomass-derived C9 alkenes. Green Chem. 2010, 12, 992–999. [Google Scholar] [CrossRef]
- Kriván, E.; Tomasek, S.; Hancsók, J. The oligomerization of high olefin containing hydrocarbon by-products to clean engine fuels. J. Clean. Prod. 2016, 136, 81–88. [Google Scholar] [CrossRef]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of g-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Malaika, A.; Rechnia-Gorący, P.; Kot, M.; Kozłowski, M. Selective and efficient dimerization of isobutene over H3PO4/activated carbon catalysts. Catal. Today 2018, 301, 266–273. [Google Scholar] [CrossRef]
- Martens, L.R.; Verduijn, J.P.; Mathys, G.M. The development of an environmental friendly catalytic system for the conversion of olefins. Catal. Today 1997, 36, 451–460. [Google Scholar] [CrossRef] [Green Version]
- De Klerk, A. Oligomerization of fischer-tropsch olefins to distillates over amorphous silica-alumina. Energy Fuels 2006, 20, 1799–1805. [Google Scholar] [CrossRef]
- Occelli, M.L.; Hsu, J.T.; Galaya, L.G. Propylene oligomerization over molecular sieves part I. Zeolite effects on reactivity and liquid product selectivities. J. Mol. Catal. 1985, 32, 377–390. [Google Scholar] [CrossRef]
- Bjørgen, M.; Lillerud, K.P.; Olsbye, U.; Bordiga, S.; Zecchina, A. 1-Butene oligomerization in bronsted acidic zeolites: Mechanistic insights from low-temperature in situ FTIR spectroscopy. J. Phys. Chem. B 2004, 108, 7862–7870. [Google Scholar] [CrossRef]
- Coma, A. Inorganic solid acids and their use in acid-catalyzed hydrocarbon reactions. Chem. Rev. 1995, 95, 559–614. [Google Scholar]
- Martínez, C.; Corma, A. Inorganic molecular sieves: Preparation, modification and industrial application in catalytic processes. Coord. Chem. Rev. 2011, 255, 1558–1580. [Google Scholar] [CrossRef] [Green Version]
- Martens, J.A.; Ravishankar, R.; Mishin, I.E.; Jacobs, P.A. Tailored alkene oligomerization with H-ZSM-57 zeolite. Angew. Chem. Int. Ed. 2000, 39, 4376–4379. [Google Scholar] [CrossRef]
- Kokotailo, G.T.; Lawton, S.L.; Olson, D.H.; Meier, W.M. Structure of synthetic zeolite zsm-5. Nature 1978, 272, 437. [Google Scholar] [CrossRef]
- Tabak, S.A.; Krambeck, F.J.; Garwood, W.E. Conversion of propylene and butylene over ZSM-5 catalyst. AIChE J. 1986, 32, 1526–1531. [Google Scholar] [CrossRef]
- Quann, R.J.; Green, L.A.; Tabak, S.A.; Krambeck, F.J. Chemistry of olefin oligomerization over ZSM-5 catalyst. Ind. Eng. Chem. Res. 1988, 27, 565–570. [Google Scholar] [CrossRef]
- Nicholas, C.P. Applications of light olefin oligomerization to the production of fuels and chemicals. Appl. Catal. A Gen. 2017, 543, 82–97. [Google Scholar] [CrossRef]
- Margarit, V.J.; Diaz-Rey, M.R.; Navarro, M.T.; Martinez, C.; Corma, A. Direct synthesis of nano-ferrierite along the 10-ring-channel direction boosts their catalytic behavior. Angew. Chem. Int. Ed. 2018, 57, 3459–3463. [Google Scholar] [CrossRef] [PubMed]
- Gallego, E.M.; Paris, C.; Diaz-Rey, M.R.; Martinez-Armero, M.E.; Martinez-Triguero, J.; Martinez, C.; Moliner, M.; Corma, A. Simple organic structure directing agents for synthesizing nanocrystalline zeolites. Chem. Sci. 2017, 8, 8138–8149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nkosi, B.; Ng, F.T.T.; Rempel, G.L. The oligomerization of butenes with partially alkali exchanged NiNaY zeolite catalysts. Appl. Catal. A Gen. 1997, 158, 225–241. [Google Scholar] [CrossRef]
- Silva, A.F.; Fernandes, A.; Antunes, M.M.; Neves, P.; Rocha, S.M.; Ribeiro, M.F.; Pillinger, M.; Ribeiro, J.; Silva, C.M.; Valente, A.A. TUD-1 type aluminosilicate acid catalysts for 1-butene oligomerisation. Fuel 2017, 209, 371–382. [Google Scholar] [CrossRef]
- Hwang, A.; Kim, S.; Kwak, G.; Kim, S.K.; Park, H.-G.; Kang, S.C.; Jun, K.-W.; Kim, Y.T. Low temperature oligomerization of ethylene over Ni/Al-KIT-6 catalysts. Catal. Lett. 2017, 147, 1303–1314. [Google Scholar] [CrossRef]
- Hulea, V.; Fajula, F. Ni-exchanged AlMCM-41—An efficient bifunctional catalyst for ethylene oligomerization. J. Catal. 2004, 225, 213–222. [Google Scholar] [CrossRef]
- Corma, A.; Martínez, C.; Doskocil, E. Designing MFI-based catalysts with improved catalyst life for oligomerization to high-quality liquid fuels. J. Catal. 2013, 300, 183–196. [Google Scholar] [CrossRef] [Green Version]
- Bertrand-Drira, C.; Cheng, X.-W.; Cacciaguerra, T.; Trens, P.; Melinte, G.; Ersen, O.; Minoux, D.; Finiels, A.; Fajula, F.; Gerardin, C. Mesoporous mordenites obtained by desilication: Mechanistic considerations and evaluation in catalytic oligomerization of pentene. Microporous Mesoporous Mater. 2015, 213, 142–149. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Song, C.; Lux, K.W.; Namazian, M.; Imam, T. Oligomerization of biomass-derived light olefins to liquid fuel: Effect of alkali treatment on the HZSM-5 catalyst. Ind. Eng. Chem. Res. 2017, 56, 12046–12055. [Google Scholar] [CrossRef]
- Keller, T.C.; Desai, K.; Mitchell, S.; Pérez-Ramírez, J. Design of base zeolite catalysts by alkali-metal grafting in alcoholic media. ACS Catal. 2015, 5, 5388–5396. [Google Scholar] [CrossRef]
- Dapsens, P.Y.; Mondelli, C.; Perez-Ramirez, J. Highly selective lewis acid sites in desilicated MFI zeolites for dihydroxyacetone isomerization to lactic acid. ChemSusChem 2013, 6, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhang, L.; Kang, J.; Peng, X.; Zhang, Q.; Wang, Y. Selective transformation of syngas into gasoline-range hydrocarbons over mesoporous H-ZSM-5-supported cobalt nanoparticles. Chem. Eur. J. 2015, 21, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.S.; Svelle, S.; Joensen, F.; Beato, P.; Christensen, C.H.; Bordiga, S.; Bjørgen, M. Assessing the acid properties of desilicated ZSM-5 by FTIR using CO and 2,4,6-trimethylpyridine (collidine) as molecular probes. Appl. Catal. A Gen. 2009, 356, 23–30. [Google Scholar] [CrossRef]
- Zhu, X.; Lobban, L.L.; Mallinson, R.G.; Resasco, D.E. Tailoring the mesopore structure of HZSM-5 to control product distribution in the conversion of propanal. J. Catal. 2010, 271, 88–98. [Google Scholar] [CrossRef]
- Sadowska, K.; Wach, A.; Olejniczak, Z.; Kuśtrowski, P.; Datka, J. Hierarchic zeolites: Zeolite ZSM-5 desilicated with NaOH and NaOH/tetrabutylamine hydroxide. Microporous Mesoporous Mater. 2013, 167, 82–88. [Google Scholar] [CrossRef]
- Vicente, J.; Gayubo, A.G.; Ereña, J.; Aguayo, A.T.; Olazar, M.; Bilbao, J. Improving the DME steam reforming catalyst by alkaline treatment of the HZSM-5 zeolite. Appl. Catal. B Environ. 2013, 130–131, 73–83. [Google Scholar] [CrossRef]
- Hoff, T.C.; Gardner, D.W.; Thilakaratne, R.; Proano-Aviles, J.; Brown, R.C.; Tessonnier, J.-P. Elucidating the effect of desilication on aluminum-rich ZSM-5 zeolite and its consequences on biomass catalytic fast pyrolysis. Appl. Catal. A Gen. 2017, 529, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Groen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Alkaline posttreatment of MFI zeolites. From accelerated screening to scale-up. Ind. Eng. Chem. Res. 2007, 46, 4193–4201. [Google Scholar] [CrossRef]
- Deng, Q.; Zhang, X.; Wang, L.; Zou, J.-J. Catalytic isomerization and oligomerization of endo-dicyclopentadiene using alkali-treated hierarchical porous HZSM-5. Chem. Eng. Sci. 2015, 135, 540–546. [Google Scholar] [CrossRef]
- Le Van Mao, R.; Nguyen, T.M.; Yao, J. Conversion of ethanol in aqueous solution over ZSM-5 zeolites: Influence of reaction parameters and catalyst acidic properties as studied by ammonia TPD technique. Appl. Catal. 1990, 61, 161–173. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Alonso, A.; Valle, B.; Aguayo, A.T.; Bilbao, J. Selective production of olefins from bioethanol on HZSM-5 zeolite catalysts treated with naoh. Appl. Catal. B Environ. 2010, 97, 299–306. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Alonso, A.; Valle, B.; Aguayo, A.T.; Bilbao, J. Deactivation kinetics of a HZSM-5 zeolite catalyst treated with alkali for the transformation of bio-ethanol into hydrocarbons. AIChE J. 2012, 58, 526–537. [Google Scholar] [CrossRef]
- Xin, H.; Li, X.; Fang, Y.; Yi, X.; Hu, W.; Chu, Y.; Zhang, F.; Zheng, A.; Zhang, H.; Li, X. Catalytic dehydration of ethanol over post-treated ZSM-5 zeolites. J. Catal. 2014, 312, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Campbell, S.M.; Bibby, D.M.; Coddington, J.M.; Howe, R.F. Dealumination of HZSM-5 zeolites, II. Methanol to gasoline conversion. J. Catal. 1996, 161, 350–358. [Google Scholar] [CrossRef]
- Muraza, O.; Sanhoob, M.A.; Siddiqui, M.A.B. Fabrication of desilicated MTW zeolite and its application in catalytic cracking of n-heptane. Adv. Powder Technol. 2016, 27, 372–378. [Google Scholar] [CrossRef]
- Aslam, W.; Siddiqui, M.A.B.; Rabindran Jermy, B.; Aitani, A.; Čejka, J.; Al-Khattaf, S. Selective synthesis of linear alkylbenzene by alkylation of benzene with 1-dodecene over desilicated zeolites. Catal. Today 2014, 227, 187–197. [Google Scholar] [CrossRef]
- Jun, Y.; Lee, S.; Lee, K.; Choi, M. Effects of secondary mesoporosity and zeolite crystallinity on catalyst deactivation of ZSM-5 in propanal conversion. Microporous Mesoporous Mater. 2017, 245, 16–23. [Google Scholar] [CrossRef]
- Mantilla, A.; Ferrat, G.; López-Ortega, A.; Romero, E.; Tzompantzi, F.; Torres, M.; Ortíz-Islas, E.; Gómez, R. Catalytic behavior of sulfated TiO2 in light olefins oligomerization. J. Mol. Catal. A Chem. 2005, 228, 333–338. [Google Scholar] [CrossRef]
- Yoon, J.W.; Jhung, S.H.; Choo, D.H.; Lee, S.J.; Lee, K.-Y.; Chang, J.-S. Oligomerization of isobutene over dealuminated Y zeolite catalysts. Appl. Catal. A Gen. 2008, 337, 73–77. [Google Scholar] [CrossRef]
- Xu, Z.; Chada, J.P.; Zhao, D.; Carrero, C.A.; Kim, Y.T.; Rosenfeld, D.C.; Rogers, J.L.; Rozeveld, S.J.; Hermans, I.; Huber, G.W. Production of linear octenes from oligomerization of 1-butene over carbon-supported cobalt catalysts. ACS Catal. 2016, 6, 3815–3825. [Google Scholar] [CrossRef]
- Brogaard, R.Y.; Olsbye, U. Ethene oligomerization in Ni-containing zeolites: Theoretical discrimination of reaction mechanisms. ACS Catal. 2016, 6, 1205–1214. [Google Scholar] [CrossRef]
- Kim, Y.T.; Chada, J.P.; Xu, Z.; Pagan-Torres, Y.J.; Rosenfeld, D.C.; Winniford, W.L.; Schmidt, E.; Huber, G.W. Low-temperature oligomerization of 1-butene with H-ferrierite. J. Catal. 2015, 323, 33–44. [Google Scholar] [CrossRef]
- Kumar, N.; Mäki-Arvela, P.; Yläsalmi, T.; Villegas, J.; Heikkilä, T.; Leino, A.R.; Kordás, K.; Salmi, T.; Yu Murzin, D. Dimerization of 1-butene in liquid phase reaction: Influence of structure, pore size and acidity of beta zeolite and MCM-41 mesoporous material. Microporous Mesoporous Mater. 2012, 147, 127–134. [Google Scholar] [CrossRef]
- Ding, K.; Zhong, Z.; Wang, J.; Zhang, B.; Addy, M.; Ruan, R. Effects of alkali-treated hierarchical HZSM-5 zeolites on the production of aromatic hydrocarbons from catalytic fast pyrolysis of waste cardboard. J. Anal. Appl. Pyrolysis 2017, 125, 153–161. [Google Scholar] [CrossRef]
- Yoon, J.; Chang, J.; Lee, H.; Kim, T.; Jhung, S. Trimerization of isobutene over a zeolite beta catalyst. J. Catal. 2007, 245, 253–256. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, S.-S.; Pinnavaia, T.J.; Tzompantzi, F.; Prince, J.; Valente, J.S. Selective isobutene oligomerization by mesoporous MSU-SBEA catalysts. J. Phys. Chem. C 2011, 115, 5809–5816. [Google Scholar] [CrossRef]
- Popov, A.G.; Pavlov, V.S.; Ivanova, I.I. Effect of crystal size on butenes oligomerization over MFI catalysts. J. Catal. 2016, 335, 155–164. [Google Scholar] [CrossRef]
- Moon, S.; Chae, H.-J.; Park, M.B. Oligomerization of light olefins over ZSM-5 and beta zeolite catalysts by modifying textural properties. Appl. Catal. A Gen. 2018, 553, 15–23. [Google Scholar] [CrossRef]
- Díaz-Rey, M.R.; Paris, C.; Martínez-Franco, R.; Moliner, M.; Martínez, C.; Corma, A. Efficient oligomerization of pentene into liquid fuels on nanocrystalline beta zeolites. ACS Catal. 2017, 7, 6170–6178. [Google Scholar] [CrossRef]
- Jin, F.; Li, Y. A FTIR and TPD examination of the distributive properties of acid sites on ZSM-5 zeolite with pyridine as a probe molecule. Catal. Today 2009, 145, 101–107. [Google Scholar] [CrossRef]

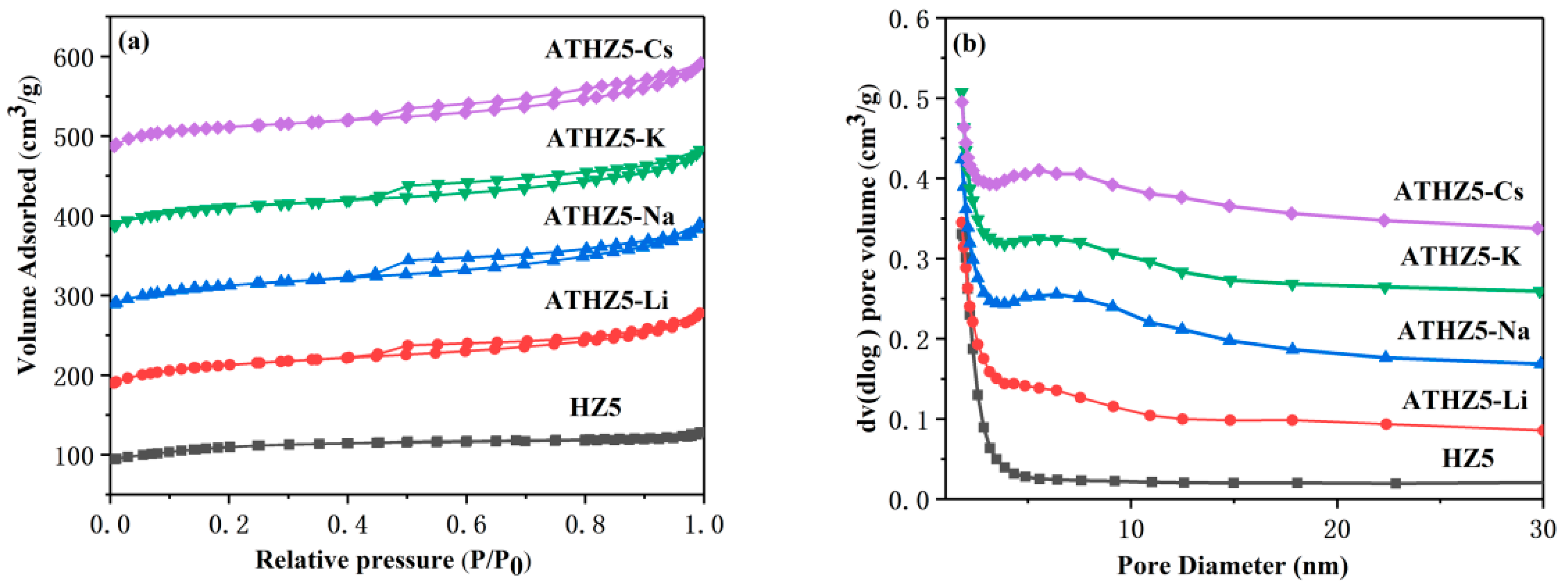

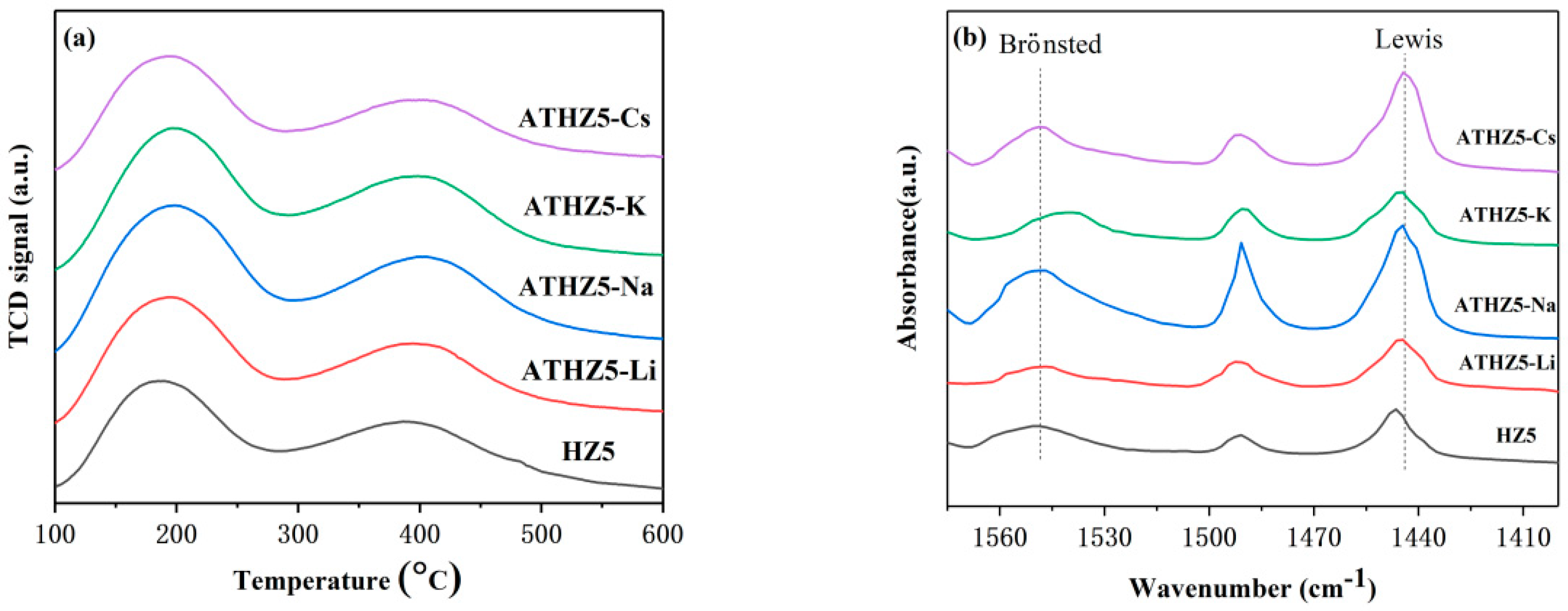
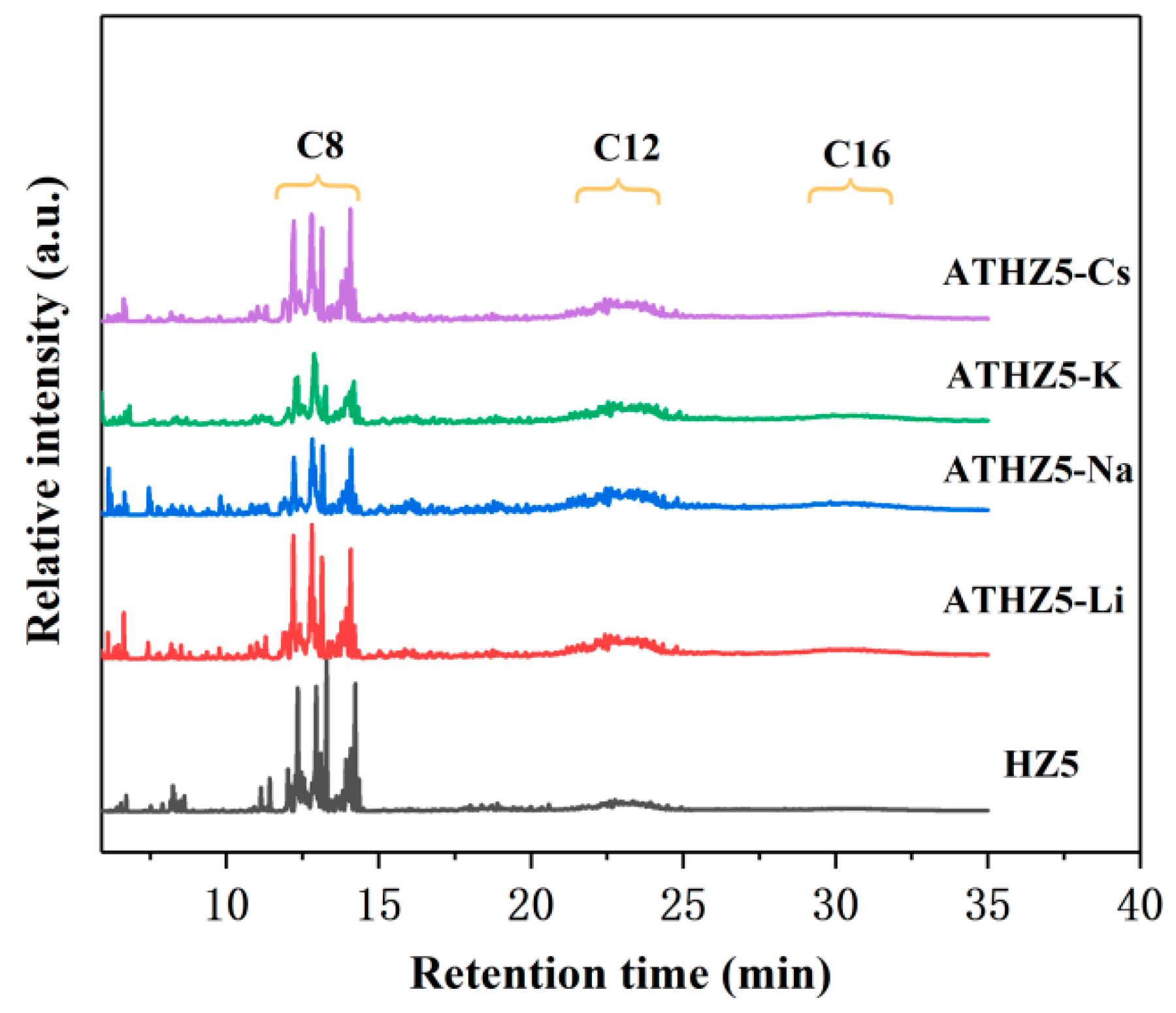
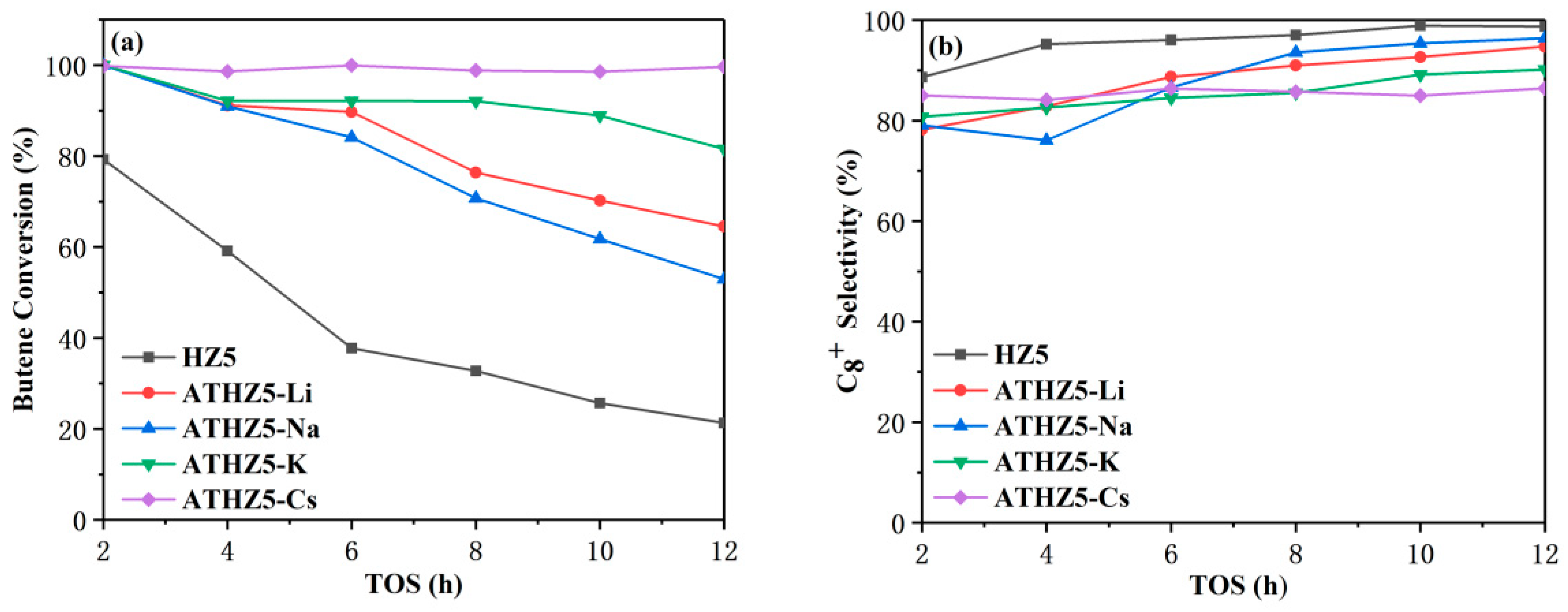

| Catalyst | Si/Al a | SBET (m2/g) b | Smicro (m2/g) c | Smeso (m2/g) c | VTotal (cm3/g) | Vmicro (cm3/g) c | Vmeso (cm3/g) d |
|---|---|---|---|---|---|---|---|
| HZ5 | 20.2 | 359.2 | 234.9 | 124.3 | 0.190 | 0.115 | 0.075 |
| ATHZ5-Li | 12.1 | 365.3 | 223.1 | 142.2 | 0.276 | 0.110 | 0.167 |
| ATHZ5-Na | 11.9 | 374.9 | 218.4 | 156.5 | 0.298 | 0.107 | 0.191 |
| ATHZ5-K | 11.8 | 373.1 | 223.8 | 149.3 | 0.286 | 0.109 | 0.177 |
| ATHZ5-Cs | 11.9 | 367.5 | 235.2 | 132.3 | 0.303 | 0.119 | 0.184 |
| Catalyst | Acidity by Strength a (mmol/g) | Acidity by Type b (mmol/g) | ||||
|---|---|---|---|---|---|---|
| Weak | Strong | Total | Brönsted | Lewis | L/B Ratio c | |
| HZ5 | 0.66 | 0.44 | 1.10 | 0.38 | 0.35 | 0.92 |
| ATHZ5-Li | 0.86 | 0.78 | 1.64 | 0.34 | 0.40 | 1.18 |
| ATHZ5-Na | 1.08 | 0.92 | 2.00 | 1.05 | 0.82 | 0.78 |
| ATHZ5-K | 0.94 | 0.91 | 1.85 | 0.4 | 0.41 | 1.03 |
| ATHZ5-Cs | 0.74 | 0.64 | 1.38 | 0.61 | 0.74 | 1.21 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Ke, M.; Song, Z.; Liu, Y.; Shan, W.; Wang, Q.; Xia, C.; Li, C.; He, C. Improvement of the Catalytic Efficiency of Butene Oligomerization Using Alkali Metal Hydroxide-Modified Hierarchical ZSM-5 Catalysts. Catalysts 2018, 8, 298. https://doi.org/10.3390/catal8080298
Zhang L, Ke M, Song Z, Liu Y, Shan W, Wang Q, Xia C, Li C, He C. Improvement of the Catalytic Efficiency of Butene Oligomerization Using Alkali Metal Hydroxide-Modified Hierarchical ZSM-5 Catalysts. Catalysts. 2018; 8(8):298. https://doi.org/10.3390/catal8080298
Chicago/Turabian StyleZhang, Lei, Ming Ke, Zhaozheng Song, Yang Liu, Wenbo Shan, Qi Wang, Chengjie Xia, Changchun Li, and Chunyu He. 2018. "Improvement of the Catalytic Efficiency of Butene Oligomerization Using Alkali Metal Hydroxide-Modified Hierarchical ZSM-5 Catalysts" Catalysts 8, no. 8: 298. https://doi.org/10.3390/catal8080298
APA StyleZhang, L., Ke, M., Song, Z., Liu, Y., Shan, W., Wang, Q., Xia, C., Li, C., & He, C. (2018). Improvement of the Catalytic Efficiency of Butene Oligomerization Using Alkali Metal Hydroxide-Modified Hierarchical ZSM-5 Catalysts. Catalysts, 8(8), 298. https://doi.org/10.3390/catal8080298




