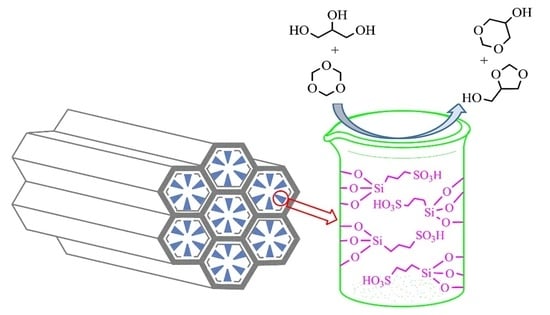
Propylsulfonic Acid Functionalized SBA-15 Mesoporous Silica as Efficient Catalysts for the Acetalization of Glycerol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Materials Characterization
2.2. Catalytic Activity for Glycerol Acetalization
2.3. Reaction Mechanism
2.4. Catalyst Reusability
3. Experimental
3.1. Chemicals and Materials
3.2. Catalysts Preparation
3.3. Catalyst Characterization
3.4. Catalytic Activity Tests
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhou, C.H.; Beltramini, J.N.; Fan, Y.X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. Appl. Catal. B Environ. 2016, 193, 75–92. [Google Scholar] [CrossRef] [Green Version]
- Katryniok, B.; Paul, S.; Dumeignil, F. Recent Developments in the Field of Catalytic Dehydration of Glycerol to Acrolein. ACS Catal. 2013, 3, 1819–1834. [Google Scholar] [CrossRef]
- Bauer, F.; Hulteberg, C. Is there a future in glycerol as a feedstock in the production of biofuels and biochemicals? Biofuels Bioprod. Biorefin. 2013, 7, 43–51. [Google Scholar] [CrossRef]
- Rühl, C.; Giljum, J. BP Energy Outlook 2030. Presented at International Association for Energy Economics, Stockholm, Sweden, 19–23 June 2011. [Google Scholar]
- Wang, Y.; Zhou, J.; Guo, X. Catalytic hydrogenolysis of glycerol to propanediols: A review. RSC Adv. 2015, 5, 74611–74628. [Google Scholar] [CrossRef]
- Kusunoki, Y.; Miyazawa, T.; Kunimori, K.; Tomishige, K. Highly active metal–acid bifunctional catalyst system for hydrogenolysis of glycerol under mild reaction conditions. Catal. Commun. 2005, 6, 645–649. [Google Scholar] [CrossRef]
- Furikado, I.; Miyazawa, T.; Koso, S.; Shimao, A.; Kunimori, K.; Tomishige, K. Catalytic performance of Rh/SiO2 in glycerol reaction under hydrogen. Green Chem. 2007, 9, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Soares, R.R.; Simonetti, D.A.; Dumesic, J.A. Glycerol as a source for fuels and chemicals by low-temperature catalytic processing. Angew. Chem. Int. Ed. 2006, 45, 3982–3985. [Google Scholar] [CrossRef] [PubMed]
- Katryniok, B.; Kimura, H.; Skrzyńska, E.; Girardon, J.S.; Fongarland, P.; Capron, M.; Ducoulombier, R.; Mimura, N.; Paul, S.; Dumeignil, F. Selective catalytic oxidation of glycerol: Perspectives for high value chemicals. Green Chem. 2011, 13, 1960–1979. [Google Scholar] [CrossRef]
- Chai, S.; Wang, H.; Liang, Y.; Xu, B. Sustainable production of acrolein: Gas-phase dehydration of glycerol over Nb2O5 catalyst. J. Catal. 2007, 250, 342–349. [Google Scholar] [CrossRef]
- Pawar, R.R.; Gosai, K.A.; Bhatt, A.S.; Kumaresan, S.; Lee, S.M.; Bajaj, H.C. Clay catalysed rapid valorization of glycerol towards cyclic acetals and ketals. RSC Adv. 2015, 5, 83985–83996. [Google Scholar] [CrossRef]
- Garcia, E.; Laca, M.; Pérez, E.; Garrido, A.; Peinado, J. New Class of Acetal Derived from Glycerin as a Biodiesel Fuel Component. Energy Fuels 2008, 22, 4274–4280. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Velty, A. Synthesis of hyacinth, vanilla, and blossom orange fragrances: The benefit of using zeolites and delaminated zeolites as catalysts. Appl. Catal. A Gen. 2004, 263, 155–161. [Google Scholar] [CrossRef]
- Climent, M.J.; Velty, A.; Corma, A. Design of a solid catalyst for the synthesis of a molecule with blossom orange scent. Green Chem. 2002, 4, 565–569. [Google Scholar] [CrossRef]
- Konwar, L.J.; Samikannu, A.; Mäki-Arvela, P.; Boström, D.; Mikkola, J.P. Lignosulfonate-based macro/mesoporous solid protonic acids for acetalization of glycerol to bio-additives. Appl. Catal. B Environ. 2018, 220, 314–323. [Google Scholar] [CrossRef]
- Da Silva, C.X.A.; Gonçalves, V.L.C.; Mota, C.J.A. Water-tolerant zeolite catalyst for the acetalisation of glycerol. Green Chem. 2009, 11, 38–41. [Google Scholar] [CrossRef]
- Trifoi, A.R.; Agachi, P.S.; Pap, T. Glycerol acetals and ketals as possible diesel additives: A review of their synthesis protocols. Renew. Sustain. Energy. Rev. 2016, 62, 804–814. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Showler, A.J.; Darley, P.A. Condensation products of glycerol with aldehydes and ketones 2-substituted m-diloxan-5-ols and 1,3-dioxolane-4-methanols. Chem. Rev. 1998, 67, 427–440. [Google Scholar] [CrossRef]
- Wang, J.; Shen, X.; Chi, R. The Synthesis Method of Glycerol Methyl Acetal. CN Patent 101525329, 9 September 2009. [Google Scholar]
- Gras, J.L.; Nouguier, R.; Mchich, M. Transacetalisation de triols a partir du dimethoxymethane. Selectivite et applications synthetiques. Tetrahedron Lett. 1987, 28, 6601–6604. [Google Scholar] [CrossRef]
- Umbarkar, S.B.; Kotbagi, T.V.; Biradar, A.V.; Pasricha, R.; Chanale, J.; Dongare, M.K.; Mamede, A.S.; Lancelot, C.; Payen, E. Acetalization of glycerol using mesoporous MoO3/SiO2 solid acid catalyst. J. Mol. Catal. A Chem. 2009, 310, 150–158. [Google Scholar] [CrossRef]
- Dodson, J.R.; Leite, T.; Pontes, N.S.; Peres Pinto, B.; Mota, C.J. Green acetylation of solketal and glycerol formal by heterogeneous acid catalysts to form a biodiesel fuel additive. ChemSusChem 2014, 7, 2728–2734. [Google Scholar] [CrossRef] [PubMed]
- Nair, G.S.; Adrijanto, E.; Alsalme, A.; Kozhevnikov, I.V.; Cooke, D.J.; Brown, D.R.; Shiju, N.R. Glycerol utilization: Solvent-free acetalisation over niobia catalysts. Catal. Sci. Technol. 2012, 2, 1173–1179. [Google Scholar] [CrossRef]
- Reddy, P.S.; Sudarsanam, P.; Mallesham, B.; Raju, G.; Reddy, B.M. Acetalisation of glycerol with acetone over zirconia and promoted zirconia catalysts under mild reaction conditions. J. Ind. Eng. Chem. 2011, 17, 377–381. [Google Scholar] [CrossRef]
- Chen, L.; Nohair, B.; Kaliaguine, S. Glycerol acetalization with formaldehyde using water-tolerant solid acids. Appl. Catal. A Gen. 2016, 509, 143–152. [Google Scholar] [CrossRef]
- Chen, L.; Nohair, B.; Zhao, D.; Kaliaguine, S. Glycerol acetalization with formaldehyde using heteropolyacid salts supported on mesostructured silica. Appl. Catal. A Gen. 2018, 549, 207–215. [Google Scholar] [CrossRef]
- Deutsch, J.; Martin, A.; Lieske, H. Investigations on heterogeneously catalysed condensations of glycerol to cyclic acetals. J. Catal. 2007, 245, 428–435. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Trewyn, B.G.; Lin, V.S.Y. Mesoporous silica nanoparticles: Structural design and applications. J. Mater. Chem. 2010, 20, 7924–7937. [Google Scholar] [CrossRef]
- Das, D.; Lee, J.F.; Cheng, S. Selective synthesis of Bisphenol-A over mesoporous MCM silica catalysts functionalized with sulfonic acid groups. J. Catal. 2004, 223, 152–160. [Google Scholar] [CrossRef]
- Dhainaut, J.; Dacquin, J.P.; Lee, A.F.; Wilson, K. Hierarchical macroporous–mesoporous SBA-15 sulfonic acidcatalysts for biodiesel synthesis. Green Chem. 2010, 12, 296–303. [Google Scholar] [CrossRef]
- Mercier, L.; Pinnavaia, T.I. Access in Mesoporous Materials: Advantages of a Uniform Pore Structure in the Design of a Heavy Metal Ion Adsorbent for Environmental Remediation. Adv. Mater. 1997, 9, 500–503. [Google Scholar] [CrossRef]
- Mercier, L.; Pinnavaia, T.J. Heavy Metal Ion Adsorbents Formed by the Grafting of a Thiol Functionality to Mesoporous Silica Molecular Sieves: Factors Affecting Hg(II) Uptake. Environ. Sci. Technol. 1998, 32, 2749–2754. [Google Scholar] [CrossRef]
- Díaz, I.; Márquez-Alvarez, C.; Mohino, F.; Pérez-Pariente, J.N.; Sastre, E. Combined Alkyl and Sulfonic Acid Functionalization of MCM-41-Type Silica. J. Catal. 2000, 193, 283–294. [Google Scholar] [CrossRef]
- Karimi, B.; Mirzaei, H.M.; Mobaraki, A.; Vali, H. Sulfonic acid-functionalized periodic mesoporous organosilicas in esterification and selective acylation reactions. Catal. Sci. Technol. 2015, 5, 3624–3631. [Google Scholar] [CrossRef]
- Jun, S.; Kim, J.M.; Ryoo, R.; Ahn, Y.S.; Han, M.H. Hydrothermal stability of MCM-48 improved by postsynthesis restructuring in salt solution. Microporous Mesoporous Mater. 2000, 41, 119–127. [Google Scholar] [CrossRef]
- Rhijn, W.M.V.; Vos, D.E.D.; Sels, B.F.; Bossaert, W.D.; Jacobs, P.A. Sulfonic acid functionalised ordered mesoporous materials as catalysts for condensation and esterification reactions. Chem. Commun. 1998, 317–318. [Google Scholar] [CrossRef]
- Karimi, B.; Mirzaei, H.M.; Mobaraki, A. Periodic mesoporous organosilica functionalized sulfonic acids as highly efficient and recyclable catalysts in biodiesel production. Catal. Sci. Technol. 2012, 2, 828–834. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, Y.; Yang, H.; Meng, Y.; Yu, C.; Tu, B.; Zhao, D. Understanding Effect of Wall Structure on the Hydrothermal Stability of Mesostructured Silica SBA-15. J. Phys. Chem. B 2005, 109, 8723–8732. [Google Scholar] [CrossRef] [PubMed]
- Pirez, C.; Lee, A.F.; Manayil, J.C.; Parlett, C.M.A.; Wilson, K. Hydrothermal saline promoted grafting: A route to sulfonic acid SBA-15 silica with ultra-high acid site loading for biodiesel synthesis. Green Chem. 2014, 16, 4506–4509. [Google Scholar] [CrossRef]
- Dacquin, J.P.; Lee, A.F.; Pirez, C.; Wilson, K. Pore-expanded SBA-15 sulfonic acid silicas for biodiesel synthesis. Chem. Commun. 2012, 48, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the porous structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Konwar, L.J.; Das, R.; Thakur, A.J.; Salminen, E.; Mäki-Arvela, P.; Kumar, N.; Mikkola, J.P.; Deka, D. Biodiesel production from acid oils using sulfonated carbon catalyst derived from oil-cake waste. J. Mol. Catal. A Chem. 2014, 388–389, 167–176. [Google Scholar] [CrossRef]
- Konwar, L.J.; Mäki-Arvela, P.; Salminen, E.; Kumar, N.; Thakur, A.J.; Mikkola, J.P.; Deka, D. Towards carbon efficient biorefining: Multifunctional mesoporous solid acids obtained from biodiesel production wastes for biomass conversion. Appl. Catal. B Environ. 2015, 176–177, 20–35. [Google Scholar] [CrossRef]
- Roberts, A.D.; Li, X.; Zhang, H. Hierarchically porous sulfur-containing activated carbon monoliths via ice-templating and one-step pyrolysis. Carbon 2015, 95, 268–278. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Zhao, Z.; Chen, Y.; Lan, J. A DFT study of the structural units in SBA-15 mesoporous molecular sieve. Comput. Theor. Chem. 2011, 963, 403–411. [Google Scholar] [CrossRef]
- Cano-Serrano, E.; Campos-Martin, J.M.; Fierro, J.L.G. Sulfonic acid-functionalized silica through quantitative oxidation of thiol groups. Chem. Commun. 2003, 0, 246–247. [Google Scholar] [CrossRef]
- da Silva Ferreira, A.C.; Barbe, J.C.; Bertrand, A. Heterocyclic Acetals from Glycerol and Acetaldehyde in Port Wines: Evolution with Aging. J. Agric. Food Chem. 2002, 50, 2560–2564. [Google Scholar] [CrossRef] [PubMed]
- Chopade, S.P.; Sharma, M.M. Acetalization of ethylene glycol with formaldehyde using cation-exchange resins as catalysts: Batch versus reactive distilltion. React. Funct. Polym. 1997, 34, 37–45. [Google Scholar] [CrossRef]
- Agirre, I.; Garcıa, I.; Requies, J.; Barrio, V.L.; Guemez, M.B.; Cambra, J.F.; Arias, P.L. Glycerol acetals, kinetic study of the reaction between glycerol and formaldehyde. Biomass Bioenergy 2011, 35, 3636–3642. [Google Scholar] [CrossRef]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 2136–2137. [Google Scholar] [CrossRef]
- Margolese, D.; Melero, J.A.; Christiansen, S.C.; Chmelka, B.F.; Stucky, G.D. Direct Syntheses of Ordered SBA-15 Mesoporous Silica Containing Sulfonic Acid Groups. Chem. Mater. 2000, 12, 2448–2459. [Google Scholar] [CrossRef]
- Burkett, S.L.; Sims, S.D.; Mann, S. Synthesis of hybrid inorganic-organic mesoporous silica by co-condensation of siloxane and organosiloxane precursors. Chem. Commun. 1996, 0, 1367–1368. [Google Scholar] [CrossRef]
- Karimi, B.; Zareyee, D. Design of a Highly Efficient and Water-Tolerant Sulfonic Acid Nanoreactor Based on Tunable Ordered Porous Silica for the von Pechmann Reaction. Org. Lett. 2008, 10, 3989–3992. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.H.; Stein, A. Comparative Studies of Grafting and Direct Syntheses of Inorganic-Organic Hybrid Mesoporous Materials. Chem. Mater. 1999, 11, 3285–3295. [Google Scholar] [CrossRef]

















| Samples | Surface Area a/m2g−1 | Pore Volume/cm3g−1 | Average Pore Diameter b/nm | Bulk S Content c/wt % | Surface S Content d/wt % | Acid Capacity e/mmol g−1 |
|---|---|---|---|---|---|---|
| SBA-15 | 541 | 1.30 | 9.8 | – | – | – |
| PrSO3H-SBA-15-Tol | 464 | 1.08 | 9.9 | 0.14 | 3.36 | 0.43 |
| PrSO3H-SBA-15-OP | 674 | 0.52 | 3.1 | 0.06 | 1.38 | 0.22 |
| PrSO3H-SBA-15-0 | 410 | 0.87 | 8.5 | 4.87 | 5.30 | 1.03 |
| PrSO3H-SBA-15-200 | 325 | 0.54 | 6.7 | 6.22 | 3.60 | 1.11 |
| PrSO3H-SBA-15-400 | 281 | 0.58 | 8.2 | 8.50 | 3.74 | 1.17 |
| PrSO3H-SBA-15-800 | 296 | 0.66 | 8.9 | 8.07 | 3.42 | 1.09 |
| KIT-6-80 | 539 | 0.57 | 4.3 | – | – | – |
| PrSO3H-KIT-6-80 | 362 | 0.37 | 4.1 | 1.34 | 2.56 | 0.67 |
| PrSO3H-KIT-6-100 | 443 | 0.42 | 3.8 | 5.90 | 5.21 | 0.60 |
| PrSO3H-KIT-6-120 | 402 | 0.44 | 4.4 | 2.03 | 4.95 | 0.55 |
| Entry | Cat | Yield of GF/% | Ratio of 5R to 6R |
|---|---|---|---|
| 1 | KIT-6-80 | 6.0 | 22:78 |
| 2 | PrSO3H-KIT-6-80 | 89.6 | 34:66 |
| 3 | PrSO3H-KIT-6-100 | 85.6 | 38:62 |
| 4 | PrSO3H-KIT-6-120 | 66.6 | 37:63 |
| 5 | PrSO3H-SBA-15-400 | 91.5 | 42:58 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Song, H.; Chen, J. Propylsulfonic Acid Functionalized SBA-15 Mesoporous Silica as Efficient Catalysts for the Acetalization of Glycerol. Catalysts 2018, 8, 297. https://doi.org/10.3390/catal8080297
Li R, Song H, Chen J. Propylsulfonic Acid Functionalized SBA-15 Mesoporous Silica as Efficient Catalysts for the Acetalization of Glycerol. Catalysts. 2018; 8(8):297. https://doi.org/10.3390/catal8080297
Chicago/Turabian StyleLi, Ruiyun, Heyuan Song, and Jing Chen. 2018. "Propylsulfonic Acid Functionalized SBA-15 Mesoporous Silica as Efficient Catalysts for the Acetalization of Glycerol" Catalysts 8, no. 8: 297. https://doi.org/10.3390/catal8080297